Abstract
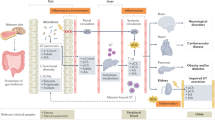
The observation that unhealthy diets (those that are low in whole grains, fruits and vegetables, and high in sugar, salt, saturated fat and ultra-processed foods) are a major risk factor for poor health outcomes has boosted interest in the concept of ‘food as medicine’. This concept is especially relevant to metabolic diseases, such as chronic kidney disease (CKD), in which dietary approaches are already used to ameliorate metabolic and nutritional complications. Increased awareness that toxic uraemic metabolites originate not only from intermediary metabolism but also from gut microbial metabolism, which is directly influenced by diet, has fuelled interest in the potential of ‘food as medicine’ approaches in CKD beyond the current strategies of protein, sodium and phosphate restriction. Bioactive nutrients can alter the composition and metabolism of the microbiota, act as modulators of transcription factors involved in inflammation and oxidative stress, mitigate mitochondrial dysfunction, act as senolytics and impact the epigenome by altering one-carbon metabolism. As gut dysbiosis, inflammation, oxidative stress, mitochondrial dysfunction, premature ageing and epigenetic changes are common features of CKD, these findings suggest that tailored, healthy diets that include bioactive nutrients as part of the foodome could potentially be used to prevent and treat CKD and its complications.
Key points
-
The foodome is the pool of all of the compounds that are present in a food sample and/or in a biological system that is interacting with the investigated food.
-
A food-as-medicine approach could be used as a novel strategy to utilize bioactive nutrients to target the uraemic phenotype in chronic kidney disease.
-
Epigenetic alterations, gut dysbiosis, mitochondrial dysfunction, inflammation, oxidative stress and premature ageing are common features of the uraemic phenotype that could potentially be targeted using a food-as-medicine approach.
-
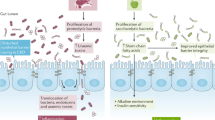
Gut dysbiosis is associated with inflammation and increased cardiovascular risk; prebiotics, probiotics, synbiotics and food components, including polyphenols, sugars and proteins, could alter the diversity of the gut microbiota and the production of uraemic toxins.
-
Senotherapeutic dietary compounds could potentially mitigate the effects of premature ageing in chronic kidney disease and associated complications, such as disturbed mitochondrial metabolism.
-
Natural bioactive compounds, including those found in turmeric, broccoli sprouts, berries, propolis and other foods, are potential nutritional therapeutic agents that could modulate the expression of pro-inflammatory transcription factors and the inflammasome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Global Burden of Diseas. Diet collaborators. health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393, 1958–1972 (2019).
Srour, B. et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the nutrinet-santé prospective cohort. JAMA Intern. Med. 180, 283–291 (2019).
World Health Organization. WHO report on the global tobacco epidemic. WHO https://www.who.int/tobacco/global_report/2017/en/ (2017).
Stenvinkel, P., Meyer, C. J., Block, G. A., Chertow, G. M., Shiels, P. G. Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2-related factor 2-lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfz120 (2019).
O’Neill, B. & Raggi, P. The ketogenic diet pros and cons. Atherosclerosis 292, 119–126 (2019).
Carriazo, S. et al. Dietary care for ADPKD patients: current status and future directions. Nutrients 11, E1576 (2019).
Torres, J. A. et al. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab. 30, 1007–1023 (2019).
de Cabo, R. & Matsson, M. P. Effects of intermittent fasting on health, aging and disease. N. Engl. J. Med. 381, 2541–2551 (2019).
Kandouz, S., Shendi, A. M., Zheng, Y., Sandeman, S. R. & Davenport, A. Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodial. Int. 20, 610–617 (2016).
Black, A. P. et al. Does low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J. Ren. Nutr. 28, 208–214 (2018).
Saglimbene, V. M. et al. Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 14, 250–260 (2019).
Sharaf, El Din, U. A., Salem, M. M. & Abdulazim, D. O. Stop chronic kidney disease progression: time is approaching. World J. Nephrol. 5, 258–273 (2016).
Adair, K. E. & Bowden, R. G. Ameliorating chronic kidney disease using a whole food plant-based diet. Nutrients 12, 1007 (2020).
Carrero, J. J. et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 16, 525–542 (2020).
Kim, H. et al. Plant-based diets and incident CKD and kidney function. Clin. J. Am. Soc. Nephrol. 14, 682–691 (2019).
Forouhi, N. G. & Unwin, N. Global diet and health: old questions, fresh evidence, and new horizons. Lancet. 393, 1916–1918 (2019).
Stockler-Pinto, M. B. et al. Brazil nut (Bertholletia excelsa, H.B.K.) improves oxidative stress and inflammation biomarkers in hemodialysis patients. Biol. Trace Elem. Res. 158, 105–112 (2014).
Khakimov, B. & Engelsen, S. B. Resveratrol in the foodomics era: 1:25,000. Ann. N. Y. Acad. Sci. 1403, 48–58 (2017).
Khakimov, B., Gurdeniz, G. & Engelsen, S. B. Trends in the application of chemometrics to foodomics studies. Acta Aliment. 44, 4–31 (2015).
Hu, E. A. et al. Dietary patterns and risk of incident chronic kidney disease: the atherosclerosis risk in communities study. Am. J. Clin. Nutr. 110, 713–721 (2019).
Kelly, J. T. et al. Healthy dietary patterns and risk of mortality and ESRD in CKD: a meta-analysis of cohort studies. Clin. J. Am. Soc. Nephrol. 12, 272–279 (2017).
Khoueiry, G. et al. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J. Ren. Nutr. 21, 438–447 (2011).
Sussman, E. J., Singh, B., Clegg, D., Palmer, B. F. & Kalantar-Zadeh, K. Let them eat healthy: can emerging potassium binders help overcome dietary potassium restrictions in chronic kidney disease? J. Ren. Nutr. https://doi.org/10.1053/j.jrn.2020.01.022 (2020).
Lehallier, B. et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 25, 1843–1850 (2019).
Clark, M. A., Springmann, M., Hill, J. & Tilman, D. Multiple health and environmental impacts of foods. Proc. Natl Acad. Sci. USA 116, 23357–23362 (2019).
