Abstract
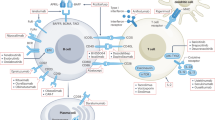
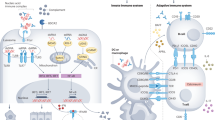
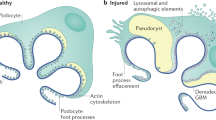
Idiopathic nephrotic syndrome (INS) describes a group of pathologies of the renal glomerulus that result in the classic triad of heavy proteinuria, oedema and hypoalbuminaemia. The disease has historically been defined by evidence of distinctive histological changes in the absence of clinical evidence of a distinct pathological driver. However, the current classification is not based on any systematic mechanistic understanding of biological processes, and therefore current treatment regimens are broad, iterative and nonspecific. Over the past 20 years delineation of the underlying biology of the target cell in INS — the glomerular podocyte — has transformed our understanding of the mechanisms that contribute to breakdown of the glomerular filtration barrier and the development of INS. It is increasingly clear that nephrotic syndrome caused by monogenic mutations is distinct from immune-driven disease, which in some cases is mediated by circulating factors that target the podocyte. The combination of systems biology and bioinformatics approaches, together with powerful laboratory models and ever-growing patient registries has potential to identify disease ‘signatures’ that reflect the underlying molecular mechanism of INS on an individual basis. Understanding of such processes could lead to the development of targeted therapies.
Key points
-
New insights suggest that nephrotic syndrome can be classified according to our understanding of underlying molecular mechanisms into genetic nephrotic syndrome, immune-based nephrotic syndrome and circulating factor disease (CFD); such a classification might aid the identification of patient subgroups who would benefit from targeted therapy.
-
Genetic advances have shown that at least 33% of cases of childhood-onset steroid-resistant nephrotic syndrome are caused by single gene mutations, and have identified specific biological pathways that could be therapeutically targeted.
-
CFD is characterized by disease recurrence after transplantation, and is associated with negative genetic testing and often secondary steroid resistance.
-
Immune-based nephrotic syndrome is acquired, and is likely to be the basis of some forms CFD, but may also account for an immune-mediated non-CFD subtype of disease; some forms of immune-based nephrotic syndrome also have known genetic associations, particularly with HLA-DR alleles.
-
Biomarkers for CFD can be derived by correlating findings from in vitro models with key clinical features; such approaches may aid the identification of patients at risk of recurrence and provide greater insights into disease processes.
-
In-depth molecular stratification will be enhanced by systems biology approaches that combine big data methodologies with advanced bioinformatics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Banh, T. H. et al. Ethnic differences in incidence and outcomes of childhood nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 11, 1760–1768 (2016).
Kim, J. S. et al. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney Int. 68, 1275–1281 (2005).
Bierzynska, A. et al. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int. 91, 937–947 (2017).
Saleem, M. A. One hundred ways to kill a podocyte. Nephrol. Dial. Transpl. 30, 1266–1271 (2015).
Hunte, W., al-Ghraoui, F. & Cohen, R. J. Secondary syphilis and the nephrotic syndrome. J. Am. Soc. Nephrol. 3, 1351–1355 (1993).
Becker, C. G. et al. Nephrotic syndrome after contact with mercury. A report of five cases, three after the use of ammoniated mercury ointment. Arch. Intern. Med. 110, 178–186 (1962).
Maas, R. J. et al. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat. Rev. Nephrol. 12, 768–776 (2016).
Report of the International Study of Kidney Disease in Children. Minimal change nephrotic syndrome in children: deaths during the first 5 to 15 years’ observation. Pediatrics 73, 497–501 (1984).
Niaudet, P. Long-term outcome of children with steroid-sensitive idiopathic nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 4, 1547–1548 (2009).
Vivarelli, M. et al. Minimal change disease. Clin. J. Am. Soc. Nephrol. 12, 332–345 (2017).
D’Agati, V. D. et al. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am. J. Kidney Dis. 43, 368–382 (2004).
Trautmann, A. et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin. J. Am. Soc. Nephrol. 10, 592–600 (2015).
Bagga, A., Sinha, A. & Moudgil, A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N. Engl. J. Med. 356, 2751–2752 (2007).
Iijima, K. et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384, 1273–1281 (2014).
Sinha, A. et al. Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol. Dial. Transpl. 30, 96–106 (2015).
