Abstract
Nuclear receptors have a broad spectrum of biological functions in normal physiology and in the pathology of various diseases, including glomerular disease. The primary therapies for many glomerular diseases are glucocorticoids, which exert their immunosuppressive and direct podocyte protective effects via the glucocorticoid receptor (GR). As glucocorticoids are associated with important adverse effects and a substantial proportion of patients show resistance to these therapies, the beneficial effects of selective GR modulators are now being explored. Peroxisome proliferator-activated receptor-γ (PPARγ) agonism using thiazolidinediones has potent podocyte cytoprotective and nephroprotective effects. Repurposing of thiazolidinediones or identification of novel PPARγ modulators are potential strategies to treat non-diabetic glomerular disease. Retinoic acid receptor-α is the key mediator of the renal protective effects of retinoic acid, and repair of the endogenous retinoic acid pathway offers another potential therapeutic strategy for glomerular disease. Vitamin D receptor, oestrogen receptor and mineralocorticoid receptor modulators regulate podocyte injury in experimental models. Further studies are needed to better understand the mechanisms of these nuclear receptors, evaluate their synergistic pathways and identify their novel modulators. Here, we focus on the role of nuclear receptors in podocyte biology and non-diabetic glomerular disease.
Key points
-
Understanding the role of nuclear receptors in podocyte biology and glomerular disease could lead to novel therapeutic strategies for glomerular disease.
-
Glucocorticoids activate the glucocorticoid receptor and have direct protective effects on podocytes in addition to their immunosuppressive effects; glucocorticoid resistance and adverse effects pose huge challenges to the therapeutic use of these agents.
-
Activation of peroxisome proliferator-activated receptor-γ using thiazolidinediones protects against podocyte injury and has beneficial effects in animal models of glomerular disease.
-
Retinoic acid receptor-α mediates the renal protective effects of retinoic acid; repair of the endogenous retinoic acid synthesis pathway might be a novel strategy to treat glomerular disease.
-
Vitamin D3 activates the vitamin D receptor and ameliorates podocyte injury in experimental models; the available data from clinical trials of vitamin D supplementation in patients with chronic kidney disease are inconclusive.
-
A deeper understanding of the mechanisms, synergistic pathways and modulated targeting of nuclear receptor pathways in podocyte biology could enable the development of more effective therapies for non-diabetic glomerular disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
Mazaira, G. I. et al. The nuclear receptor field: a historical overview and future challenges. Nucl. Receptor Res. 5, 101320 (2018).
Jensen, E. V. On the mechanism of estrogen action. Perspect. Biol. Med. 6, 47–59 (1962).
Hollenberg, S. M. et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 318, 635–641 (1985).
Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell 97, 161–163 (1999).
Glass, C. K. & Ogawa, S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat. Rev. Immunol. 6, 44–55 (2006).
Ogawa, S. et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122, 707–721 (2005).
Cain, D. W. & Cidlowski, J. A. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 29, 545–556 (2015).
Zhou, J. & Cidlowski, J. A. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 70, 407–417 (2005).
Weikum, E. R., Knuesel, M. T., Ortlund, E. A. & Yamamoto, K. R. Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 18, 159–174 (2017).
Pratt, W. B. & Toft, D. O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18, 306–360 (1997).
Zhao, X., Hwang, D. Y. & Kao, H. Y. The role of glucocorticoid receptors in podocytes and nephrotic syndrome. Nucl. Receptor Res. 5, 101323 (2018).
Rhen, T. & Cidlowski, J. A. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723 (2005).
Schacke, H., Docke, W. D. & Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 96, 23–43 (2002).
Ponticelli, C. & Locatelli, F. Glucocorticoids in the treatment of glomerular diseases: pitfalls and pearls. Clin. J. Am. Soc. Nephrol. 13, 815–822 (2018).
Ito, K., Chung, K. F. & Adcock, I. M. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 117, 522–543 (2006).
Barnes, P. J. & Adcock, I. M. Glucocorticoid resistance in inflammatory diseases. Lancet 373, 1905–1917 (2009).
Cavallo, T., Graves, K. & Granholm, N. A. Murine lupus nephritis. Effects of glucocorticoid on circulating and tissue-bound immunoreactants. Lab. Invest. 49, 476–481 (1983).
Cavallo, T., Graves, K. & Granholm, N. A. Murine lupus nephritis. Effects of glucocorticoid on glomerular permeability. Lab. Invest. 50, 378–384 (1984).
Holdsworth, S. R. & Bellomo, R. Differential effects of steroids on leukocyte-mediated glomerulonephritis in the rabbit. Kidney Int. 26, 162–169 (1984).
Ito, M., Aono, Y., Suzuki, A., Nagamatsu, T. & Suzuki, Y. Accelerated passive Heymann nephritis in rats as an experimental model for membranous glomerulonephritis and effects of azathioprine and prednisolone on the nephritis. Jpn. J. Pharmacol. 49, 101–110 (1989).
Fujiwara, Y. An ultrastructural study of the effect of the steroid in puromycin aminonucleoside nephrosis rats. Virchows Arch. A Pathol. Anat. Histopathol. 405, 11–24 (1984).
Kawamura, T., Yoshioka, T., Bills, T., Fogo, A. & Ichikawa, I. Glucocorticoid activates glomerular antioxidant enzymes and protects glomeruli from oxidant injuries. Kidney Int. 40, 291–301 (1991).
Agrawal, S. et al. Pioglitazone enhances the beneficial effects of glucocorticoids in experimental nephrotic syndrome. Sci. Rep. 6, 24392 (2016).
Bertani, T. et al. Steroids and Adriamycin nephrosis. Appl. Pathol. 2, 32–38 (1984).
Pippin, J. W. et al. Inducible rodent models of acquired podocyte diseases. Am. J. Physiol. Ren. Physiol. 296, F213–F229 (2009).
Zhou, H. et al. Loss of the podocyte glucocorticoid receptor exacerbates proteinuria after injury. Sci. Rep. 7, 9833 (2017).
Kuppe, C. et al. Investigations of glucocorticoid action in GN. J. Am. Soc. Nephrol. 28, 1408–1420 (2017).
