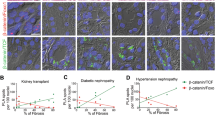
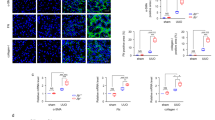
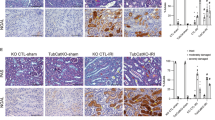
Abstract
The WNT–β-catenin system is an evolutionary conserved signalling pathway that is of particular importance for morphogenesis and cell organization during embryogenesis. The system is usually suppressed in adulthood; however, it can be re-activated in organ injury and regeneration. WNT-deficient mice display severe kidney defects at birth. Transient WNT–β-catenin activation stimulates tissue regeneration after acute kidney injury, whereas sustained (uncontrolled) WNT–β-catenin signalling promotes kidney fibrosis in chronic kidney disease (CKD), podocyte injury and proteinuria, persistent tissue damage during acute kidney injury and cystic kidney diseases. Additionally, WNT–β-catenin signalling is involved in CKD-associated vascular calcification and mineral bone disease. The WNT–β-catenin pathway is tightly regulated, for example, by proteins of the Dickkopf (DKK) family. In particular, DKK3 is released by ‘stressed’ tubular epithelial cells; DKK3 drives kidney fibrosis and is associated with short-term risk of CKD progression and acute kidney injury. Thus, targeting the WNT–β-catenin pathway might represent a promising therapeutic strategy in kidney injury and associated complications.
Key points
-
WNT–β-catenin is key regulator of embryogenesis and tissue organization.
-
WNT–β-catenin can be re-expressed in adult life in a variety of disease conditions.
-
Transient WNT–β-catenin signalling induces repair and regeneration during acute kidney injury, whereas sustained (uncontrolled) WNT–β-catenin signalling promotes kidney fibrosis, podocyte damage and mineral bone disease associated with chronic kidney disease.
-
Dickkopf 3 is a WNT–β-catenin modulator that drives kidney fibrosis and represents a novel marker for chronic kidney disease progression and acute kidney injury.
-
Several WNT–β-catenin signalling inhibitors are in development, of which so far only the sclerostin-inhibiting antibody is available for treatment of osteoporosis.
-
Future studies will improve understanding of the regulation of WNT–β-catenin and set the stage for specific therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nusse, R. & Varmus, H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31, 99–109 (1982).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Edeling, M., Ragi, G., Huang, S., Pavenstadt, H. & Susztak, K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 12, 426–439 (2016).
Karner, C. M. et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet. 41, 793–799 (2009).
MacDonald, B. T., Tamai, K. & He, X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009).
Niehrs, C. & Shen, J. Regulation of Lrp6 phosphorylation. Cell Mol. Life Sci. 67, 2551–2562 (2010).
Clevers, H. & Nusse, R. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Ma, L. & Wang, H. Y. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J. Biol. Chem. 282, 28980–28990 (2007).
Yang, Y. & Mlodzik, M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt). Annu. Rev. Cell Dev. Biol. 31, 623–646 (2015).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Harterink, M. & Korswagen, H. C. Dissecting the Wnt secretion pathway: key questions on the modification and intracellular trafficking of Wnt proteins. Acta Physiol. 204, 8–16 (2012).
Lorenowicz, M. J. & Korswagen, H. C. Sailing with the Wnt: charting the Wnt processing and secretion route. Exp. Cell Res. 315, 2683–2689 (2009).
Langton, P. F., Kakugawa, S. & Vincent, J. P. Making, exporting, and modulating Wnts. Trends Cell Biol. 26, 756–765 (2016).
Panakova, D., Sprong, H., Marois, E., Thiele, C. & Eaton, S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435, 58–65 (2005).
Mulligan, K. A. et al. Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility. Proc. Natl Acad. Sci. USA 109, 370–377 (2012).
Stanganello, E. et al. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat. Commun. 6, 5846 (2015).
Gross, J. C., Chaudhary, V., Bartscherer, K. & Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 14, 1036–1045 (2012).
Xu, Y. K. & Nusse, R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr. Biol. 8, R405–R406 (1998).
Pinson, K. I., Brennan, J., Monkley, S., Avery, B. J. & Skarnes, W. C. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538 (2000).