Trachtman, H. et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J. Am. Soc. Nephrol. 29, 2745–2754 (2018).
Fan, X. et al. SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. JCI Insight 1, e86934 (2016).
Welsh, G. I. & Saleem, M. A. The podocyte cytoskeleton–key to a functioning glomerulus in health and disease. Nat. Rev. Nephrol. 8, 14–21 (2012).
Kreidberg, J. A. et al. WT-1 is required for early kidney development. Cell 74, 679–691 (1993).
Kestila, M. et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Welsh, G. I. & Saleem, M. A. Nephrin — signature molecule of the glomerular podocyte? J. Pathol. 220, 328–337 (2010).
Simons, M. et al. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am. J. Pathol. 159, 1069–1077 (2001).
Schwarz, K. et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J. Clin. Invest. 108, 1621–1629 (2001).
Weber, S. et al. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 66, 571–579 (2004).
Shih, N. Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312–315 (1999).
Bierzynska, A. et al. MAGI2 mutations cause congenital nephrotic syndrome. J. Am. Soc. Nephrol. 28, 1614–1621 (2017).
van Duijn, T. J. et al. Rac1 recruits the adapter protein CMS/CD2AP to cell-cell contacts. J. Biol. Chem. 285, 20137–20146 (2010).
Huber, T. B. et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell. Biol. 23, 4917–4928 (2003).
Akilesh, S., Koziell, A. & Shaw, A. S. Basic science meets clinical medicine: identification of a CD2AP-deficient patient. Kidney Int. 72, 1181–1183 (2007).
Gigante, M. et al. CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS). Nephrol. Dial. Transpl. 24, 1858–1864 (2009).
Akilesh, S. et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 121, 4127–4137 (2011).
Gee, H. Y. et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J. Clin. Invest. 123, 3243–3253 (2013).
Harris, J. J. et al. Active proteases in nephrotic plasma lead to a podocin-dependent phosphorylation of VASP in podocytes via protease activated receptor-1. J. Pathol. 229, 660–671 (2013).
Pozzi, A. et al. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev. Biol. 316, 288–301 (2008).
Kanasaki, K. et al. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev. Biol. 313, 584–593 (2008).
Wei, C. et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 14, 55–63 (2008).
Wei, C. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 17, 952–960 (2011).
Lin, Y., Rao, J., Zha, X. & Xu, H. Angiopoietin-like 3 induces podocyte F-actin rearrangement through integrin alpha(V)beta(3)/FAK/PI3K pathway-mediated Rac1 activation. Biomed Res. Int. 2013, 135608 (2013).
Ashraf, S. et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Invest. 123, 5179–5189 (2013).
Heeringa, S. F. et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 121, 2013–2024 (2011).
Diomedi-Camassei, F. et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 18, 2773–2780 (2007).
Lopez, L. C. et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 79, 1125–1129 (2006).
Braun, D. A. et al. Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat. Genet. 48, 457–465 (2016).
Shalhoub, R. J. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 2, 556–560 (1974).
Eremina, V. & Quaggin, S. E. The role of VEGF-A in glomerular development and function. Curr. Opin. Nephrol. Hypertens. 13, 9–15 (2004).
Daehn, I. et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Invest. 124, 1608–1621 (2014).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Keir, L. S. et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J. Clin. Invest. 127, 199–214 (2017).
Guan, Z., VanBeusecum, J. P. & Inscho, E. W. Endothelin and the renal microcirculation. Semin. Nephrol. 35, 145–155 (2015).
Lenoir, O. et al. Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 25, 1050–1062 (2014).
Barton, M. Therapeutic potential of endothelin receptor antagonists for chronic proteinuric renal disease in humans. Biochim. Biophys. Acta 1802, 1203–1213 (2010).
Coward, R. J. et al. The human glomerular podocyte is a novel target for insulin action. Diabetes 54, 3095–3102 (2005).
Welsh, G. I. et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12, 329–340 (2010).
Keir, L. S., Marks, S. D. & Kim, J. J. Shigatoxin-associated hemolytic uremic syndrome: current molecular mechanisms and future therapies. Drug Des. Dev. Ther. 6, 195–208 (2012).
Psotka, M. A. et al. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect. Immun. 77, 959–969 (2009).
Davin, J. C. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr. Nephrol. 31, 207–215 (2016).
