Abstract
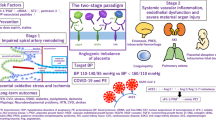
Pre-eclampsia is a complication of pregnancy that is associated with substantial maternal and fetal morbidity and mortality. The disease presents with new-onset hypertension and often proteinuria in the mother, which can progress to multi-organ dysfunction, including hepatic, renal and cerebral disease, if the fetus and placenta are not delivered. Maternal endothelial dysfunction due to circulating factors of fetal origin from the placenta is a hallmark of pre-eclampsia. Risk factors for the disease include maternal comorbidities, such as chronic kidney disease, hypertension and obesity; a family history of pre-eclampsia, nulliparity or multiple pregnancies; and previous pre-eclampsia or intrauterine fetal growth restriction. In the past decade, the discovery and characterization of novel antiangiogenic pathways have been particularly impactful both in increasing understanding of the disease pathophysiology and in directing predictive and therapeutic efforts. In this Review, we discuss the pathogenic role of antiangiogenic proteins released by the placenta in the development of pre-eclampsia and review novel therapeutic strategies directed at restoring the angiogenic imbalance observed during pre-eclampsia. We also highlight other notable advances in the field, including the identification of long-term maternal and fetal risks conferred by pre-eclampsia.
Key points
-
Pre-eclampsia is defined as new-onset hypertension and proteinuria or other end-organ damage such as to the liver or brain occurring after 20 weeks of pregnancy.
-
Pre-eclampsia is characterized by defective placentation, placental ischaemia, abnormal spiral artery remodelling, oxidative stress at the maternal–fetal interface and angiogenic imbalance in the maternal circulation with ensuing endothelial and end-organ damage.
-
High levels of antiangiogenic factors and low levels of proangiogenic factors are useful biomarkers for the early detection and prognosis of pre-eclampsia; these markers also serve as theranostics in clinical trials.
-
Delivery is currently the only definitive treatment for pre-eclampsia; aspirin is recommended for prevention of pre-eclampsia in women at high risk.
-
Potential therapeutic strategies for pre-eclampsia include targeted apheresis, antibody therapies, RNA interference and small-molecule inhibitors of factors that have a role in placental dysfunction.
-
Evidence is emerging of long-term increased risk of cardiovascular and kidney disease in women who have experienced pre-eclampsia; pre-eclampsia is also an important risk factor for neonatal respiratory distress syndrome and bronchopulmonary dysplasia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
08 May 2019
In the version of this article originally published online, the date when Francois Mauriceau published one of the earliest descriptions of pre-eclampsia was incorrectly stated to be 1637, which is actually his year of birth. The work was published in 1668. This error has been corrected in the PDF and HTML versions of the article.
References
Wallis, A. B., Saftlas, A. F., Hsia, J. & Atrash, H. K. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am. J. Hypertens. 21, 521–526 (2008).
Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33, 130–137 (2009).
Ananth, C. V., Keyes, K. M. & Wapner, R. J. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 347, f6564 (2013).
Abalos, E., Cuesta, C., Grosso, A. L., Chou, D. & Say, L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 1–7 (2013).
Kuklina, E. V., Ayala, C. & Callaghan, W. M. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet. Gynecol. 113, 1299–1306 (2009).
Task Force on Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet. Gynecol. 122, 1122–1131 (2013). This paper highlights changes in diagnostic criteria for pre-eclampsia and summarizes the current recommendations for the management of patients with pre-eclampsia.
Bell, M. J. A historical overview of preeclampsia-eclampsia. J. Obstet. Gynecol. Neonatal Nurs. 39, 510–518 (2010).
Roberts, J. M. et al. Preeclampsia: an endothelial cell disorder. Am. J. Obstet. Gynecol. 161, 1200–1204 (1989).
Karumanchi, S. A. Angiogenic factors in preeclampsia: from diagnosis to therapy. Hypertension 67, 1072–1079 (2016).
Hod, T., Cerdeira, A. S. & Karumanchi, S. A. Molecular mechanisms of preeclampsia. Cold Spring Harb. Perspect. Med. 5, a023473 (2015).
Romero, R. & Chaiworapongsa, T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J. Clin. Invest. 123, 2775–2777 (2013).
Ghulmiyyah, L. & Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 36, 56–59 (2012).
Shahul, S. et al. Racial disparities in comorbidities, complications, and maternal and fetal outcomes in women with preeclampsia/eclampsia. Hypertens. Pregnancy 34, 506–515 (2015).