Oishi, I. et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cell 8, 645–654 (2003).
Malinauskas, T., Aricescu, A. R., Lu, W., Siebold, C. & Jones, E. Y. Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nat. Struct. Mol. Biol. 18, 886–893 (2011).
Hsieh, J. C. et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398, 431–436 (1999).
Joiner, D. M., Ke, J., Zhong, Z., Xu, H. E. & Williams, B. O. LRP5 and LRP6 in development and disease. Trends Endocrinol. Metab. 24, 31–39 (2013).
Niida, A. et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene 23, 8520–8526 (2004).
Agapova, O. A., Fang, Y., Sugatani, T., Seifert, M. E. & Hruska, K. A. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney Int. 89, 1231–1243 (2016).
Li, Z. et al. (Pro)renin receptor is an amplifier of Wnt/beta-catenin signaling in kidney injury and fibrosis. J. Am. Soc. Nephrol. 28, 2393–2408 (2017).
Riediger, F. et al. Prorenin receptor is essential for podocyte autophagy and survival. J. Am. Soc. Nephrol. 22, 2193–2202 (2011).
Kinouchi, K. et al. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ. Res. 107, 30–34 (2010).
Zhou, D., Tan, R. J., Zhou, L., Li, Y. & Liu, Y. Kidney tubular beta-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci. Rep. 3, 1878 (2013).
Maarouf, O. H. et al. Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J. Am. Soc. Nephrol. 27, 781–790 (2016).
DiRocco, D. P., Kobayashi, A., Taketo, M. M., McMahon, A. P. & Humphreys, B. D. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. 24, 1399–1412 (2013).
Beaton, H. et al. Wnt6 regulates epithelial cell differentiation and is dysregulated in renal fibrosis. Am. J. Physiol. Ren. Physiol. 311, F35–F45 (2016).
Zhou, D. et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 82, 537–547 (2012).
Wang, Z. et al. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J. Am. Soc. Nephrol. 20, 1919–1928 (2009).
Sinha, D. et al. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am. J. Physiol. Ren. Physiol. 288, F703–F713 (2005).
Nlandu-Khodo, S. et al. Tubular beta-catenin and FoxO3 interactions protect in chronic kidney disease. JCI Insight 5, e135454 (2020).
Luo, C. et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 29, 1238–1256 (2018).
Li, X. et al. Activation of Wnt5a-Ror2 signaling associated with epithelial-to-mesenchymal transition of tubular epithelial cells during renal fibrosis. Genes Cell 18, 608–619 (2013).
Strutz, F. & Zeisberg, M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J. Am. Soc. Nephrol. 17, 2992–2998 (2006).
Zhou, D. et al. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J. Am. Soc. Nephrol. 28, 2322–2336 (2017).
Zhou, D. et al. Fibroblast-specific beta-catenin signaling dictates the outcome of AKI. J. Am. Soc. Nephrol. 29, 1257–1271 (2018).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Lin, S. L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl Acad. Sci. USA 107, 4194–4199 (2010).
Lu, X. et al. Opposing actions of renal tubular- and myeloid-derived porcupine in obstruction-induced kidney fibrosis. Kidney Int. 96, 1308–1319 (2019).
Wong, D. W. L. et al. Activated renal tubular Wnt/beta-catenin signaling triggers renal inflammation during overload proteinuria. Kidney Int. 93, 1367–1383 (2018).
Lever, J. M. et al. Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 4, e12553 (2019).
Feng, Y., Liang, Y., Ren, J. & Dai, C. Canonical Wnt signaling promotes macrophage proliferation during kidney fibrosis. Kidney Dis. 4, 95–103 (2018).
Feng, Y. et al. Wnt/beta-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J. Am. Soc. Nephrol. 29, 182–193 (2018).
Feng, Y. et al. The signaling protein Wnt5a promotes TGFbeta1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J. Biol. Chem. 293, 19290–19302 (2018).
Zhou, D., Tan, R. J., Fu, H. & Liu, Y. Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Lab. Invest. 96, 156–167 (2016).
Terada, Y. et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J. Am. Soc. Nephrol. 14, 1223–1233 (2003).