Bierzynska, A. & Saleem, M. A. Deriving and understanding the risk of post-transplant recurrence of nephrotic syndrome in the light of current molecular and genetic advances. Pediatr. Nephrol. 33, 2027–2035 (2018).
Gallon, L. et al. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N. Engl. J. Med. 366, 1648–1649 (2012).
Hinkes, B. et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 38, 1397–1405 (2006).
Wasilewska, A. M., Kuroczycka-Saniutycz, E. & Zoch-Zwierz, W. Effect of cyclosporin A on proteinuria in the course of glomerulopathy associated with WT1 mutations. Eur. J. Pediatr. 170, 389–391 (2011).
Kuusniemi, A. M. et al. Plasma exchange and retransplantation in recurrent nephrosis of patients with congenital nephrotic syndrome of the Finnish type (NPHS1). Transplantation 83, 1316–1323 (2007).
Gbadegesin, R. et al. A new locus for familial FSGS on chromosome 2P. J. Am. Soc. Nephrol. 21, 1390–1397 (2010).
Bertelli, R. et al. Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am. J. Kidney Dis. 41, 1314–1321 (2003).
Billing, H. et al. NPHS2 mutation associated with recurrence of proteinuria after transplantation. Pediatr. Nephrol. 19, 561–564 (2004).
Becker-Cohen, R. et al. Recurrent nephrotic syndrome in homozygous truncating NPHS2 mutation is not due to anti-podocin antibodies. Am. J. Transpl. 7, 256–260 (2007).
Hocker, B. et al. Recurrence of proteinuria 10 years post-transplant in NPHS2-associated focal segmental glomerulosclerosis after conversion from cyclosporin A to sirolimus. Pediatr. Nephrol. 21, 1476–1479 (2006).
Ruf, R. G. et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J. Am. Soc. Nephrol. 15, 722–732 (2004).
Caridi, G. et al. Heterozygous NPHS1 or NPHS2 mutations in responsive nephrotic syndrome and the multifactorial origin of proteinuria. Kidney Int. 66, 1715–1716 (2004).
Dorval, G. et al. Clinical and genetic heterogeneity in familial steroid-sensitive nephrotic syndrome. Pediatr. Nephrol. 33, 473–483 (2018).
Gee, H. Y. et al. Mutations in EMP2 cause childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 94, 884–890 (2014).
Gee, H. Y. et al. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J. Clin. Investig. 125, 2375–2384 (2015).
Xing, C. Y. et al. Direct effects of dexamethasone on human podocytes. Kidney Int. 70, 1038–1045 (2006).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
McCarthy, H. J. et al. Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 8, 637–648 (2013).
Sen, E. S. et al. Clinical genetic testing using a custom-designed steroid-resistant nephrotic syndrome gene panel: analysis and recommendations. J. Med. Genet. 54, 795–804 (2017).
Lee, H. et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 312, 1880–1887 (2014).
Sadowski, C. E. et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol. 26, 1279–1289 (2015).
Gribouval, O. et al. Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int. 94, 1013–1022 (2018).
Yao, T. et al. Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin. J. Am. Soc. Nephrol. 14, 213–223 (2019).
Robson, K. J. et al. HLA and kidney disease: from associations to mechanisms. Nat. Rev. Nephrol. 14, 636–655 (2018).
Jia, X. Y. et al. Strong association of the HLA-DR/DQ locus with childhood steroid-sensitive nephrotic syndrome in the Japanese population. J. Am. Soc. Nephrol. 29, 2189–2199 (2018).
Debiec, H. et al. Transethnic, genome-wide analysis reveals immune-related risk alleles and phenotypic correlates in pediatric steroid-sensitive nephrotic syndrome. J. Am. Soc. Nephrol. 29, 2000–2013 (2018).
Crawford, B. D. et al. Evaluating Mendelian nephrotic syndrome genes for evidence for risk alleles or oligogenicity that explain heritability. Pediatr. Nephrol. 32, 467–476 (2017).
Kienzl-Wagner, K., Waldegger, S. & Schneeberger, S. Disease recurrence-the sword of Damocles in kidney transplantation for primary focal segmental glomerulosclerosis. Front. Immunol. 10, 1669 (2019).
Ding, W. Y. et al. Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J. Am. Soc. Nephrol. 25, 1342–1348 (2014).
Sinha, A. et al. Disease course in steroid sensitive nephrotic syndrome. Indian Pediatr. 49, 881–887 (2012).