Ransom, R. F., Lam, N. G., Hallett, M. A., Atkinson, S. J. & Smoyer, W. E. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 68, 2473–2483 (2005).
Wada, T., Pippin, J. W., Marshall, C. B., Griffin, S. V. & Shankland, S. J. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J. Am. Soc. Nephrol. 16, 2615–2625 (2005).
Ransom, R. F., Vega-Warner, V., Smoyer, W. E. & Klein, J. Differential proteomic analysis of proteins induced by glucocorticoids in cultured murine podocytes. Kidney Int. 67, 1275–1285 (2005).
Wada, T., Pippin, J. W., Nangaku, M. & Shankland, S. J. Dexamethasone’s prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron Exp. Nephrol. 109, e8–e19 (2008).
Ohashi, T., Uchida, K., Uchida, S., Sasaki, S. & Nitta, K. Dexamethasone increases the phosphorylation of nephrin in cultured podocytes. Clin. Exp. Nephrol. 15, 688–693 (2011).
Mallipattu, S. K. et al. Kruppel-like factor 15 mediates glucocorticoid-induced restoration of podocyte differentiation markers. J. Am. Soc. Nephrol. 28, 166–184 (2017).
Lewko, B. et al. Dexamethasone-dependent modulation of cyclic GMP synthesis in podocytes. Mol. Cell Biochem. 409, 243–253 (2015).
Xie, H. et al. Inhibition of microRNA-30a prevents puromycin aminonucleoside-induced podocytic apoptosis by upregulating the glucocorticoid receptor alpha. Mol. Med. Rep. 12, 6043–6052 (2015).
Wu, J. et al. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J. Am. Soc. Nephrol. 25, 92–104 (2014).
Guess, A. et al. Dose- and time-dependent glucocorticoid receptor signaling in podocytes. Am. J. Physiol. Ren. Physiol. 299, F845–F853 (2010).
Agrawal, S., Guess, A. J., Benndorf, R. & Smoyer, W. E. Comparison of direct action of thiazolidinediones and glucocorticoids on renal podocytes: protection from injury and molecular effects. Mol. Pharmacol. 80, 389–399 (2011).
Agrawal, S., Guess, A. J., Chanley, M. A. & Smoyer, W. E. Albumin-induced podocyte injury and protection are associated with regulation of COX-2. Kidney Int. 86, 1150–1160 (2014).
Luetscher, J. A. Jr. & Deming, Q. B. Treatment of nephrosis with cortisone. J. Clin. Invest. 29, 1576–1587 (1950).
Nourbakhsh, N. & Mak, R. H. Steroid-resistant nephrotic syndrome: past and current perspectives. Pediatric Health Med. Ther. 8, 29–37 (2017).
Canetta, P. A. & Radhakrishnan, J. The evidence-based approach to adult-onset idiopathic nephrotic syndrome. Front. Pediatr. 3, 78 (2015).
Agrawal, S. et al. Predicting and defining steroid resistance in pediatric nephrotic syndrome using plasma proteomics. Kidney Int. Rep. 5, 66–80 (2020).
Gooding, J. R. et al. Predicting and defining steroid resistance in pediatric nephrotic syndrome using plasma metabolomics. Kidney Int. Rep. 5, 81–93 (2020).
Bennett, M. R. et al. A novel biomarker panel to identify steroid resistance in childhood idiopathic nephrotic syndrome. Biomarker Insights 12, 1177271917695832 (2017).
Saleem, M. A. Molecular stratification of idiopathic nephrotic syndrome. Nat. Rev. Nephrol. 15, 750–765 (2019).
Newton, R. & Holden, N. S. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol. Pharmacol. 72, 799–809 (2007).
Gessi, S., Merighi, S. & Borea, P. A. Glucocorticoid’s pharmacology: past, present and future. Curr. Pharm. Des. 16, 3540–3553 (2010).
Zhu, Y., Alvares, K., Huang, Q., Rao, M. S. & Reddy, J. K. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J. Biol. Chem. 268, 26817–26820 (1993).
Chen, F., Law, S. W. & O’Malley, B. W. Identification of two mPPAR related receptors and evidence for the existence of five subfamily members. Biochem. Biophys. Res. Commun. 196, 671–677 (1993).
Bookout, A. L. et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126, 789–799 (2006).
Miglio, G. et al. The subtypes of peroxisome proliferator-activated receptors expressed by human podocytes and their role in decreasing podocyte injury. Br. J. Pharmacol. 162, 111–125 (2011).
Mukherjee, R., Jow, L., Croston, G. E. & Paterniti, J. R. Jr Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARγ2 versus PPARγ1 and activation with retinoid X receptor agonists and antagonists. J. Biol. Chem. 272, 8071–8076 (1997).
Aprile, M. et al. PPARγΔ5, a naturally occurring dominant-negative splice isoform, impairs PPARγ function and adipocyte differentiation. Cell Rep. 25, 1577–1592.e6 (2018).
Sabatino, L. et al. A novel peroxisome proliferator-activated receptor gamma isoform with dominant negative activity generated by alternative splicing. J. Biol. Chem. 280, 26517–26525 (2005).
Varanasi, U. et al. Identification of a peroxisome proliferator-responsive element upstream of the human peroxisomal fatty acyl coenzyme A oxidase gene. J. Biol. Chem. 271, 2147–2155 (1996).
Chandra, V. et al. Structure of the intact PPAR-γ-RXR–nuclear receptor complex on DNA. Nature 456, 350–356 (2008).
Leo, C. & Chen, J. D. The SRC family of nuclear receptor coactivators. Gene 245, 1–11 (2000).
Siersbaek, R. et al. Molecular architecture of transcription factor hotspots in early adipogenesis. Cell Rep. 7, 1434–1442 (2014).
Dubois-Chevalier, J. et al. A dynamic CTCF chromatin binding landscape promotes DNA hydroxymethylation and transcriptional induction of adipocyte differentiation. Nucleic Acids Res. 42, 10943–10959 (2014).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Lin, J., Puigserver, P., Donovan, J., Tarr, P. & Spiegelman, B. M. Peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β), a novel PGC-1-related transcription coactivator associated with host cell factor. J. Biol. Chem. 277, 1645–1648 (2002).