Xiao, L. et al. Sustained activation of Wnt/beta-catenin signaling drives AKI to CKD progression. J. Am. Soc. Nephrol. 27, 1727–1740 (2016).
Chen, S. et al. Tenascin-C protects against acute kidney injury by recruiting Wnt ligands. Kidney Int. 95, 62–74 (2019).
Wang, D., Dai, C., Li, Y. & Liu, Y. Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 80, 1159–1169 (2011).
Shkreli, M. et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat. Med. 18, 111–119 (2011).
Dai, C. et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 20, 1997–2008 (2009).
Zhou, L. & Liu, Y. Wnt/beta-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat. Rev. Nephrol. 11, 535–545 (2015).
Kato, H. et al. Wnt/beta-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J. Biol. Chem. 286, 26003–26015 (2011).
Zhou, L. et al. Wnt/beta-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int. 95, 830–845 (2019).
Jiang, L. et al. Calmodulin-dependent protein kinase II/cAMP response element-binding protein/Wnt/beta-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J. Biol. Chem. 288, 23368–23379 (2013).
He, W., Kang, Y. S., Dai, C. & Liu, Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 22, 90–103 (2011).
Bergmann, C. et al. Polycystic kidney disease. Nat. Rev. Dis. Primers 4, 50 (2018).
Lancaster, M. A. et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 15, 1046–1054 (2009).
Feng, S. et al. The sorting nexin 3 retromer pathway regulates the cell surface localization and activity of a Wnt-activated polycystin channel complex. J. Am. Soc. Nephrol. 28, 2973–2984 (2017).
Kim, S. et al. The polycystin complex mediates Wnt/Ca2+ signalling. Nat. Cell Biol. 18, 752–764 (2016).
Papakrivopoulou, E., Dean, C. H., Copp, A. J. & Long, D. A. Planar cell polarity and the kidney. Nephrol. Dial. Transpl. 29, 1320–1326 (2014).
Simons, M. et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 37, 537–543 (2005).
Otto, E. A. et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 34, 413–420 (2003).
Li, A. et al. Canonical Wnt inhibitors ameliorate cystogenesis in a mouse ortholog of human ADPKD. JCI Insight 3, e95874 (2018).
Surendran, K., Schiavi, S. & Hruska, K. A. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 16, 2373–2384 (2005).
He, W. et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 (2009).
Miao, J. et al. Wnt/beta-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 18, e13004 (2019).
Li, S. Y. et al. Four-and-a-half LIM domains protein 2 is a coactivator of Wnt signaling in diabetic kidney disease. J. Am. Soc. Nephrol. 26, 3072–3084 (2015).
Zhao, Y. et al. Wnt/beta-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int. 95, 815–829 (2019).
Zhang, P., Cai, Y., Soofi, A. & Dressler, G. R. Activation of Wnt11 by transforming growth factor-beta drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells. J. Biol. Chem. 287, 21290–21302 (2012).
Nlandu-Khodo, S. et al. Blocking TGF-beta and beta-catenin epithelial crosstalk exacerbates CKD. J. Am. Soc. Nephrol. 28, 3490–3503 (2017).
Qiao, X. et al. Redirecting TGF-beta signaling through the beta-catenin/foxo complex prevents kidney fibrosis. J. Am. Soc. Nephrol. 29, 557–570 (2018).
Satoh, M. et al. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am. J. Physiol. Ren. Physiol. 303, F1641–F1651 (2012).
Zhou, L., Li, Y., Zhou, D., Tan, R. J. & Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J. Am. Soc. Nephrol. 24, 771–785 (2013).
Cheng, R., Ding, L., He, X., Takahashi, Y. & Ma, J. X. Interaction of PPARα with the canonic Wnt pathway in the regulation of renal fibrosis. Diabetes 65, 3730–3743 (2016).
Madan, B. et al. Experimental inhibition of porcupine-mediated Wnt O-acylation attenuates kidney fibrosis. Kidney Int. 89, 1062–1074 (2016).
He, X. et al. Pigment epithelium-derived factor, a noninhibitory serine protease inhibitor, is renoprotective by inhibiting the Wnt pathway. Kidney Int. 91, 642–657 (2017).
Hao, S. et al. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 22, 1642–1653 (2011).