Buscher, A. K. et al. Rapid response to cyclosporin A and favorable renal outcome in nongenetic versus genetic steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 11, 245–253 (2016).
Savin, V. J. et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N. Engl. J. Med. 334, 878–883 (1996).
Cattran, D. et al. Serial estimates of serum permeability activity and clinical correlates in patients with native kidney focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 14, 448–453 (2003).
Trachtman, H. et al. Glomerular permeability activity: prevalence and prognostic value in pediatric patients with idiopathic nephrotic syndrome. Am. J. Kidney Dis. 44, 604–610 (2004).
Saleem, M. A. et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 (2002).
Srivastava, P. et al. Development of a novel cell-based assay to diagnose recurrent focal segmental glomerulosclerosis patients. Kidney Int. 95, 708–716 (2019).
Kitzler, T. M. et al. Use of genomic and functional analysis to characterize patients with steroid-resistant nephrotic syndrome. Pediatr. Nephrol. 33, 1741–1750 (2018).
Bitzan, M. et al. TNF alpha pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and beta 3 integrin activation. Pediatr. Nephrol. 27, 2217–2226 (2012).
Coward, R. J. et al. Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, podocin, and CD2 associated protein in cultured human podocytes. J. Am. Soc. Nephrol. 16, 629–637 (2005).
May, C. J. et al. Human Th17 cells produce a soluble mediator that increases podocyte motility via signalling pathways which mimic PAR-1 activation. Am. J. Physiol. Renal Physiol. 317, F913–F921 (2019).
Yu, C. C. et al. Abatacept in B7-1-positive proteinuric kidney disease. N. Engl. J. Med. 369, 2416–2423 (2013).
Novelli, R., Benigni, A. & Remuzzi, G. The role of B7-1 in proteinuria of glomerular origin. Nat. Rev. Nephrol. 14, 589–596 (2018).
Kim, E. Y., Roshanravan, H. & Dryer, S. E. Changes in podocyte TRPC channels evoked by plasma and sera from patients with recurrent FSGS and by putative glomerular permeability factors. Biochim. Biophys. Acta 1863, 2342–2354 (2017).
Morath, C. et al. Management of severe recurrent focal segmental glomerulosclerosis through circulating soluble urokinase receptor modification. Am. J. Ther. 20, 226–229 (2013).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Farmer, L. K., et al. TRPC6 binds to and activates calpain, independent of its channel activity, and regulates podocyte cytoskeleton, cell adhesion, and motility. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2018070729 (2019).
Verheijden, K. A. T. et al. The calcium-dependent protease calpain-1 links TRPC6 activity to podocyte injury. J. Am. Soc. Nephrol. 29, 2099–2109 (2018).
Veit, G. et al. Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat. Med. 24, 1732–1742 (2018).
Roselli, S. et al. Plasma membrane targeting of podocin through the classical exocytic pathway: effect of NPHS2 mutations. Traffic 5, 37–44 (2004).
Nathwani, A. C., Davidoff, A. M. & Tuddenham, E. G. D. Advances in gene therapy for hemophilia. Hum. Gene Ther. 28, 1004–1012 (2017).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Hale, L. J. et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat. Commun. 9, 5167 (2018).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Sharmin, S. et al. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J. Am. Soc. Nephrol. 27, 1778–1791 (2016).
Taguchi, A. & Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21, 730–773 (2017).
Xinaris, C. et al. Functional human podocytes generated in organoids from amniotic fluid stem cells. J. Am. Soc. Nephrol. 27, 1400–1411 (2016).
Merchant, M. L. et al. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat. Rev. Nephrol. 13, 731–749 (2017).
Lai, Z. W., Petrera, A. & Schilling, O. Protein amino-terminal modifications and proteomic approaches for N-terminal profiling. Curr. Opin. Chem. Biol. 24, 71–79 (2015).
Lange, P. F. & Overall, C. M. Protein TAILS: when termini tell tales of proteolysis and function. Curr. Opin. Chem. Biol. 17, 73–82 (2013).
Turk, B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Discov. 5, 785–799 (2006).
Wiita, A. P. et al. Circulating proteolytic signatures of chemotherapy-induced cell death in humans discovered by N-terminal labeling. Proc. Natl Acad. Sci. USA 111, 7594–7599 (2014).