Tomaru, T. et al. Isolation and characterization of a transcriptional cofactor and its novel isoform that bind the deoxyribonucleic acid-binding domain of peroxisome proliferator-activated receptor-γ. Endocrinology 147, 377–388 (2006).
Zhu, Y. et al. Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a coactivator for PPAR. J. Biol. Chem. 275, 13510–13516 (2000).
Ricote, M. & Glass, C. K. PPARs and molecular mechanisms of transrepression. Biochim. Biophys. Acta 1771, 926–935 (2007).
Glass, C. K. & Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 10, 365–376 (2010).
Viswakarma, N. et al. Coactivators in PPAR-regulated gene expression. PPAR Res. 2010, 250126 (2010).
Moran-Salvador, E. et al. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 25, 2538–2550 (2011).
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994).
Lefterova, M. I., Haakonsson, A. K., Lazar, M. A. & Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 25, 293–302 (2014).
Sun, X., Han, R., Wang, Z. & Chen, Y. Regulation of adiponectin receptors in hepatocytes by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Diabetologia 49, 1303–1310 (2006).
Stewart, W. C., Morrison, R. F., Young, S. L. & Stephens, J. M. Regulation of signal transducers and activators of transcription (STATs) by effectors of adipogenesis: coordinate regulation of STATs 1, 5A, and 5B with peroxisome proliferator-activated receptor-γ and C/AAAT enhancer binding protein-α. Biochim. Biophys. Acta 1452, 188–196 (1999).
Olsen, H. & Haldosen, L. A. Peroxisome proliferator-activated receptor gamma regulates expression of signal transducer and activator of transcription 5A. Exp. Cell Res. 312, 1371–1380 (2006).
Prost, S. et al. Human and simian immunodeficiency viruses deregulate early hematopoiesis through a Nef/PPARγ/STAT5 signaling pathway in macaques. J. Clin. Invest. 118, 1765–1775 (2008).
Heikkinen, S., Auwerx, J. & Argmann, C. A. PPARgamma in human and mouse physiology. Biochim. Biophys. Acta 1771, 999–1013 (2007).
Sato, K. et al. Expression of peroxisome proliferator-activated receptor isoform proteins in the rat kidney. Hypertens. Res. 27, 417–425 (2004).
Yang, T. et al. Expression of peroxisomal proliferator-activated receptors and retinoid X receptors in the kidney. Am. J. Physiol. 277, F966–F973 (1999).
Henique, C. et al. Nuclear factor erythroid 2-related factor 2 drives podocyte-specific expression of peroxisome proliferator-activated receptor γ essential for resistance to crescentic GN. J. Am. Soc. Nephrol. 27, 172–188 (2016).
Long, Q. et al. Peroxisome proliferator-activated receptor-γ increases adiponectin secretion via transcriptional repression of endoplasmic reticulum chaperone protein ERp44. Endocrinology 151, 3195–3203 (2010).
Choi, J. H. et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466, 451–456 (2010).
Rutkowski, J. M. et al. Adiponectin promotes functional recovery after podocyte ablation. J. Am. Soc. Nephrol. 24, 268–282 (2013).
Pascual, G. et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 437, 759–763 (2005).
Tsukahara, T. et al. Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARγ by cyclic phosphatidic acid. Mol. Cell 39, 421–432 (2010).
McIntyre, T. M. et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proc. Natl Acad. Sci. USA 100, 131–136 (2003).
Ristow, M., Muller-Wieland, D., Pfeiffer, A., Krone, W. & Kahn, C. R. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N. Engl. J. Med. 339, 953–959 (1998).
Barroso, I. et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402, 880–883 (1999).
Nikiforova, M. N. et al. RAS point mutations and PAX8-PPARγ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 88, 2318–2326 (2003).
Marques, A. R. et al. Expression of PAX8-PPARγ1 rearrangements in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 87, 3947–3952 (2002).
Rochel, N. et al. Recurrent activating mutations of PPARγ associated with luminal bladder tumors. Nat. Commun. 10, 253 (2019).
Halstead, A. M. et al. Bladder-cancer-associated mutations in RXRA activate peroxisome proliferator-activated receptors to drive urothelial proliferation. eLife 6, e30862 (2017).
Toffoli, B. et al. Nephropathy in Pparg-null mice highlights PPARγ systemic activities in metabolism and in the immune system. PLoS ONE 12, e0171474 (2017).
Chinetti, G., Fruchart, J. C. & Staels, B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 49, 497–505 (2000).
Nakamura, T. et al. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism 50, 1193–1196 (2001).
Sarafidis, P. A., Stafylas, P. C., Georgianos, P. I., Saratzis, A. N. & Lasaridis, A. N. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am. J. Kidney Dis. 55, 835–847 (2010).
Schneider, C. A. et al. Effect of pioglitazone on cardiovascular outcome in diabetes and chronic kidney disease. J. Am. Soc. Nephrol. 19, 182–187 (2008).
Kernan, W. N. et al. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 374, 1321–1331 (2016).
Young, L. H. et al. Cardiac outcomes after ischemic stroke or transient ischemic attack: effects of pioglitazone in patients with insulin resistance without diabetes mellitus. Circulation 135, 1882–1893 (2017).
Liu, J. & Wang, L. N. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack. Cochrane Database Syst. Rev. 10, CD010693 (2019).
Zhou, Y. et al. Pioglitazone for the primary and secondary prevention of cardiovascular and renal outcomes in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis. J. Clin. Endocrinol. Metab. 105, dgz252 (2020).
Nissen, S. E. & Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 356, 2457–2471 (2007).
Wallach, J. D. et al. Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses. BMJ 368, l7078 (2020).
van Wijk, J. P., de Koning, E. J., Martens, E. P. & Rabelink, T. J. Thiazolidinediones and blood lipids in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 23, 1744–1749 (2003).
Deeg, M. A. et al. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30, 2458–2464 (2007).
Mamtani, R. et al. Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J. Natl Cancer Inst. 104, 1411–1421 (2012).
Graham, D. J. et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 304, 411–418 (2010).
Buckingham, R. E. et al. Peroxisome proliferator-activated receptor-γ agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes 47, 1326–1334 (1998).