Zoccali, C. et al. The systemic nature of CKD. Nat. Rev. Nephrol. 13, 344–358 (2017).
Krishnan, V., Bryant, H. U. & Macdougald, O. A. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 116, 1202–1209 (2006).
Clement-Lacroix, P. et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl Acad. Sci. USA 102, 17406–17411 (2005).
Tu, X. et al. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc. Natl Acad. Sci. USA 112, E478–E486 (2015).
Holmen, S. L. et al. Essential role of beta-catenin in postnatal bone acquisition. J. Biol. Chem. 280, 21162–21168 (2005).
Kramer, I. et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 30, 3071–3085 (2010).
Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell… and more. Endocr. Rev. 34, 658–690 (2013).
Bonewald, L. F. & Johnson, M. L. Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615 (2008).
van Lierop, A. H., Appelman-Dijkstra, N. M. & Papapoulos, S. E. Sclerostin deficiency in humans. Bone 96, 51–62 (2017).
Li, X. et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 23, 860–869 (2008).
Winkler, D. G. et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 22, 6267–6276 (2003).
Wijenayaka, A. R. et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One 6, e25900 (2011).
Grotewold, L., Theil, T. & Ruther, U. Expression pattern of Dkk-1 during mouse limb development. Mech. Dev. 89, 151–153 (1999).
Morvan, F. et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 21, 934–945 (2006).
Nakajima, H. et al. Wnt modulators, SFRP-1, and SFRP-2 are expressed in osteoblasts and differentially regulate hematopoietic stem cells. Biochem. Biophys. Res. Commun. 390, 65–70 (2009).
Ominsky, M. S. et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J. Bone Miner. Res. 26, 1012–1021 (2011).
Feng, G., Chang-Qing, Z., Yi-Min, C. & Xiao-Lin, L. Systemic administration of sclerostin monoclonal antibody accelerates fracture healing in the femoral osteotomy model of young rats. Int. Immunopharmacol. 24, 7–13 (2015).
Jin, H. et al. Anti-DKK1 antibody promotes bone fracture healing through activation of beta-catenin signaling. Bone 71, 63–75 (2015).
Ominsky, M. S., Boyce, R. W., Li, X. & Ke, H. Z. Effects of sclerostin antibodies in animal models of osteoporosis. Bone 96, 63–75 (2017).
Moe, S. M. et al. Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy. J. Bone Miner. Res. 30, 499–509 (2015).
Fang, Y. et al. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J. Am. Soc. Nephrol. 25, 1760–1773 (2014).
Carrillo-Lopez, N. et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 90, 77–89 (2016).
Sabbagh, Y. et al. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J. Bone Miner. Res. 27, 1757–1772 (2012).
Huang, J. C. et al. PTH differentially regulates expression of RANKL and OPG. J. Bone Miner. Res. 19, 235–244 (2004).
Keller, H. & Kneissel, M. SOST is a target gene for PTH in bone. Bone 37, 148–158 (2005).
Bellido, T. et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146, 4577–4583 (2005).
Guo, J. et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 11, 161–171 (2010).
Kulkarni, N. H. et al. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell Biochem. 95, 1178–1190 (2005).
Malluche, H. H., Mawad, H. & Monier-Faugere, M. C. The importance of bone health in end-stage renal disease: out of the frying pan, into the fire? Nephrol. Dial. Transpl. 19, i9–i13 (2004).
Yang, C. Y. et al. Circulating Wnt/beta-catenin signalling inhibitors and uraemic vascular calcifications. Nephrol. Dial. Transpl. 30, 1356–1363 (2015).
Ho, T. Y. et al. Evaluation of the association of Wnt signaling with coronary artery calcification in patients on dialysis with severe secondary hyperparathyroidism. BMC Nephrol. 20, 345 (2019).
Albanese, I. et al. Role of noncanonical Wnt signaling pathway in human aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 37, 543–552 (2017).
Cai, T. et al. WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 345, 206–217 (2016).
Freise, C., Kretzschmar, N. & Querfeld, U. Wnt signaling contributes to vascular calcification by induction of matrix metalloproteinases. BMC Cardiovasc. Disord. 16, 185 (2016).
Shao, J. S. et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Invest. 115, 1210–1220 (2005).
Al-Aly, Z. et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 27, 2589–2596 (2007).