Stenvinkel, P. The one health concept — the health of humans is intimately linked with the health of animals and a sustainable environment. J. Int. Med. 287, 223–225 (2020).
Stenvinkel, P. et al. A planetary health perspective for kidney disease. Kidney Int. 98, 261–265 (2020).
Dinkova-Kostova, A. T. & Abramov, A. Y. The emerging role of Nrf2 in mitochondrial function. Free. Radic. Biol. Med. 88, 179–188 (2015).
Fulop, G. et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 40, 513–521 (2018).
Mischke, M. & Plösch, T. The gut microbiota and their metabolites: potential implications for the host epigenome. Adv. Exp. Med. Biol. 902, 33–44 (2016).
Shiels, P. G., McGuinness, D., Eriksson, M., Kooman, J. P. & Stenvinkel, P. The role of epigenetics in renal ageing. Nat. Rev. Nephrol. 13, 471–482 (2017).
Shiels, P. G., Buchanan, S., Selman, C. & Stenvinkel, P. Allostatic load and ageing: linking the microbiome and nutrition with age-related health. Biochem. Soc. Trans. 47, 1165–1172 (2019).
Witasp, A. et al. Current epigenetic aspects the clinical kidney researcher should embrace. Clin. Sci. 131, 1649–1667 (2017).
Larkin, B. P., Glastras, S. J., Chen, H., Pollock, C. A. & Saad, S. DNA methylation and the potential role of demethylating agents in prevention of progressive chronic kidney disease. FASEB J. 32, 5215–5226 (2018).
O’Toole, P. W. & Shiels, P. G. The role of the microbiota in sedentary lifestyle disorders and ageing: lessons from the animal kingdom. J. Intern. Med. 287, 271–282 (2020).
Kooman, J. P. et al. Inflammation and premature aging in advanced chronic kidney disease. Am. J. Physiol. Ren. Physiol. 313, F938–F950 (2017).
Cañadas-Garre, M., Anderson, K., McGoldrick, J., Maxwell, A. P. & McKnight, A. J. Genomic approaches in the search for molecular biomarkers in chronic kidney disease. J. Transl. Med. 16, 292 (2018).
Wing, M. R. et al. Chronic renal insufficiency cohort (CRIC) study. DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study. Nephrol. Dial. Transplant. 29, 864–872 (2014).
Suliman, M. E., Bárány, P., Kalantar-Zadeh, K., Lindholm, B. & Stenvinkel, P. Homocysteine in uraemia — a puzzling and conflicting story. Nephrol. Dial. Transplant. 20, 16–21 (2005).
Ingrosso, D. et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet. 361, 1693–1699 (2003).
McGuinness, D. et al. A molecular signature for delayed graft function. Aging Cell 17, e12825 (2018).
Stenvinkel, P. et al. Impact of inflammation on epigenetic DNA methylation – a novel risk factor for cardiovascular disease? J. Intern. Med. 261, 488–499 (2007).
McGuinness, D. et al. Identification of molecular markers of delayed graft function based on the regulation of biological ageing. PLoS ONE 11, e0146378 (2016).
Chu, A. Y. et al. Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat. Commun. 8, 1286 (2017).
Crider, K. S., Yang, T. P., Berry, R. J. & Bailey, L. B. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 3, 21–38 (2012).
Friso, S., Udali, S., De Santis, D. & Choi, S. W. One-carbon metabolism and epigenetics. Mol. Asp. Med. 24, 28–36 (2017).
Chu, D. M., Wahlqvist, M. L., Chang, H. Y., Yeh, N. H. & Lee, M. S. Choline and betaine food sources and intakes in Taiwanese. Asia Pac. J. Clin. Nutr. 21, 547–557 (2012).
Mafra, D. et al. Methyl donor nutrients in chronic kidney disease: impact on the epigenetic landscape. J. Nutr. 149, 372–380 (2019).
Clifford, T., Howatson, G., West, D. J. & Stevenson, E. J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 14, 2801–2822 (2015).
Du, J. et al. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 10, E131 (2018).
Missailidis, C. et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE 11, e0141738 (2016).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Tang, W. H., Kitai, T. & Hazen, S. L. Gut microbiota in cardiovascular health and disease. Circ. Res. 120, 1183–1196 (2017).
Meijers, B., Evenepoel, P. & Anders, H. J. Intestinal microbiome and fitness in kidney disease. Nat. Rev. Nephrol. 15, 531–545 (2019).
Vaziri, N. D., Zhao, Y. Y. & Pahl, M. V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 31, 737–746 (2015).
Al-Khodor, S. & Shatat, I. F. Gut microbiome and kidney disease: a bidirectional relationship. Pediatr. Nephrol. 32, 921–931 (2017).
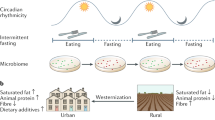
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Hida, M. et al. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74, 349–355 (1996).
Taki, K., Takayama, F. & Niwa, T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemi a in hemodialysis patients. J. Ren. Nutr. 15, 77–80 (2005).
Ranganathan, N. et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 27, 634–647 (2010).
Miranda Alatriste, P. V., Urbina Arronte, R., Gomez Espinosa, C. O. & Espinosa Cuevas, M. de L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 29, 582–590 (2014).
Natarajan, R. et al. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed. Res. Int. 2014, 568571 (2014).
Borges, N. A. et al. Probiotic supplementation in chronic kidney disease: a double-blind, randomized, placebo-controlled trial. J. Ren. Nutr. 28, 28–36 (2018).
Singh, S. P., Jadaun, J. S., Narnoliya, L. K. & Pandey, A. Prebiotic oligosaccharides: special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 183, 613–635 (2017).
Walker, A. W. et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5, 220–230 (2011).
Lecerf, J. M. et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and imune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 108, 1847–1858 (2012).
Graf, D. et al. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 26, 26164 (2015).
Upadhyaya, B. et al. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 6, 28797 (2016).
Poesen, R. et al. The Influence of CKD on Colonic Microbial Metabolism. J. Am. Soc. Nephrol. 27, 1389–1399 (2016).
Khosroshahi, H. T. et al. Effect of high amylose resistant starch (HAMRS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: a randomized clinical trial. Hemodial. Int. 22, 492–500 (2018).
Esgalhado, M. et al. Resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 13, 6508–6516 (2018).