Cha, D. R. et al. Peroxisome proliferator activated receptor α/γ dual agonist tesaglitazar attenuates diabetic nephropathy in db/db mice. Diabetes 56, 2036–2045 (2007).
Tanimoto, M. et al. Effect of pioglitazone on the early stage of type 2 diabetic nephropathy in KK/Ta mice. Metabolism 53, 1473–1479 (2004).
Calkin, A. C. et al. PPAR-α and -γ agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol. Dial. Transplant. 21, 2399–2405 (2006).
Straus, D. S. & Glass, C. K. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551–558 (2007).
Bouhlel, M. A. et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 (2007).
Chinetti, G. et al. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 273, 25573–25580 (1998).
Ma, L. J., Marcantoni, C., Linton, M. F., Fazio, S. & Fogo, A. B. Peroxisome proliferator-activated receptor-γ agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int. 59, 1899–1910 (2001).
Yang, H. C. et al. The PPARγ agonist pioglitazone ameliorates aging-related progressive renal injury. J. Am. Soc. Nephrol. 20, 2380–2388 (2009).
Yang, H. C., Ma, L. J., Ma, J. & Fogo, A. B. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int. 69, 1756–1764 (2006).
Liu, H. F. et al. Thiazolidinedione attenuate proteinuria and glomerulosclerosis in Adriamycin-induced nephropathy rats via slit diaphragm protection. Nephrology 15, 75–83 (2010).
Zuo, Y. et al. Protective effects of PPARγ agonist in acute nephrotic syndrome. Nephrol. Dial. Transplant. 27, 174–181 (2012).
Haraguchi, K., Shimura, H. & Onaya, T. Suppression of experimental crescentic glomerulonephritis by peroxisome proliferator-activated receptor (PPAR)γ activators. Clin. Exp. Nephrol. 7, 27–32 (2003).
Chafin, C. et al. Deletion of PPAR-γ in immune cells enhances susceptibility to antiglomerular basement membrane disease. J. Inflamm. Res. 3, 127–134 (2010).
Cho, H. Y. et al. Nrf2-regulated PPARγ expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care Med. 182, 170–182 (2010).
Cho, H. Y., Reddy, S. P., Debiase, A., Yamamoto, M. & Kleeberger, S. R. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic. Biol. Med. 38, 325–343 (2005).
Huang, J., Tabbi-Anneni, I., Gunda, V. & Wang, L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1211–G1221 (2010).
Sonneveld, R. et al. Sildenafil prevents podocyte injury via PPAR-γ-mediated TRPC6 inhibition. J. Am. Soc. Nephrol. 28, 1491–1505 (2017).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Paueksakon, P., Revelo, M. P., Ma, L. J., Marcantoni, C. & Fogo, A. B. Microangiopathic injury and augmented PAI-1 in human diabetic nephropathy. Kidney Int. 61, 2142–2148 (2002).
Okada, T. et al. Thiazolidinediones ameliorate diabetic nephropathy via cell cycle-dependent mechanisms. Diabetes 55, 1666–1677 (2006).
Kanjanabuch, T. et al. PPAR-γ agonist protects podocytes from injury. Kidney Int. 71, 1232–1239 (2007).
Miglio, G. et al. Protective effects of peroxisome proliferator-activated receptor agonists on human podocytes: proposed mechanisms of action. Br. J. Pharmacol. 167, 641–653 (2012).
Miceli, I. et al. Stretch reduces nephrin expression via an angiotensin II-AT(1)-dependent mechanism in human podocytes: effect of rosiglitazone. Am. J. Physiol. Ren. Physiol. 298, F381–F390 (2010).
Zhu, C. et al. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARγ. Am. J. Pathol. 178, 2020–2031 (2011).
Zhou, Z. et al. MicroRNA-27a promotes podocyte injury via PPARγ-mediated β-catenin activation in diabetic nephropathy. Cell Death Dis. 8, e2658 (2017).
Ahmadian, M. et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 (2013).
Burris, T. P. et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 65, 710–778 (2013).
Choi, J. H. et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 477, 477–481 (2011).
Marciano, D. P. et al. Pharmacological repression of PPARγ promotes osteogenesis. Nat. Commun. 6, 7443 (2015).
Allenby, G. et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl Acad. Sci. USA 90, 30–34 (1993).
Xu, Q. et al. Retinoids in nephrology: promises and pitfalls. Kidney Int. 66, 2119–2131 (2004).
Fisher, G. J. et al. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J. Biol. Chem. 269, 20629–20635 (1994).
Fitzgerald, P., Teng, M., Chandraratna, R. A., Heyman, R. A. & Allegretto, E. A. Retinoic acid receptor alpha expression correlates with retinoid-induced growth inhibition of human breast cancer cells regardless of estrogen receptor status. Cancer Res. 57, 2642–2650 (1997).
Jones, K. A. et al. Localization of the retinoid X receptor alpha gene (RXRA) to chromosome 9q34. Ann. Hum. Genet. 57, 195–201 (1993).
Harrison, E. H. Mechanisms of digestion and absorption of dietary vitamin A. Annu. Rev. Nutr. 25, 87–103 (2005).
Kawaguchi, R. et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 (2007).
Liu, L. & Gudas, L. J. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J. Biol. Chem. 280, 40226–40234 (2005).
Blaner, W. S. et al. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J. Biol. Chem. 269, 16559–16565 (1994).
Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 (2008).
Touma, S. E., Perner, S., Rubin, M. A., Nanus, D. M. & Gudas, L. J. Retinoid metabolism and ALDH1A2 (RALDH2) expression are altered in the transgenic adenocarcinoma mouse prostate model. Biochem. Pharmacol. 78, 1127–1138 (2009).
Gronemeyer, H. & Miturski, R. Molecular mechanisms of retinoid action. Cell Mol. Biol. Lett. 6, 3–52 (2001).
Na, S. Y. et al. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J. Biol. Chem. 274, 7674–7680 (1999).
Benkoussa, M., Brand, C., Delmotte, M. H., Formstecher, P. & Lefebvre, P. Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol. Cell Biol. 22, 4522–4534 (2002).