Meyer-Schwesinger, C. et al. Ubiquitin C-terminal hydrolase-l1 activity induces polyubiquitin accumulation in podocytes and increases proteinuria in rat membranous nephropathy. Am. J. Pathol. 178, 2044–2057 (2011).
Meyer-Schwesinger, C. The ubiquitin-proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 15, 393–411 (2019).
Spath, M. R. et al. The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int. 95, 333–349 (2019).
Karpman, D., Stahl, A. L. & Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 13, 545–562 (2017).
Hogan, M. C. et al. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int. 85, 1225–1237 (2014).
Erkan, E. et al. Distinct urinary lipid profile in children with focal segmental glomerulosclerosis. Pediatr. Nephrol. 31, 581–588 (2016).
Shah, L., et al. LDL-apheresis-induced remission of focal segmental glomerulosclerosis recurrence in pediatric renal transplant recipients. Pediatr. Nephrol. https://doi.org/10.1007/s00467-019-04296-6 (2019).
Kaisar, M. et al. Plasma degradome affected by variable storage of human blood. Clin. Proteom. 13, 26 (2016).
Trautmann, A., Lipska-Zietkiewicz, B. S. & Schaefer, F. Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the PodoNet Registry. Front. Pediatr. 6, 200 (2018).
Gadegbeku, C. A. et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 83, 749–756 (2013).
Dossier, C. et al. Five-year outcome of children with idiopathic nephrotic syndrome: the NEPHROVIR population-based cohort study. Pediatr. Nephrol. 34, 671–678 (2019).
Scheufele, E. et al. tranSMART: an open source knowledge management and high content data analytics platform. AMIA Jt. Summits Transl. Sci. Proc. 2014, 96–101 (2014).
Ding, W. Y. et al. Big data and stratified medicine: what does it mean for children? Arch. Dis. Child. 104, 389–394 (2019).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Agius, P., Ying, Y. & Campbell, C. Bayesian unsupervised learning with multiple data types. Stat. Appl. Genet. Mol. Biol. 8, 27 (2009).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Perez, V. et al. Comparative differential proteomic analysis of minimal change disease and focal segmental glomerulosclerosis. BMC Nephrol. 18, 49 (2017).
Choi, Y. W. et al. Potential urine proteomics biomarkers for primary nephrotic syndrome. Clin. Proteom. 14, 18 (2017).
Huang, Z., Zhang, Y., Zhou, J. & Zhang, Y. Urinary exosomal miR-193a can be a potential biomarker for the diagnosis of primary focal segmental glomerulosclerosis in children. Biomed. Res. Int. 2017, 7298160 (2017).
Ramezani, A. et al. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur. J. Clin. Invest. 45, 394–404 (2015).
Lee, J. E. et al. Systematic biomarker discovery and coordinative validation for different primary nephrotic syndromes using gas chromatography-mass spectrometry. J. Chromatogr. A 1453, 105–115 (2016).
Sui, W. et al. Circulating microRNAs as potential biomarkers for nephrotic syndrome. Iran. J. Kidney Dis. 8, 371–376 (2014).
Lu, M. et al. Differentially expressed microRNAs in kidney biopsies from various subtypes of nephrotic children. Exp. Mol. Pathol. 99, 590–595 (2015).
Nafar, M. et al. The novel diagnostic biomarkers for focal segmental glomerulosclerosis. Int. J. Nephrol. 2014, 574261 (2014).
Pant, P. et al. Serum sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of patients with membranous nephropathy and focal and segmental glomerulosclerosis. Saudi J. Kidney Dis. Transpl. 27, 539–545 (2016).
Suresh, C. P. et al. Differentially expressed urinary biomarkers in children with idiopathic nephrotic syndrome. Clin. Exp. Nephrol. 20, 273–283 (2016).
Kalantari, S. et al. Predictive urinary biomarkers for steroid-resistant and steroid-sensitive focal segmental glomerulosclerosis using high resolution mass spectrometry and multivariate statistical analysis. BMC Nephrol. 15, 141 (2014).
Nickavar, A. et al. Urine neutrophil gelatinase associated lipocalin to creatinine ratio: a novel index for steroid response in idiopathic nephrotic syndrome. Indian J. Pediatr. 83, 18–21 (2016).
Gopal, N. et al. Assay of urinary protein-bound sialic acid can differentiate steroidsensitive nephrotic syndrome from steroid-resistant cases. Saudi J. Kidney Dis. Transpl. 27, 37–40 (2016).