Khosroshahi, H. T. et al. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease: a randomized clinical trial. J. Renal Inj. Prev. 5, 162–167 (2016).
Gibson, G. R. et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Tsai, Y. L. et al. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 26, 3 (2019).
Al-Sheraji, S. H. et al. Prebiotics as functional foods: a review. J. Func. Foods. 5, 1542–1553 (2013).
Moraes, C., Borges, N. A. & Mafra, D. Resistant starch for modulation of gut microbiota: Promising adjuvant therapy for chronic kidney disease patients? Eur. J. Nutr. 55, 1813–1821 (2016).
Cruz-Mora, J. et al. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J. Ren. Nutr. 24, 330–335 (2014).
Dehghani, H. H. F., Mozaffari-Khosravi, H., Nouri-Majelan, N. & Dehghani, A. Synbiotic supplementations for azotemia in patients with chronic kidney disease: a randomized controlled trial. Iran. J. Kidney Dis. 10, 351–357 (2016).
Rossi, M. et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin. J. Am. Soc. Nephrol. 11, 223–231 (2016).
McFarlane, C., Ramos, C. I., Johnson, D. W. & Campbell, K. L. Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J. Ren. Nutr. 29, 209–220 (2019).
Rinninella, E. et al. The role of diet, micronutrients and the gut microbiota in age-related macular degeneration: new perspectives from the gut–retina axis. Nutrients 10, 1677 (2018).
Rosas-Villegas, A. et al. Differential effect of sucrose and fructose in combination with a high fat diet on intestinal microbiota and kidney oxidative stress. Nutrients 9, E393 (2017).
Do, M. H., Lee, E., Oh, M. J., Kim, Y. & Park, H. Y. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10, 761 (2018).
Rysz, J., Franczyk, B., Ciałkowska-Rysz, A. & Gluba-Brzózka, A. The effect of diet on the survival of patients with chronic kidney disease. Nutrients 9, E495 (2017).
Scott, K. P., Gratz, S. W., Sheridan, P. O., Flint, H. J. & Duncan, S. H. The influence of diet on the gut microbiota. Pharmacol. Res. 69, 52–60 (2013).
Ercolini, D. & Fogliano, V. Food design to feed the human gut microbiota. J. Agric. Food Chem. 66, 3754–3758 (2018).
Evenepoel, P., Meijers, B. K., Bammens, B. R. & Verbeke, K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. Suppl. 114, S12–S19 (2009).
Mafra, D., Barros, A. F. & Fouque, D. Dietary protein metabolism by gut microbiota and its consequences for chronic kidney disease patients. Future Microbiol. 8, 1317–1323 (2013).
Swain Ewald, H. A. & Ewald, P. W. Natural selection, the microbiome, and public health. Yale J. Biol. Med. 91, 445–455 (2018).
Nyangale, E. P., Mottram, D. S. & Gibson, G. R. J. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. Proteome Res. 11, 5573–5585 (2012).
Madsen, L., Myrmel, L. S., Fjære, E., Liaset, B. & Kristiansen, K. Links between dietary protein sources, the gut microbiota, and obesity. Front. Physiol. 8, 1047 (2017).
Zhao, J., Zhang, X., Liu, H., Brown, M. A. & Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 20, 145–154 (2019).
Song, M. & Chan, A. T. Diet, gut microbiota, and colorectal cancer prevention: a review of potential mechanisms and promising targets for future research. Curr. Colorectal Cancer Rep. 13, 429–439 (2017).
Mafra, D. et al. Red meat intake in chronic kidney disease patients: two sides of the coin. Nutrition 46, 26–32 (2018).
Zhu, Y. et al. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front. Microbiol. 8, 1395 (2017).
Ge, Y. et al. Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur. J. Nutr. 58, 2625–2638 (2018).
Wisniewski, P. J., Dowden, R. A. & Campbell, S. C. Role of dietary lipids in modulating inflammation through the gut microbiota. Nutrients 11, 117 (2019).
Wan, Y. et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 68, 1417–1429 (2019).
Lam, Y. Y. et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity 23, 1429–1439 (2015).
Costantini, L., Molinari, R., Farinon, B. & Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 18, 2645 (2017).
Martín-Peláez, S. et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 56, 119–131 (2017).
Prieto, I. et al. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 13, e0190368 (2018).
Martínez, N. et al. Refined versus extra virgin olive oil high-fat diet impact on intestinal microbiota of mice and its relation to different physiological variables. Microorganisms 7, 61 (2019).
Sarmugam, R. & Worsley, A. Current levels of salt knowledge: a review of the literature. Nutrients 6, 5534–5559 (2014).
Hu, J. et al. Enteric dysbiosis-linked gut barrier disruption triggers early renal injury induced by chronic high salt feeding in mice. Exp. Mol. Med. 49, e370 (2017).
Vazquez-Gutierrez, P. et al. Bifidobacteria strains isolated from stools of iron deficient infants can efficiently sequester iron. BMC Microbiol. 15, 3 (2015).
Richard, S. A. et al. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the peruvian amazon. Am. J. Trop. Med. Hyg. 75, 126–132 (2006).
Chang, S. et al. Supplementing iron and zinc: double blind, randomized evaluation of separate or combined delivery. Eur. J. Clin. Nutr. 64, 153–160 (2010).
Nchito, M., Friis, H., Michaelsen, K. F., Mubila, L. & Olsen, A. Iron supplementation increases small intestine permeability in primary schoolchildren in Lusaka, Zambia. Trans. R. Soc. Trop. Med. Hyg. 100, 791–794 (2006).
Zimmermann, M. B. et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 92, 1406–1415 (2010).
Kortman, G. A. et al. Microbial metabolism shifts towards an adverse profile with supplementary iron in the TIM-2 in vitro model of the human colon. Front. Microbiol. 6, 1481 (2016).
Chiang, C. K., Tanaka, T., Inagi, R., Fujita, T. & Nangaku, M. Indoxyl sulfate, a representative uremic toxin, suppresses erythropoietin production in a HIF-dependent manner. Lab. Invest. 91, 1564–1571 (2011).
Bonan, N. B., Steiner, Y. M. & Kuntsevich, V. Uremic toxicity-induced eryptosis and monocyte modulation: the erythrophagocytosis as a novel pathway to renal anemia. Blood Purif. 41, 317–323 (2016).