Simonson, M. S. Anti-AP-1 activity of all-trans retinoic acid in glomerular mesangial cells. Am. J. Physiol. 267, F805–F815 (1994).
Wang, R. et al. All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor κB (NFκB) signaling. J. Biol. Chem. 290, 22532–22542 (2015).
Balmer, J. E. & Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 43, 1773–1808 (2002).
Canon, E., Cosgaya, J. M., Scsucova, S. & Aranda, A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol. Biol. Cell 15, 5583–5592 (2004).
Lonze, B. E. & Ginty, D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 (2002).
Zhao, Q. et al. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia 18, 285–292 (2004).
Parrella, E. et al. Phosphodiesterase IV inhibition by piclamilast potentiates the cytodifferentiating action of retinoids in myeloid leukemia cells. Cross-talk between the cAMP and the retinoic acid signaling pathways. J. Biol. Chem. 279, 42026–42040 (2004).
Boskovic, G., Desai, D. & Niles, R. M. Regulation of retinoic acid receptor α by protein kinase C in B16 mouse melanoma cells. J. Biol. Chem. 277, 26113–26119 (2002).
Hughes, P. J., Zhao, Y., Chandraratna, R. A. & Brown, G. Retinoid-mediated stimulation of steroid sulfatase activity in myeloid leukemic cell lines requires RARα and RXR and involves the phosphoinositide 3-kinase and ERK-MAP kinase pathways. J. Cell. Biochem. 97, 327–350 (2006).
Evans, T. R. & Kaye, S. B. Retinoids: present role and future potential. Br. J. Cancer 80, 1–8 (1999).
Cabezas-Wallscheid, N. et al. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell 169, 807–823.e19 (2017).
Clemens, G. et al. The action of all-trans-retinoic acid (ATRA) and synthetic retinoid analogues (EC19 and EC23) on human pluripotent stem cells differentiation investigated using single cell infrared microspectroscopy. Mol. Biosyst. 9, 677–692 (2013).
Uray, I. P., Dmitrovsky, E. & Brown, P. H. Retinoids and rexinoids in cancer prevention: from laboratory to clinic. Semin. Oncol. 43, 49–64 (2016).
Simoni, D. & Tolomeo, M. Retinoids, apoptosis and cancer. Curr. Pharm. Des. 7, 1823–1837 (2001).
Kitamura, M. et al. Intervention by retinoic acid in oxidative stress-induced apoptosis. Nephrol. Dial., Transplant. 17 (Suppl 9), 84–87 (2002).
Riahi, R. R., Bush, A. E. & Cohen, P. R. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am. J. Clin. Dermatol. 17, 265–276 (2016).
Beckenbach, L., Baron, J. M., Merk, H. F., Loffler, H. & Amann, P. M. Retinoid treatment of skin diseases. Eur. J. Dermatol. 25, 384–391 (2015).
Tallman, M. S. All-trans-retinoic acid in acute promyelocytic leukemia and its potential in other hematologic malignancies. Semin. Hematol. 31, 38–48 (1994).
Brzezinski, P., Borowska, K., Chiriac, A. & Smigielski, J. Adverse effects of isotretinoin: a large, retrospective review. Dermatol. Ther. 30, e12483 (2017).
Lehrke, I. et al. Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am. J. Physiol. Ren. Physiol. 282, F741–F751 (2002).
Wagner, J. et al. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J. Am. Soc. Nephrol. 11, 1479–1487 (2000).
Moreno-Manzano, V. et al. Retinoids as a potential treatment for experimental puromycin-induced nephrosis. Br. J. Pharmacol. 139, 823–831 (2003).
Inagaki, T. et al. The retinoic acid-responsive proline-rich protein is identified in promyeloleukemic HL-60 cells. J. Biol. Chem. 278, 51685–51692 (2003).
Schaier, M. et al. Isotretinoin alleviates renal damage in rat chronic glomerulonephritis. Kidney Int. 60, 2222–2234 (2001).
Perez de Lema, G. et al. Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease. Kidney Int. 66, 1018–1028 (2004).
Vaughan, M. R. et al. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int. 68, 133–144 (2005).
Morath, C. et al. Effects of retinoids on the TGF-beta system and extracellular matrix in experimental glomerulonephritis. J. Am. Soc. Nephrol. 12, 2300–2309 (2001).
Dechow, C. et al. Effects of all-trans retinoic acid on renin-angiotensin system in rats with experimental nephritis. Am. J. Physiol. Ren. Physiol. 281, F909–F919 (2001).
Moreno-Manzano, V., Ishikawa, Y., Lucio-Cazana, J. & Kitamura, M. Suppression of apoptosis by all-trans-retinoic acid. Dual intervention in the c-Jun n-terminal kinase-AP-1 pathway. J. Biol. Chem. 274, 20251–20258 (1999).
Xu, Q., Konta, T. & Kitamura, M. Retinoic acid regulation of mesangial cell apoptosis. Exp. Nephrol. 10, 171–175 (2002).
Ratnam, K. K. et al. Role of the retinoic acid receptor-α in HIV-associated nephropathy. Kidney Int 79, 624–634 (2011).
Dai, Y. et al. Retinoic acid improves nephrotoxic serum-induced glomerulonephritis through activation of podocyte retinoic acid receptor α. Kidney Int. 92, 1444–1457 (2017).
Mallipattu, S. K. & He, J. C. The beneficial role of retinoids in glomerular disease. Front. Med. 2, 16 (2015).
Shankland, S. J., Pippin, J. W., Reiser, J. & Mundel, P. Podocytes in culture: past, present, and future. Kidney Int. 72, 26–36 (2007).
Lazzeri, E., Peired, A. J., Lasagni, L. & Romagnani, P. Retinoids and glomerular regeneration. Semin. Nephrol. 34, 429–436 (2014).
He, J. C. et al. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J. Am. Soc. Nephrol. 18, 93–102 (2007).
Zhang, J. et al. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp. Nephrol. 121, e23–e37 (2012).
Merlet-Benichou, C., Vilar, J., Lelievre-Pegorier, M. & Gilbert, T. Role of retinoids in renal development: pathophysiological implication. Curr. Opin. Nephrol. Hypertens. 8, 39–43 (1999).