Zhang, H. et al. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/beta-catenin signaling. Toxicol. Lett. 284, 29–36 (2018).
Grande, M. T. et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 21, 989–997 (2015).
Lovisa, S. et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 21, 998–1009 (2015).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Simon-Tillaux, N. & Hertig, A. Snail and kidney fibrosis. Nephrol. Dial. Transpl. 32, 224–233 (2017).
Zhou, B. P. et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6, 931–940 (2004).
Yook, J. I., Li, X. Y., Ota, I., Fearon, E. R. & Weiss, S. J. Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 280, 11740–11748 (2005).
Matsui, I. et al. Snail, a transcriptional regulator, represses nephrin expression in glomerular epithelial cells of nephrotic rats. Lab. Invest. 87, 273–283 (2007).
Li, C. & Siragy, H. M. High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-beta-catenin-snail signaling pathway. PLoS One 9, e89233 (2014).
Li, X., Deng, W., Lobo-Ruppert, S. M. & Ruppert, J. M. Gli1 acts through Snail and E-cadherin to promote nuclear signaling by beta-catenin. Oncogene 26, 4489–4498 (2007).
Zhou, D. et al. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J. Am. Soc. Nephrol. 28, 598–611 (2017).
Tan, R. J. & Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Ren. Physiol. 302, F1351–F1361 (2012).
Villa-Morales, M. & Fernandez-Piqueras, J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets 16, 85–101 (2012).
Hu, C. et al. Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr. Med. Chem. 22, 2858–2870 (2015).
Tan, R. J. et al. Tubular injury triggers podocyte dysfunction by beta-catenin-driven release of MMP-7. JCI Insight 4, e122399 (2019).
He, W. et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/beta-catenin activity in CKD. J. Am. Soc. Nephrol. 23, 294–304 (2012).
Yang, X. et al. Urinary matrix metalloproteinase 7 and prediction of IgA nephropathy progression. Am. J. Kidney Dis. 75, 384–393 (2020).
Fu, H. et al. Matrix metalloproteinase-7 protects against acute kidney injury by priming renal tubules for survival and regeneration. Kidney Int. 95, 1167–1180 (2019).
Yang, X. et al. Urinary matrix metalloproteinase-7 predicts severe AKI and poor outcomes after cardiac surgery. J. Am. Soc. Nephrol. 28, 3373–3382 (2017).
Hall, G., Wang, L. & Spurney, R. F. TRPC channels in proteinuric kidney diseases. Cells 9, 44 (2019).
Wang, L. et al. Gq signaling causes glomerular injury by activating TRPC6. J. Clin. Invest. 125, 1913–1926 (2015).
Zhou, L. et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J. Am. Soc. Nephrol. 26, 107–120 (2015).
Floege, J. Antagonism of canonical Wnt/beta-catenin signaling: taking RAS blockade to the next level? J. Am. Soc. Nephrol. 26, 3–5 (2015).
Yao, L. et al. Fibroblast-specific plasminogen activator inhibitor-1 depletion ameliorates renal interstitial fibrosis after unilateral ureteral obstruction. Nephrol. Dial. Transpl. 34, 2042–2050 (2019).
Liu, Y. et al. Inhibition of PAI-1 attenuates perirenal fat inflammation and the associated nephropathy in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 316, E260–E267 (2019).
Gu, C., Zhang, J., Noble, N. A., Peng, X. R. & Huang, Y. An additive effect of anti-PAI-1 antibody to ACE inhibitor on slowing the progression of diabetic kidney disease. Am. J. Physiol. Ren. Physiol. 311, F852–F863 (2016).
Kobayashi, N. et al. Podocyte injury-driven intracapillary plasminogen activator inhibitor type 1 accelerates podocyte loss via uPAR-mediated beta1-integrin endocytosis. Am. J. Physiol. Ren. Physiol. 308, F614–F626 (2015).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Mao, B. et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325 (2001).
Pollard, S. L. & Holland, P. W. Evidence for 14 homeobox gene clusters in human genome ancestry. Curr. Biol. 10, 1059–1062 (2000).
Luke, G. N. et al. Dispersal of NK homeobox gene clusters in amphioxus and humans. Proc. Natl Acad. Sci. USA 100, 5292–5295 (2003).