Kortman, G. A. M., Reijnders, D. & Swinkels, D. W. Oral iron supplementation: Potential implications for the gut microbiome and metabolome in patients with CKD. Hemodial. Int. 21, S28–S36 (2017).
Bondonno, N. P. et al. Flavonoid intake is associated with lower mortality in the Danish diet cancer and health cohort. Nat. Commun. 10, 3651 (2019).
Ozdal, T. et al. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 8, 78 (2016).
Singh, R. et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 10, 89 (2019).
Tzounis, X. et al. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 93, 62–72 (2011).
Lee, S. et al. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J. Nutr. 148, 209–219 (2018).
Wu, W.-K. et al. Dietary allicin reduces transformation of L-carnitine to TMAO through impact on gut microbiota. J. Func. Foods 15, 408–417 (2015).
Chen, M. L. et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 7, e02210–e02215 (2016).
Cardona, F., Andrés-Lacueva, C., Tulipani, S., Tinahones, F. J. & Queipo-Ortuño, M. I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 24, 1415–1422 (2013).
Pfeffer, M., Ziesenitz, S. C. & Siebert, G. Acesulfame K cyclamate and saccharin inhibit the anaerobic fermentation of glucose by intestinal bacteria. Z. Ernahrungswiss. 24, 231–235 (1985).
Wang, Q. P., Browman, D., Herzog, H. & Neely, G. G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 13, e0199080 (2018).
Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
Abou-Donia, M. B., El-Masry, E. M., Abdel-Rahman, A. A., McLendon, R. E. & Schiffman, S. S. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J. Toxicol. Environ. Health A 71, 1415–1429 (2008).
Daly, K., Darby, A. C. & Shirazi-Beechey, S. P. Low calorie sweeteners and gut microbiota. Physiol. Behav. 164, 494–500 (2016).
Pepino, M. Y. Metabolic effects of non-nutritive sweeteners. Physiol. Behav. 152, 450–455 (2015).
Martínez-Carrillo, B. E. et al. Effect of chronic consumption of sweeteners on microbiota and immunity in the small intestine of young mice. Int. J. Food Sci. 2019, 9619020 (2019).
Garcia-Mantrana, I., Selma-Royo, M., Alcantara, C. & Collado, M. C. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 9, 890 (2018).
Bischoff, S. Microbiota and aging. Curr. Opin. Clin. Nutr. Metab. Care. 19, 26–30 (2016).
Van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Mosteiro, L. et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, aaf4445 (2016).
Coppé, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Biran, A. et al. Quantitative identification of senescent cells in aging and disease. Aging Cell. 16, 661–671 (2017).
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. EBioMedicine 21, 21–28 (2017).
de Kok, M. J. et al. The neglectable impact of delayed graft function on long-term graft survival in kidneys donated after circulatory death associates with superior organ resilience. Ann. Surg. 270, 877–883 (2019).
Kooman, J. P., Kotanko, P., Schols, A. M., Shiels, P. G. & Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10, 732–742 (2014).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell. Res. 25, 585–621 (1961).
Teo, Y. V. et al. Notch signaling mediates secondary senescence. Cell Rep. 27, 997–1007.e5 (2019).
Robinson, M. W. et al. Non cell autonomous upregulation of CDKN2 transcription linked to progression of chronic hepatitis C disease. Aging Cell 12, 1141–1143 (2013).
Sturmlechner, I., Durik, M., Sieben, C. J., Baker, D. J. & van Deursen, J. M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 13, 77–89 (2017).
Palmer, A. K., Gustafson, B., Kirkland, J. L. & Smith, U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia 62, 1835–1841 (2019).
Stenvinkel, P. et al. CDKN2A/p16INK4a expression is associated with vascular progeria in chronic kidney disease. Aging 9, 494–507 (2017).
Tchkonia, T. & Kirkland, J. L. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA 320, 1319–1320 (2018).
Gurău, F. et al. Anti-senescence compounds: a potential nutraceutical approach to healthy aging. Ageing Res. Rev. 46, 14–31 (2018).
Li, W. Emerging senolytic agents derived from natural products. Mech. Ageing Dev. 181, 1–6 (2019).
Senger, D. R., Li, D., Jaminet, S.-C. & Cao, S. Activation of the Nrf2 cell defense pathway by ancient foods: disease prevention by important molecules and microbes lost from the modern Western diet. PLoS ONE 11, e0148042 (2016).
Gómez-Linton, D. R. et al. Some naturally occurring compounds that increase longevity and stress resistance in model organisms of aging. Biogerontology 20, 583–603 (2019).
He, J. et al. The resistant effect of SIRT1 in oxidative stress-induced senescence of rat nucleus pulposus cell is regulated by Akt-FoxO1 pathway. Biosci. Rep. 39, BSR20190112 (2019).
Man, A. W. C., Li, H. & Xia, N. The role of sirtuin1 in regulating endothelial function, arterial remodeling and vascular aging. Front. Physiol. 10, 1173 (2019).
Shiels, P. G. et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS ONE 6, e22521 (2011).
McGuinness, D. et al. Socio-economic status is associated with epigenetic differences in the pSoBid cohort. Int. J. Epidemiol. 41, 151–160 (2012).
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018).
Singh, S., Garg, G., Singh, A. K., Bissoyi, A. & Rizvi, S. I. Fisetin, a potential caloric restriction mimetic, attenuates senescence biomarkers in rat erythrocytes. Biochem. Cell Biol. 97, 480–487 (2019).
Shi, Y. S. et al. Fisetin attenuates metabolic dysfunction in mice challenged with a high-fructose diet. J. Agric. Food Chem. 66, 8291–8298 (2018).
Bondonno, N. P. et al. Fruit intake and abdominal aortic calcification in elderly women: a prospective cohort study. Nutrients 8, 159 (2016).
Xu, X. et al. Effects of dietary apple polyphenols supplementation on hepatic fat deposition and antioxidant capacity in finishing pigs. Animals 9, 937 (2019).
Proshkina, E. et al. Geroprotective and radioprotective activity of quercetin, (-)-epicatechin, and ibuprofen in Drosophila melanogaster. Front. Pharmacol. 7, 505 (2016).