Gilbert, T. Vitamin A and kidney development. Nephro. Dial. Transplant. 17 (Suppl 9), 78–80 (2002).
Mallipattu, S. K. et al. Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J. Biol. Chem. 287, 19122–19135 (2012).
Peired, A. et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J. Am. Soc. Nephrol. 24, 1756–1768 (2013).
Standeven, A. M., Teng, M. & Chandraratna, R. A. Lack of involvement of retinoic acid receptor α in retinoid-induced skin irritation in hairless mice. Toxicol. Lett. 92, 231–240 (1997).
Nagpal, S. & Chandraratna, R. A. Recent developments in receptor-selective retinoids. Curr. Pharm. Des. 6, 919–931 (2000).
Advani, A. et al. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc. Natl Acad. Sci. USA 104, 14448–14453 (2007).
Zhong, Y. et al. Novel retinoic acid receptor alpha agonists for treatment of kidney disease. PLoS ONE 6, e27945 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT00098020 (2017).
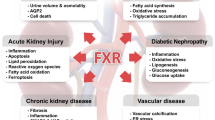
Jiang, T. et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 (2007).
Wang, X. X. et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59, 2916–2927 (2010).
Wang, X. X. et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am. J. Physiol. Ren. Physiol. 297, F1587–F1596 (2009).
NIH Office of Dietary Supplements. Vitamin D. ODS https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#en1 (2020).
Perez-Gomez, M. V., Ortiz-Arduan, A. & Lorenzo-Sellares, V. Vitamin D and proteinuria: a critical review of molecular bases and clinical experience. Nefrologia 33, 716–726 (2013).
Pike, J. W. & Meyer, M. B. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol. Metab. Clin. North Am. 39, 255–269 (2010).
Sertznig, P., Seifert, M., Tilgen, W. & Reichrath, J. Peroxisome proliferator-activated receptor (PPAR) and vitamin D receptor (VDR) signaling pathways in melanoma cells: promising new therapeutic targets? J. Steroid Biochem. Mol. Biol. 121, 383–386 (2010).
Chang, S. W. & Lee, H. C. Vitamin D and health – the missing vitamin in humans. Pediatr. Neonatol. 60, 237–244 (2019).
Wang, W., Zhang, J., Wang, H., Wang, X. & Liu, S. Vitamin D deficiency enhances insulin resistance by promoting inflammation in type 2 diabetes. Int. J. Clin. Exp. Pathol. 12, 1859–1867 (2019).
Talaei, A., Mohamadi, M. & Adgi, Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol. Metab. Syndr. 5, 8 (2013).
Mutt, S. J. et al. Vitamin D deficiency induces insulin resistance and re-supplementation attenuates hepatic glucose output via the PI3K-AKT-FOXO1 mediated pathway. Mol. Nutr. Food Res. 64, e1900728 (2020).
Sonneveld, R. et al. Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease. Am. J. Pathol. 182, 1196–1204 (2013).
Wang, X. X. et al. Vitamin D receptor agonist doxercalciferol modulates dietary fat-induced renal disease and renal lipid metabolism. Am. J. Physiol. Ren. Physiol. 300, F801–F810 (2011).
Garsen, M. et al. Vitamin D attenuates proteinuria by inhibition of heparanase expression in the podocyte. J. Pathol. 237, 472–481 (2015).
Branisteanu, D. D., Leenaerts, P., van Damme, B. & Bouillon, R. Partial prevention of active Heymann nephritis by 1α, 25 dihydroxyvitamin D3. Clin. Exp. Immunol. 94, 412–417 (1993).
Makibayashi, K. et al. A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am. J. Pathol. 158, 1733–1741 (2001).
Zou, M. S. et al. 1,25-Dihydroxyvitamin D3 decreases adriamycin-induced podocyte apoptosis and loss. Int. J. Med. Sci. 7, 290–299 (2010).
Zou, M. S. et al. 1,25-Dihydroxyvitamin D3 ameliorates podocytopenia in rats with adriamycin-induced nephropathy. Intern. Med. 49, 2677–2686 (2010).
Schwarz, U. et al. Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 53, 1696–1705 (1998).
Sanchez-Nino, M. D. et al. Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol. Dial. Transplant. 26, 1797–1802 (2011).
Agarwal, R. Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin. J. Am. Soc. Nephrol. 4, 1523–1528 (2009).
Chandel, N. et al. Vitamin D receptor deficit induces activation of renin angiotensin system via SIRT1 modulation in podocytes. Exp. Mol. Pathol. 102, 97–105 (2017).
Chandel, N. et al. Epigenetic modulation of human podocyte vitamin D receptor in HIV milieu. J. Mol. Biol. 427, 3201–3215 (2015).
Xu, L. et al. Vitamin D and its receptor regulate lipopolysaccharide-induced transforming growth factor-β, angiotensinogen expression and podocytes apoptosis through the nuclear factor-κB pathway. J. Diabetes Investig. 7, 680–688 (2016).
Cheng, X., Zhao, X., Khurana, S., Bruggeman, L. A. & Kao, H. Y. Microarray analyses of glucocorticoid and vitamin D3 target genes in differentiating cultured human podocytes. PLoS ONE 8, e60213 (2013).
He, W., Kang, Y. S., Dai, C. & Liu, Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 22, 90–103 (2011).
Khurana, S., Bruggeman, L. A. & Kao, H. Y. Nuclear hormone receptors in podocytes. Cell Biosci. 2, 33 (2012).
Li, Y. C. Vitamin D receptor signaling in renal and cardiovascular protection. Semin. Nephrol. 33, 433–447 (2013).
de Borst, M. H. et al. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J. Am. Soc. Nephrol. 24, 1863–1871 (2013).
Melamed, M. L. & Thadhani, R. I. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin. J. Am. Soc. Nephrol. 7, 358–365 (2012).
de Boer, I. H. et al. Effect of Vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA 322, 1899–1909 (2019).
Fishbane, S. et al. Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trial. Am. J. Kidney Dis. 54, 647–652 (2009).
Alborzi, P. et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension 52, 249–255 (2008).
de Zeeuw, D. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376, 1543–1551 (2010).