Bennett, M. R. et al. Urinary vitamin D-binding protein as a biomarker of steroid-resistant nephrotic syndrome. Biomark. Insights 11, 1–6 (2016).
Badr, H. S., El-Hawy, M. A. & Helwa, M. A. P-glycoprotein activity in steroid-responsive vs. steroid-resistant nephrotic syndrome. Indian J. Pediatr. 83, 1222–1226 (2016).
Turolo, S. et al. SXR rs3842689: a prognostic factor for steroid sensitivity or resistance in pediatric idiopathic nephrotic syndrome. Pharmacogenomics 17, 1227–1233 (2016).
Mishra, O. P. et al. Toll-like receptor 3 (TLR-3), TLR-4 and CD80 expression in peripheral blood mononuclear cells and urinary CD80 levels in children with idiopathic nephrotic syndrome. Pediatr. Nephrol. 32, 1355–1361 (2017).
Gopal, N. et al. Assay of urinary protein carbonyl content can predict the steroid dependence and resistance in children with idiopathic nephrotic syndrome. Saudi J. Kidney Dis. Transpl. 28, 268–272 (2017).
Bennett, M. R. et al. A novel biomarker panel to identify steroid resistance in childhood idiopathic nephrotic syndrome. Biomark. Insights 12, 1–11 (2017).
Watany, M. M. & El-Horany, H. E. Nephronectin (NPNT) and the prediction of nephrotic syndrome response to steroid treatment. Eur. J. Hum. Genet. 26, 1354–1360 (2018).
Andersen, R. F. et al. Plasma and urine proteomic profiles in childhood idiopathic nephrotic syndrome. Proteom. Clin. Appl. 6, 382–393 (2012).
Chan, C. Y. et al. T lymphocyte activation markers as predictors of responsiveness to rituximab among patients with FSGS. Clin. J. Am. Soc. Nephrol. 11, 1360–1368 (2016).
Kuribayashi-Okuma, E. et al. Proteomics approach identifies factors associated with the response to low-density lipoprotein apheresis therapy in patients with steroid-resistant nephrotic syndrome. Ther. Apher. Dial. 20, 174–182 (2016).
Kalantari, S. et al. Urinary prognostic biomarkers in patients with focal segmental glomerulosclerosis. Nephrourol. Mon. 6, e16806 (2014).
Lopez-Hellin, J. et al. A form of apolipoprotein a-I is found specifically in relapses of focal segmental glomerulosclerosis following transplantation. Am. J. Transpl. 13, 493–500 (2013).
Puig-Gay, N. et al. Apolipoprotein A-Ib as a biomarker of focal segmental glomerulosclerosis recurrence after kidney transplantation: diagnostic performance and assessment of its prognostic value – a multi-centre cohort study. Transpl. Int. 32, 313–322 (2019).
Acknowledgements
Funders for studies referenced from the author’s laboratory include the Medical Research Council (UK), Kidney Research UK, Nephrotic Syndrome Trust (NeST), NIHR-TRC, and Kids Kidney Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks R. Gbadegesin, K. Iijima and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NURTuRE: www.nurturebiobank.org
Glossary
- Proteostasis
-
The concept of protein homeostasis, involving protein biogenesis, folding, trafficking and degradation of proteins present within and outside the cell.
- Tryptic peptides
-
Peptides generated by the action of the protease trypsin.
Rights and permissions
About this article
Cite this article
Saleem, M.A. Molecular stratification of idiopathic nephrotic syndrome. Nat Rev Nephrol 15, 750–765 (2019). https://doi.org/10.1038/s41581-019-0217-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0217-5
This article is cited by
-
Hidden genetics behind glomerular scars: an opportunity to understand the heterogeneity of focal segmental glomerulosclerosis?
Pediatric Nephrology (2024)
-
Rationale and design of the Japanese Biomarkers in Nephrotic Syndrome (J-MARINE) study
Clinical and Experimental Nephrology (2024)
-
Engineering antioxidant ceria-zirconia nanomedicines for alleviating podocyte injury in rats with adriamycin-induced nephrotic syndrome
Journal of Nanobiotechnology (2023)
-
Cell and gene therapy for kidney disease
Nature Reviews Nephrology (2023)
-
Mendelian steroid resistant nephrotic syndrome in childhood: is it as common as reported?
Pediatric Nephrology (2023)