Yang, H., Song, Y., Liang, Y. N. & Li, R. Quercetin treatment improves renal function and protects the kidney in a rat model of adenine-induced chronic kidney disease. Med. Sci. Monit. 24, 4760–4766 (2018).
Stenvinkel, P. & Haase, V. H. Inflamed fat and mitochondrial dysfunction in end-stage renal disease links to hypoxia-could curcumin be of benefit? Nephrol. Dial. Transplant. 32, 909–912 (2017).
Takano, K., Tatebe, J., Washizawa, N. & Morita, T. Curcumin inhibits age-related vascular changes in aged mice fed a high-fat diet. Nutrients 10, E1476 (2018).
La Fata, G., Seifert, N., Weber, P. & Mohajeri, M. H. Vitamin E supplementation delays cellular senescence in vitro. Biomed. Res. Int. 2015, 563247 (2015).
Malavolta, M. et al. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Exp. Gerontol. 99, 35–45 (2017).
Jankowska, M., Rutkowski, B. & Dębska-Ślizień, A. Vitamins and microelement bioavailability in different stages of chronic kidney disease. Nutrients 9, E282 (2017).
Galvan, D. L., Green, N. H. & Danesh, F. R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 92, 1051–1057 (2017).
Li, Q., Zhang, A., Xing, C. & Yuan, Y. Disruption of mitochondrial homeostasis in chronic kidney disease: a mini-review. Histol. Histopathol. 34, 835–842 (2019).
Sergi, D. et al. Mitochondrial (dys)function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 10, 532 (2019).
Serrano, J. C. E., Cassanye, A., Martín-Gari, M., Granado-Serrano, A. B. & Portero-Otín, M. Effect of dietary bioactive compounds on mitochondrial and metabolic flexibility. Diseases 4, 14 (2016).
Mafra, D. et al. Bioactive food and exercise in chronic kidney disease: targeting the mitochondria. Eur. J. Clin. Invest. 48, e13020 (2018).
Martinez Cantarin, M. et al. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol. Dial. Transplant. 32, 943–951 (2017).
Liu, C. et al. Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am. J. Physiol. Renal Physiol. 317, F1122–F1131 (2019).
Clark, A. & Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 8, 319 (2017).
Mafra, D., Borges, N. A., Lindholm, B. & Stenvinkel, P. Mitochondrial dysfunction and gut microbiota imbalance: an intriguing relationship in chronic kidney disease. Mitochondrion. 47, 206–209 (2019).
Schrauwen, P., Schrauwen-Hinderling, V., Hoeks, J. & Hesselink, M. K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta 1801, 266–271 (2010).
Yuzefovych, L., Wilson, G. & Rachek, L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 299, E1096–E1105 (2010).
Lanza, I. R. et al. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. Am. J. Physiol. Endocrinol. Metab. 304, E1391–E1403 (2013).
Motawi, T. M. K., Hashem, R. M., Rashed, L. A. & El-Razek, S. M. A. Comparative study between the effect of the peroxisome proliferator activated receptor-α ligands fenofibrate and n-3 polyunsaturated fatty acids on activation of 5′-AMP-activated protein kinase-α1 in high-fat fed rats. J. Pharm. Pharmacol. 61, 1339–1346 (2009).
Sun, X. & Zemel, M. B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. 6, 26 (2009).
Sun, X. & Zemel, M. B. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids 42, 297–305 (2017).
Sharma, S. & Black, S. M. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 6, e31–e39 (2009).
Steiber, A., Kerner, J. & Hoppel, C. L. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol. Asp. Med. 25, 455–473 (2004).
Yang, S.-K. et al. Effect of L-carnitine therapy on patients in maintenance hemodialysis: a systematic review and meta-analysis. J. Nephrol. 27, 317–329 (2014).
Makrecka-Kuka, M. et al. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol. Lett. 267, 32–38 (2017).
Vallance, H. D. et al. Marked elevation in plasma trimethylamine-N-oxide (TMAO) in patients with mitochondrial disorders treated with oral l-carnitine. Mol. Genet. Metab. Rep. 15, 130–133 (2018).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Jonsson, A. L. & Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 14, 79–87 (2017).
Wang, Z. et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 40, 583–594 (2019).
Zhong, V. W. et al. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern. Med. 80, 503–512 (2020).
Gross, J. L. et al. Effect of a chicken-based diet on renal function and lipid profile in patients with type 2 diabetes: a randomized crossover trial. Diabetes Care 25, 645–651 (2002).
Bolati, D., Shimizu, H., Yisireyili, M., Nishijima, F. & Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol. 14, 56 (2013).
Enoki, Y. et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J. Cachexia Sarcopenia Muscle 8, 735–747 (2017).
Gao, Z. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009).
Bajpai, P., Darra, A. & Agrawal, A. Microbe-mitochondrion crosstalk and health: an emerging paradigm. Mitochondrion 39, 20–25 (2018).
Tirosh, O., Levy, E. & Reifen, R. High selenium diet protects against TNBS-induced acute inflammation, mitochondrial dysfunction, and secondary necrosis in rat colon. Nutrition 23, 878–886 (2017).
Zhang, C. et al. Selenium triggers Nrf2-mediated protection against cadmium-induced chicken hepatocyte autophagy and apoptosis. Toxicol. In Vitro 44, 349–356 (2017).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Yuan, Y. et al. Activation of peroxisome proliferator activated receptor-g coactivator 1a ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 82, 771–789 (2012).
Chang, Y. P. et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 230, 1567–1579 (2015).
Hui, Y. et al. Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochem. 119, 392–399 (2017).
Den Hartogh, D. J. & Tsiani, E. Health benefits of resveratrol in kidney disease: evidence from in vitro and in vivo studies. Nutrients 11, 1624 (2019).
Wu, M. et al. Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation. Nephrol. Dial. Transplant. 31, 1826–1834 (2016).
Alvarenga, L. A. et al. Curcumin — a promising nutritional strategy for chronic kidney disease patients. J. Func. Foods 40, 715–721 (2018).
Correa, F. et al. Curcumin maintains cardiac and mitochondrial function in chronic kidney disease. Free. Radic. Biol. Med. 61, 119–12 (2013).