Kramer, H., Berns, J. S., Choi, M. J., Martin, K. & Rocco, M. V. 25-Hydroxyvitamin D testing and supplementation in CKD: an NKF-KDOQI controversies report. Am. J. Kidney Dis. 64, 499–509 (2014).
Le Menuet, D., Viengchareun, S., Muffat-Joly, M., Zennaro, M. C. & Lombes, M. Expression and function of the human mineralocorticoid receptor: lessons from transgenic mouse models. Mol. Cell. Endocrinol. 217, 127–136 (2004).
Arriza, J. L. et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237, 268–275 (1987).
Funder, J. W., Pearce, P. T., Smith, R. & Smith, A. I. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242, 583–585 (1988).
Buonafine, M., Bonnard, B. & Jaisser, F. Mineralocorticoid receptor and cardiovascular disease. Am. J. Hypertens. 31, 1165–1174 (2018).
Nishiyama, A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens. Res. 42, 293–300 (2019).
Aldigier, J. C., Kanjanbuch, T., Ma, L. J., Brown, N. J. & Fogo, A. B. Regression of existing glomerulosclerosis by inhibition of aldosterone. J. Am. Soc. Nephrol. 16, 3306–3314 (2005).
Fujihara, C. K. et al. A novel aldosterone antagonist limits renal injury in 5/6 nephrectomy. Sci. Rep. 7, 7899 (2017).
Zhou, X., Ono, H., Ono, Y. & Frohlich, E. D. Aldosterone antagonism ameliorates proteinuria and nephrosclerosis independent of glomerular dynamics in L-NAME/SHR model. Am. J. Nephrol. 24, 242–249 (2004).
Zitt, E. et al. The selective mineralocorticoid receptor antagonist eplerenone is protective in mild anti-GBM glomeru-lonephritis. Int. J. Clin. Exp. Pathol. 4, 606–615 (2011).
Qin, D. et al. Aldosterone mediates glomerular inflammation in experimental mesangial proliferative glomerulonephritis. J. Nephrol. 26, 199–206 (2013).
Nagase, M. et al. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 47, 1084–1093 (2006).
Shibata, S., Nagase, M., Yoshida, S., Kawachi, H. & Fujita, T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49, 355–364 (2007).
Chen, C. et al. Aldosterone induces apoptosis in rat podocytes: role of PI3-K/Akt and p38MAPK signaling pathways. Nephron Exp. Nephrol. 113, e26–e34 (2009).
Nagase, M. et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J. Am. Soc. Nephrol. 17, 3438–3446 (2006).
Takagi, N. et al. Mineralocorticoid receptor blocker protects against podocyte-dependent glomerulosclerosis. Nephron Extra 2, 17–26 (2012).
Shibata, S. et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat. Med. 14, 1370–1376 (2008).
Barrera-Chimal, J., Girerd, S. & Jaisser, F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int. 96, 302–319 (2019).
Currie, G. et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 17, 127 (2016).
Bolignano, D., Palmer, S. C., Navaneethan, S. D. & Strippoli, G. F. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 4, CD007004 (2014).
Navaneethan, S. D., Nigwekar, S. U., Sehgal, A. R. & Strippoli, G. F. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 4, 542–551 (2009).
Greene, E. L., Kren, S. & Hostetter, T. H. Role of aldosterone in the remnant kidney model in the rat. J. Clin. Invest. 98, 1063–1068 (1996).
Rocha, R., Chander, P. N., Zuckerman, A. & Stier, C. T. Jr. Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 33, 232–237 (1999).
Deroo, B. J. & Korach, K. S. Estrogen receptors and human disease. J. Clin. Invest. 116, 561–570 (2006).
Kummer, S. et al. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PLoS ONE 6, e27457 (2011).
Gross, M. L. et al. Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRsp rats. J. Am. Soc. Nephrol. 15, 348–358 (2004).
Doublier, S. et al. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 79, 404–413 (2011).
Gluhovschi, G. et al. Chronic kidney disease and the involvement of estrogen hormones in its pathogenesis and progression. Rom. J. Intern. Med. 50, 135–144 (2012).
Carrero, J. J., Hecking, M., Chesnaye, N. C. & Jager, K. J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 14, 151–164 (2018).
Lee, W. L. et al. The benefits of estrogen or selective estrogen receptor modulator on kidney and its related disease–chronic kidney disease–mineral and bone disorder: osteoporosis. J. Chin. Med. Assoc. 76, 365–371 (2013).
Melamed, M. L. et al. Raloxifene, a selective estrogen receptor modulator, is renoprotective: a post-hoc analysis. Kidney Int. 79, 241–249 (2011).
Zhang, J. et al. DNA binding alters coactivator interaction surfaces of the intact VDR-RXR complex. Nat. Struct. Mol. Biol. 18, 556–563 (2011).
Weiss, K. et al. Effect of synthetic ligands of PPAR α, β/δ, γ, RAR, RXR and LXR on the fatty acid composition of phospholipids in mice. Lipids 46, 1013–1020 (2011).
Trasino, S. E., Tang, X. H., Jessurun, J. & Gudas, L. J. Retinoic acid receptor β2 agonists restore glycaemic control in diabetes and reduce steatosis. Diabetes, Obes. Metab. 18, 142–151 (2016).
Ling, J. & Kumar, R. Crosstalk between NFkB and glucocorticoid signaling: a potential target of breast cancer therapy. Cancer Lett. 322, 119–126 (2012).
Lu, T. C. et al. Retinoic acid utilizes CREB and USF1 in a transcriptional feed-forward loop in order to stimulate MKP1 expression in human immunodeficiency virus-infected podocytes. Mol. Cell Biol. 28, 5785–5794 (2008).
Cohen, C. D. et al. Comparative promoter analysis allows de novo identification of specialized cell junction-associated proteins. Proc. Natl Acad. Sci. USA 103, 5682–5687 (2006).
Guo, Y. et al. Podocyte-specific induction of kruppel-like factor 15 restores differentiation markers and attenuates kidney injury in proteinuric kidney disease. J. Am. Soc. Nephrol. 29, 2529–2545 (2018).
Endlich, N., Nobiling, R., Kriz, W. & Endlich, K. Expression and signaling of parathyroid hormone-related protein in cultured podocytes. Exp. Nephrol. 9, 436–443 (2001).