Mao, B. et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417, 664–667 (2002).
Mao, B. & Niehrs, C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene 302, 179–183 (2003).
Federico, G. et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight 1, e84916 (2016).
Yu, B. et al. A cytokine-like protein dickkopf-related protein 3 is atheroprotective. Circulation 136, 1022–1036 (2017).
Issa Bhaloo, S. et al. Binding of Dickkopf-3 to CXCR7 enhances vascular progenitor cell migration and degradable graft regeneration. Circ. Res. 123, 451–466 (2018).
Ren, S. et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc. Natl Acad. Sci. USA 110, 1440–1445 (2013).
Johnson, B. G. et al. Connective tissue growth factor domain 4 amplifies fibrotic kidney disease through activation of LDL receptor-related protein 6. J. Am. Soc. Nephrol. 28, 1769–1782 (2017).
Lin, C. L. et al. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J. Am. Soc. Nephrol. 21, 124–135 (2010).
Liu, M. et al. Genetic variation of DKK3 may modify renal disease severity in ADPKD. J. Am. Soc. Nephrol. 21, 1510–1520 (2010).
Wong, D. W. et al. Downregulation of renal tubular Wnt/beta-catenin signaling by Dickkopf-3 induces tubular cell death in proteinuric nephropathy. Cell Death Dis. 7, e2155 (2016).
Lipphardt, M. et al. Dickkopf-3 in aberrant endothelial secretome triggers renal fibroblast activation and endothelial-mesenchymal transition. Nephrol. Dial. Transpl. 34, 49–62 (2019).
Schunk, S. J., Speer, T., Petrakis, I. & Fliser, D. Dickkopf 3 — a novel biomarker of the ‘kidney injury continuum’. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfaa003 (2020).
Zewinger, S. et al. Dickkopf-3 (DKK3) in urine identifies patients with short-term risk of eGFR Loss. J. Am. Soc. Nephrol. 29, 2722–2733 (2018).
Rauen, T. et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N. Engl. J. Med. 373, 2225–2236 (2015).
Schunk, S. J. et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet 394, 488–496 (2019).
Zarbock, A. et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 313, 2133–2141 (2015).
McClung, M. R. et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420 (2014).
Cosman, F. et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016).
Langdahl, B. L. et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 390, 1585–1594 (2017).
Saag, K. G. et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 377, 1417–1427 (2017).
Chatterjee, S. & Sil, P. C. Targeting the crosstalks of Wnt pathway with Hedgehog and Notch for cancer therapy. Pharmacol. Res. 142, 251–261 (2019).
Acknowledgements
S.J.S., T.S., J.F. and D.F. are supported by the Deutsche Forschungsgemeinschaft (DFG SFB-TRR 219).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, made a substantial contribution to discussion of content, wrote and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interest
D.F. is affiliated with DiaRen. S.J.S, J.F. and T.S. declare no competing interests.
Additional information
Peer-review information
Nature Reviews Nephrology thanks Xiao Meng, Youhua Liu and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schunk, S.J., Floege, J., Fliser, D. et al. WNT–β-catenin signalling — a versatile player in kidney injury and repair. Nat Rev Nephrol 17, 172–184 (2021). https://doi.org/10.1038/s41581-020-00343-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-00343-w
This article is cited by
-
The pathogenic role of succinate-SUCNR1: a critical function that induces renal fibrosis via M2 macrophage
Cell Communication and Signaling (2024)
-
WNT-dependent interaction between inflammatory fibroblasts and FOLR2+ macrophages promotes fibrosis in chronic kidney disease
Nature Communications (2024)
-
Aloperine Alleviates Myocardial Injury Induced by Myocardial Ischemia and Reperfusion by Activating the ERK1/2/β-catenin Signaling Pathway
Cardiovascular Drugs and Therapy (2024)
-
Phillygenin Inhibits TGF-β1-induced Hepatic Stellate Cell Activation and Inflammation: Regulation of the Bax/Bcl-2 and Wnt/β-catenin Pathways
Inflammation (2024)
-
Scutellarin ameliorates diabetic nephropathy via TGF-β1 signaling pathway
Natural Products and Bioprospecting (2024)