Hernandez-Resendiz, S. et al. Cardioprotection by curcumin post-treatment in rats with established chronic kidney disease. Cardiovasc. Drugs Ther. 29, 111–120 (2015).
Sudirman, S., Lai, C. S., Yan, Y. L., Yeh, H. I. & Kong, Z. L. Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model. Sci. Rep. 9, 15233 (2019).
Ghosh, S. S., Gehr, T. W. & Ghosh, S. Curcumin and chronic kidney disease (CKD): major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 19, 20139–20156 (2014).
Cuomo, F., Perugini, L., Marconi, E., Messia, M. C. & Lopez, F. Enhanced curcumin bioavailability through nonionic surfactant/caseinate mixed nanoemulsions. J. Food Sci. 84, 2584–2591 (2019).
Vecchione, R. et al. Curcumin bioavailability from oil in water nano-emulsions: In vitro and in vivo study on the dimensional, compositional and interactional dependence. J. Control. Rel. 233, 88–100 (2016).
Chakraborty, M., Bhattacharjee, A. & Kamath, J. V. Cardioprotective effect of curcumin and piperine combination against cyclophosphamide-induced cardiotoxicity. Indian J. Pharmacol. 49, 65–70 (2017).
Borlinghaus, J., Albrecht, F., Gruhlke, M. C., Nwachukwu, I. D. & Slusarenko, A. J. Allicin: chemistry and biological properties. Molecules 19, 12591–12618 (2014).
Salehi, B. et al. Allicin and health: a comprehensive review. Trends Food Sci. Technol. 86, 502–516 (2019).
Supakul, L. et al. Protective effects of garlic extract on cardiac function, heart rate variability, and cardiac mitochondria in obese insulin-resistant rats. Eur. J. Nutr. 53, 919–928 (2014).
Zhang, M. et al. Allicin decreases lipopolysaccharide-induced oxidative stress and inflammation in human umbilical vein endothelial cells through suppression of mitochondrial dysfunction and activation of Nrf2. Cell. Physiol. Biochem. 41, 2255–2267 (2017).
García Trejo, E. M. A. et al. The beneficial effects of allicin in chronic kidney disease are comparable to losartan. Int. J. Mol. Sci. 18, 1980 (2017).
Granata, S. et al. Mitochondria: a new therapeutic target in chronic kidney disease. Nutr. Metab. 12, 49 (2015).
Perez-Cruz, I., Carcamo, J. M. & Golde, D. W. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood 102, 336–343 (2013).
Hao, J. et al. Role of vitamin C in cardioprotection of ischemia/reperfusion injury by activation of mitochondrial KATP channel. Chem. Pharm. Bull. 64, 548–557 (2016).
D’Costa, M. R. et al. Oxalosis associated with high-dose vitamin C ingestion in a peritoneal dialysis patient. Am. J. Kidney Dis. 74, 417–420 (2019).
Chang, H., Wang, Y., Yin, X., Liu, X. & Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 17, 471 (2017).
Kubiliene, L. et al. Comparison of aqueous, polyethylene glycol-aqueous and ethanolic propolis extracts: antioxidant and mitochondria modulating properties. BMC Complement. Altern. Med. 18, 165 (2018).
Silveira, M. A. D. et al. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. BMC Nephrol. 20, 140 (2019).
Nadia, B. H. et al. Disruption of mitochondrial membrane potential by ferulenol and restoration by propolis extract: antiapoptotic role of propolis. Acta Biol. Hung. 60, 385–398 (2009).
Ulusoy, H. B., Öztürk, İ. & Sönmez, M. F. Protective effect of propolis on methotrexate-induced kidney injury in the rat. Ren. Fail. 38, 744–750 (2016).
Pedruzzi, L. M., Stockler-Pinto, M. B., Leite, M. Jr. & Mafra, D. Nrf2-keap1 system versus NF-κB: the good and the evil in chronic kidney disease? Biochimie 94, 2461–2466 (2012).
Franceschi, C. et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Sato, Y. & Yanagita, M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 15, 625–640 (2019).
Schmitz, M. L., Weber, A., Roxlau, T., Gaestel, M. & Kracht, M. Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochim. Biophys. Acta 1813, 2165–2175 (2011).
Armutcu, F. Organ crosstalk: the potent roles of inflammation and fibrotic changes in the course of organ interactions. Inflamm. Res. 68, 825–839 (2019).
Zhang, J. et al. Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res. Rev. 25, 55–69 (2016).
Bordoni, A. et al. Dairy products and inflammation: a review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 57, 2497–2525 (2017).
Bolori, P. et al. Adherence to a healthy plant diet may reduce inflammatory factors in obese and overweight women-a cross-sectional study. Diabetes Metab. Syndr. 13, 2795–2802 (2019).
Galiè, S. et al. Impact of nutrition on telomere health: systematic review of observational cohort studies and randomized clinical trials. Adv. Nutr. 11, 576–601 (2019).
Hussain, T. et al. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 7432797 (2016).
Marx, W. et al. The effect of polyphenol-rich interventions on cardiovascular risk factors in haemodialysis: a systematic review and meta-analysis. Nutrients 9, 1345 (2017).
Bellezza, I. et al. Nrf2-Keap1 signaling in oxidative and reductive stress. Bioch. Biophys. Acta Mol. Cell Res. 1865, 721–733 (2018).
Battino, M. et al. Nrf2 as regulator of innate immunity: a molecular Swiss army knife! Biotech. Adv. 36, 358–370 (2018).
Ahmed, S. M. U., Luo, L., Namani, A., Wang, X. J. & Tang, X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 585–597 (2017).
Dinkova-Kostova, A. T., Fahey, J. W., Kostov, R. V. & Kensler, T. W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 69, 257–269 (2017).
Martini, S. et al. Integrative biology identifies shared transcriptional networks in CKD. J. Am. Soc. Nephrol. 25, 2559–2572 (2014).
Wu, K. C., McDonald, P. R., Liu, J. & Klaassen, C. D. Screening of natural compounds as activators of the keap1-Nrf2 pathway. Planta Med. 80, 97–104 (2014).
Smith, R. E. The effects of dietary supplements that overactivate the Nrf2/ARE system. Curr. Med. Chem. 27, 2077–2094 (2020).