Endlich, N. & Endlich, K. cAMP pathway in podocytes. Microsc. Res. Tech. 57, 228–231 (2002).
Azeloglu, E. U. et al. Interconnected network motifs control podocyte morphology and kidney function. Sci. Signal. 7, ra12 (2014).
Zhong, Y. et al. Roflumilast enhances the renal protective effects of retinoids in an HIV-1 transgenic mouse model of rapidly progressive renal failure. Kidney Int. 81, 856–864 (2012).
Shibata, S. et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor–dependent pathway. J. Clin. Invest. 121, 3233–3243 (2011).
Wang, X. X., Jiang, T. & Levi, M. Nuclear hormone receptors in diabetic nephropathy. Nat. Rev. Nephrol. 6, 342–351 (2010).
Yang, J., Zhou, Y. & Guan, Y. PPARγ as a therapeutic target in diabetic nephropathy and other renal diseases. Curr. Opin. Nephrol. Hypertens. 21, 97–105 (2012).
Ishibashi, Y. et al. PEDF inhibits AGE-induced podocyte apoptosis via PPAR-gamma activation. Microvasc. Res. 85, 54–58 (2013).
Lee, E. Y. et al. Peroxisome proliferator-activated receptor-δ activation ameliorates albuminuria by preventing nephrin loss and restoring podocyte integrity in type 2 diabetes. Nephrol. Dial. Transplant. 27, 4069–4079 (2012).
Wang, Y. et al. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J. Am. Soc. Nephrol. 23, 1977–1986 (2012).
Guo, J. et al. GSK-3β and vitamin D receptor are involved in β-catenin and Snail signaling in high glucose-induced epithelial-mesenchymal transition of mouse podocytes. Cell Physiol. Biochem. 33, 1087–1096 (2014).
Verouti, S. N. et al. Vitamin D receptor activators upregulate and rescue podocalyxin expression in high glucose-treated human podocytes. Nephron Exp. Nephrol. 122, 36–50 (2012).
Guo, J. et al. VDR activation reduces proteinuria and high-glucose-induced injury of kidneys and podocytes by regulating Wnt signaling pathway. Cell Physiol. Biochem. 43, 39–51 (2017).
Toyonaga, J. et al. Spironolactone inhibits hyperglycemia-induced podocyte injury by attenuating ROS production. Nephrol. Dial. Transplant. 26, 2475–2484 (2011).
Han, S. Y. et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J. Am. Soc. Nephrol. 17, 1362–1372 (2006).
Zhou, G., Johansson, U., Peng, X. R., Bamberg, K. & Huang, Y. An additive effect of eplerenone to ACE inhibitor on slowing the progression of diabetic nephropathy in the db/db mice. Am. J. Transl. Res. 8, 1339–1354 (2016).
Nishiyama, A. et al. Mineralocorticoid receptor blockade enhances the antiproteinuric effect of an angiotensin II blocker through inhibiting podocyte injury in type 2 diabetic rats. J. Pharmacol. Exp. Ther. 332, 1072–1080 (2010).
Schjoedt, K. J. et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 70, 536–542 (2006).
Mehdi, U. F., Adams-Huet, B., Raskin, P., Vega, G. L. & Toto, R. D. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 20, 2641–2650 (2009).
Bakris, G. L. et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 314, 884–894 (2015).
Doublier, S., Lupia, E., Catanuto, P. & Elliot, S. J. Estrogens and progression of diabetic kidney damage. Curr. Diabetes Rev. 7, 28–34 (2011).
Han, S. Y. et al. Effect of retinoic acid in experimental diabetic nephropathy. Immunol. Cell Biol. 82, 568–576 (2004).
Wang, X. X. et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J. Am. Soc. Nephrol. 29, 118–137 (2018).
Acknowledgements
The authors’ work is supported by funding from the American Heart Association Career Development Award (18CDA34110287) and by the Nationwide Children’s Hospital to S.A.; VA Merit Award IBX000345C, NIH 1R01DK088541 and NIH P01DK56492 to J.C.H.; and by Institut National de Santé et de la Recherche Médicale (INSERM) and research grants from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ ERC grant agreement no. 107037) and SWITCHES grant (ANR12-BSV1-0039-03) from l’Agence Nationale de la Recherche (ANR) of France to P.-L.T.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data, and writing, reviewing and editing the article. S.A. focused on GR, PPARγ, ER and VDR, and drafted the figures. P.-L.T. focused on PPARγ and MR, and J.C.H. focused on RAR and RXR.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks P. Brinkkoetter, C. Faul and M. Moeller for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Co-regulators
-
Co-activators or co-repressors that bind to a nuclear receptor in the absence or presence of ligands enabling activation or repression of gene transcription.
- Sumoylation
-
A reversible post-translational protein modification that consists of the covalent labelling of a small protein called SUMO to lysine residues of selected target proteins. Sumoylation is a well-characterized regulator of nuclear functions.
- Transactivation
-
The most common mechanism of nuclear receptor action that involves direct binding of the nuclear receptor to a DNA hormone response element.
- Transrepression
-
The process by which nuclear receptors bind to and deactivate other transcription factors; for example, the glucocorticoid receptor and PPARγ can suppress target genes by inhibiting the activities of other transcription factors, such as AP-1, in a ligand-dependent manner; transrepression does not require the nuclear receptor to bind DNA.
Rights and permissions
About this article
Cite this article
Agrawal, S., He, J.C. & Tharaux, PL. Nuclear receptors in podocyte biology and glomerular disease. Nat Rev Nephrol 17, 185–204 (2021). https://doi.org/10.1038/s41581-020-00339-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-00339-6
This article is cited by
-
Vitamin D receptor attenuate ischemia-reperfusion kidney injury via inhibiting ATF4
Cell Death Discovery (2023)
-
Pioglitazone enhances proteinuria reduction in complicated pediatric nephrotic syndrome
Pediatric Nephrology (2023)
-
Signaling pathways of chronic kidney diseases, implications for therapeutics
Signal Transduction and Targeted Therapy (2022)
-
Preparation of single-cell suspensions of mouse glomeruli for high-throughput analysis
Nature Protocols (2021)