Davinelli, S., Willcox, C. & Scapagnini, G. Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun. Ageing. 9, 9 (2012).
Stenvinkel, P. et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat. Rev. Nephrol. 14, 265–284 (2018).
Paunkov, A., Chartoumpekis, D. V., Ziros, P. G. & Sykiotis, G. P. A bibliometric review of the Keap1/Nrf2 pathway and its related antioxidant compounds. Antioxidants. 8, E353 (2019).
Saraiva, J. A. et al. Effects of low protein diet on nuclear factor erythroid 2-related factor 2 gene expression in nondialysis chronic kidney disease patients. J. Ren. Nutr. 30, 46–52 (2020).
McClelland, R. et al. Accelerated ageing and renal dysfunction links lower socioeconomic status and dietary phosphate intake. Aging 8, 1135–1149 (2016).
Rebholz, C. M. et al. DASH (dietary approaches to stop hypertension) diet and risk of subsequent kidney disease. Am. J. Kidney Dis. 68, 853–861 (2016).
Bomback, A. S. et al. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 77, 609–616 (2010).
Yang, M. et al. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed. Pharmacother. 105, 1283–1290 (2018).
Woo, M., Kim, M., Noh, J. S. & Song, Y. O. Kimchi methanol extracts attenuate hepatic steatosis induced by high cholesterol diet in low-density lipoprotein receptor knockout mice through inhibition of endoplasmic reticulum stress. J. Funct. Foods 32, 218–222 (2017).
Shin, J. H. et al. Nrf2-Heme oxygenase-1 attenuates high-glucose-induced epithelial-to-mesenchymal transition of renal tubule cells by inhibiting ROS-mediated PI3K/Akt/GSK-3β Signaling. J. Diabetes Res. 2019, 2510105 (2019).
Axelsson, A. S. et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 9, eaah4477 (2017).
Gigliotti, J. C. et al. GSTM1 deletion exaggerates kidney injury in experimental mouse models and confers the protective effect of cruciferous vegetables in mice and humans. J. Am. Soc. Nephrol. 31, 102–116 (2020).
Zhang, W., Li, Y., Ding, H., Du, Y. & Wang, L. Hydrogen peroxide prevents vascular calcification induced ROS production by regulating Nrf-2 pathway. Ren. Fail. 38, 1099–1106 (2016).
Dai, L., Qureshi, A. R., Witasp, A., Lindholm, B. & Stenvinkel, P. Early vascular ageing and cellular senescence in chronic kidney disease. Comput. Struct. Biotechnol. J. 17, 721–729 (2019).
Kunnumakkara, A. B. et al. Chronic diseases, inflammation, and spices: how are they linked? J. Transl. Med. 16, 14 (2018).
Tsui, P.-F., Lin, C.-S., Ho, L.-J. & Lai, J.-H. Spices and atherosclerosis. Nutrients 10, 1724 (2018).
Nilius, B. & Appendino, G. Spices: the savory and beneficial science of pungency. Rev. Physiol. Biochem. Pharmacol. 164, 1–76 (2013).
Kocaadam, B. & Şanlier, N. Curcumin, an active component of turmeric (Curcuma Longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 57, 2889–2895 (2017).
White, C. M., Pasupuleti, V., Roman, Y. M., Li, Y. & Hernandez, A. V. Oral Turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 146, 104280 (2019).
Ali, B. H. et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 122, 65–73 (2018).
Jiménez-Osorio, A. S. et al. The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: a pilot study. J. Ren. Nutr. 26, 237–44 (2016).
Ghosh, S. S., He, H., Wang, J., Gehr, T. W. & Ghosh, S. Curcumin-mediated regulation of intestinal barrier function: the mechanism underlying its beneficial effects. Tissue Barriers 6, e1425085 (2018).
Hami, M. et al. The effect of curcumin in prevention of contrast nephropathy following coronary angiography or angioplasty in CKD patients. Iran. J. Kidney Dis. 13, 304–309 (2019).
Weir, M. A. et al. Micro-particle curcumin for the treatment of chronic kidney disease-1: study protocol for a multicenter clinical trial. Can. J. Kidney Health Dis. 5, (2018).
Wang, Y. et al. Epigallocatechin-3-gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic Clin. Pharmacol. Toxicol. 117, 164–172 (2015).
Jhee, H. J. et al. Effects of coffee intake on incident chronic kidney disease: a community-based prospective cohort study. Am. J. Med. 131, 1482–1490 (2018).
Liang, N. & Kitts, D. D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 8, 16 (2015).
Priftis, A., Angeli-Terzidou, A. E., Veskoukis, A. S., Spandidos, D. A. & Kouretas, D. Cell-specific and roasting-dependent regulation of the Keap1/Nrf2 pathway by coffee extracts. Mol. Med. Rep. 17, 8325–8331 (2018).
Rassaf, T. et al. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double-blind, randomized, placebo-controlled trial. Clin. J. Am. Soc. Nephrol. 11, 108–118 (2016).
Hariri, M. & Ghiasvand, R. Cinnamon and chronic diseases. Adv. Exp. Med. Biol. 929, 1–24 (2016).
Nabavi, S. F. et al. Nrf2 as molecular target for polyphenols: a novel therapeutic strategy in diabetic retinopathy. Crit. Rev. Clin. Lab. Sci. 53, 293–312 (2016).
Kalt, W. et al. Recent research on the health benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 11, 224–236 (2019).
Cassidy, A. et al. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 127, 188–196 (2013).
Moriwaki, S. et al. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS ONE 9, e97177 (2014).
Tang, J. S. et al. Bioavailable blueberry-derived phenolic acids at physiological concentrations enhance Nrf2-regulated antioxidant responses in human vascular endothelial cells. Mol. Nutr. Food Res. 62, 1–7 (2018).
Pan, J. et al. Pterostilbene, a bioactive component of blueberries, alleviates renal fibrosis in a severe mouse model of hyperuricemic nephropathy. Biomed. Pharmacother. 109, 1802–1808 (2018).
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20, 3328 (2019).
Komada, T. & Muruve, D. A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 15, 501–520 (2019).
Alvarenga, L. et al. Can nutritional interventions modulate the activation of the NLRP3 inflammasome in chronic kidney disease? Food Res. Int. https://doi.org/10.1053/j.jrn.2020.01.022 (2020).
Hennig, P. et al. The crosstalk between Nrf2 and inflammasomes. Int. J. Mol. Sci. 19, 562 (2018).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167 (2008).
Senkus, K. E. & Crowe-White, K. M. Influence of mouth rinse use on the enterosalivary pathway and blood pressure regulation: a systematic review. Crit. Rev. Food Sci. Nutr. 2019, 1–13 (2019).
Bonilla, O. D. A. et al. Dietary nitrate from beetroot juice for hypertension: a systematic review. Biomolecules 8, E134 (2018).
Bryan, N. S. Functional nitric oxide nutrition to combat cardiovascular disease. Curr. Atheroscler. Rep. 20, 21 (2018).
Sweazea, K. L., Johnston, C. S., Miller, B. & Gumpricht, E. Nitrate-rich fruit and vegetable supplement reduces blood pressure in normotensive healthy young males without significantly altering flow-mediated vasodilation: a randomized, double-blinded, controlled trial. J. Nutr. Metab. 2018, 1729653 (2018).
Edwards, M., Czank, C., Woodward, G. M., Cassidy, A. & Kay, C. D. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J. Agric. Food Chem. 63, 2423–2431 (2015).
Curtis, P. P. et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 109, 1535–1545 (2019).
Lundberg, J. O., Carlström, M. & Weitzberg, E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 28, 9–22 (2018).
El Gamal, A. A. et al. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediators Inflamm. 2014, 983952 (2014).
Bahadoran, Z. et al. Association between dietary intakes of nitrate and nitrite and the risk of hypertension and chronic kidney disease: Tehran Lipid and Glucose Study. Nutrients 21, E811 (2016).
Kemmner, S. et al. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: a pilot study. Nitric Oxide 64, 7–15 (2017).
Lobel, L., Cao, Y.G., Fenn, K., Glickman, J.N. & Garrett, W.S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 369, 1518–1524 (2020).
Acknowledgements
D.M. receives support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant no. 302034/2018-8) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, grant no. E-26/202.24/2019). P.S. receives funding from the Strategic Research Program in Diabetes at Karolinska Institutet (Swedish Research Council grant no. 2009-1068), the European Union’s Horizon 2020 research and innovation Program under the Marie Skłodowska-Curie grant agreement no. 722609; International Network for Training on Risks of Vascular Intimal Calcification and roads to Regression of Cardiovascular Disease (INTRICARE). Baxter Novum is the result of a grant from Baxter Healthcare to the Karolinska Institutet.
Author information
Authors and Affiliations
Contributions
D.M., N.A.B., B.L., P.G.S. and P.S. researched the data for the article. All authors contributed to discussions of the content, wrote the text and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.L.’s research is funded by Baxter Healthcare. P.S. is on the scientific advisory boards of REATA, Baxter Healthcare and AstraZeneca. P.G.S. is funded through PhD studentships supported by 4D Pharma and Constant Pharma. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks N. Vaziri, A. Cupisti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
INTRICARE: www.intricare.eu
Supplementary information
Glossary
- Uraemic toxins
-
Biologically active compounds that are removed from the blood by healthy kidneys but can accumulate and exert toxic effects in the setting of kidney dysfunction.
- Foodomics
-
The application of omics technologies to study food and nutrition.
- Planetary health
-
The health of human civilization and the state of the natural systems on which it depends.
- Methylome
-
The complement of methylation-based modifications in a genome or in a particular cell.
- Diseaseome
-
The network of genes and pathways that is associated with human disease.
- Osmoprotectant
-
Organic molecules, such as betaine, sugars and amino acids, that have neutral charge and maintain the integrity of cells exposed to osmotic stress.
- One-carbon metabolism
-
A metabolic process that interlinks the methionine and folate cycles, which provide methyl groups for synthesis of DNA and maintenance of the epigenetic landscape.
- Prebiotics
-
Non-digestible compounds in food that stimulate the growth of beneficial microorganisms such as bacteria and fungi.
- Probiotics
-
Live microorganisms such as bacteria and yeasts that are thought to have health benefits when consumed.
- Synbiotics
-
Dietary supplements that contain a combination of prebiotics and probiotics.
- Salutogenic
-
Factors that maintain and promote human health.
- Eryptosis
-
A type of programmed cell death that occurs in erythrocytes.
- Nutraceuticals
-
Products derived from food that provide health benefits.
- Senotherapeutics
-
Agents that target senescent cells, such as geroprotectors (which prevent or reverse the senescent state), senescence-associated secretory phenotype inhibitors, senolytics (which induce the death of senescent cells), senomorphics (which suppress senescent phenotypes without killing cells) and gene therapy strategies (which increase resistance to ageing).
- Inflammageing
-
Inflammation that occurs during ageing or age-related diseases.
- Blue zones
-
Regions in the world where people have high life expectancy, such as Okinawa (Japan), Loma Linda (USA), Sardinia (Italy), Nicoya (Costa Rica) and Icaria (Greece).
- Kimchi
-
A traditional, heavily seasoned Korean dish made with vegetables such as napa cabbage and radish that are fermented by mainly lactic acid bacteria.
- Natto
-
A traditional Japanese dish made with soybeans that are fermented by Bacillus subtilis.
Rights and permissions
About this article
Cite this article
Mafra, D., Borges, N.A., Lindholm, B. et al. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol 17, 153–171 (2021). https://doi.org/10.1038/s41581-020-00345-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-00345-8
This article is cited by
-
Uremic toxins mediate kidney diseases: the role of aryl hydrocarbon receptor
Cellular & Molecular Biology Letters (2024)
-
Cellular senescence of renal tubular epithelial cells in acute kidney injury
Cell Death Discovery (2024)
-
Synergistic anti-diabetic effect of phloroglucinol and total procyanidin dimer isolated from Vitis vinifera methanolic seed extract potentiates via suppressing oxidative stress: in-vitro evaluation studies
3 Biotech (2024)
-
Mediterranean diet in the targeted prevention and personalized treatment of chronic diseases: evidence, potential mechanisms, and prospects
EPMA Journal (2024)
-
Inflammation and gut dysbiosis as drivers of CKD–MBD
Nature Reviews Nephrology (2023)