Lo, J. O., Mission, J. F. & Caughey, A. B. Hypertensive disease of pregnancy and maternal mortality. Curr. Opin. Obstet. Gynecol. 25, 124–132 (2013).
Zhang, J., Meikle, S. & Trumble, A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens. Pregnancy 22, 203–212 (2003).
Robillard, P. Y., Dekker, G., Iacobelli, S. & Chaouat, G. An essay of reflection: why does preeclampsia exist in humans, and why are there such huge geographical differences in epidemiology? J. Reprod. Immunol. 114, 44–47 (2016).
Lisonkova, S. & Joseph, K. S. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 209, 544.e1–544.e12 (2013).
Rasmussen, S., Irgens, L. M. & Espinoza, J. Maternal obesity and excess of fetal growth in pre-eclampsia. BJOG 121, 1351–1357 (2014).
Chaiworapongsa, T. et al. Differences and similarities in the transcriptional profile of peripheral whole blood in early and late-onset preeclampsia: insights into the molecular basis of the phenotype of preeclampsiaa. J. Perinat. Med. 41, 485–504 (2013).
Hutcheon, J. A., Lisonkova, S. & Joseph, K. S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 391–403 (2011).
Bartsch, E., Medcalf, K. E., Park, A. L. & Ray, J. G. & High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353, i1753 (2016).
Bdolah, Y. et al. Circulating angiogenic proteins in trisomy 13. Am. J. Obstet. Gynecol. 194, 239–245 (2006).
Skjaerven, R. et al. Recurrence of pre-eclampsia across generations: exploring fetal and maternal genetic components in a population based cohort. BMJ 331, 877 (2005).
Heyborne, K. Paternal and maternal components of the predisposition to preeclampsia. N. Engl. J. Med. 345, 149; author reply 150 (2001).
Mogren, I., Hogberg, U., Winkvist, A. & Stenlund, H. Familial occurrence of preeclampsia. Epidemiology 10, 518–522 (1999).
Gray, K. J., Saxena, R. & Karumanchi, S. A. Genetic predisposition to preeclampsia is conferred by fetal DNA variants near FLT1, a gene involved in the regulation of angiogenesis. Am. J. Obstet. Gynecol. 218, 211–218 (2018).
McGinnis, R. et al. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat. Genet. 49, 1255–1260 (2017). This large clinical genome-wide association study suggests that dysregulation at the FLT1 locus in the fetal genome (likely in the placenta) is a fundamental molecular defect in pre-eclampsia.
Goel, A. et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation 132, 1726–1733 (2015).
Acosta-Sison, H. The relationship of hydatidiform mole to pre-eclampsia and eclampsia; a study of 85 cases. Am. J. Obstet. Gynecol. 71, 1279–1282 (1956).
Hecht, J. L., Zsengeller, Z. K., Spiel, M., Karumanchi, S. A. & Rosen, S. Revisiting decidual vasculopathy. Placenta 42, 37–43 (2016).
Brosens, I., Robertson, W. B. & Dixon, H. G. The physiological response of the vessels of the placental bed to normal pregnancy. J. Pathol. Bacteriol. 93, 569–579 (1967).
Young, J. The aetiology of eclampsia and albuminuria and their relation to accidental haemorrhage: (an anatomical and experimental investigation). Proc. R. Soc. Med. 7, 307–348 (1914).
Robertson, W. B., Brosens, I. & Dixon, H. G. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J. Pathol. Bacteriol. 93, 581–592 (1967).
Brosens, I. A., Robertson, W. B. & Dixon, H. G. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet. Gynecol. Annu. 1, 177–191 (1972).
Zhou, Y. et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Invest. 99, 2139–2151 (1997).
Zhou, Y., Damsky, C. H. & Fisher, S. J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Invest. 99, 2152–2164 (1997). This paper demonstrates for the first time that invasive cytotrophoblasts fail to acquire endothelial markers in women with pre-eclampsia.
Meekins, J. W., Pijnenborg, R., Hanssens, M., McFadyen, I. R. & van Asshe, A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 101, 669–674 (1994).
Burton, G. J., Woods, A. W., Jauniaux, E. & Kingdom, J. C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30, 473–482 (2009).
Lyall, F., Robson, S. C. & Bulmer, J. N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 62, 1046–1054 (2013).
Cindrova-Davies, T. et al. Energy status and HIF signalling in chorionic villi show no evidence of hypoxic stress during human early placental development. Mol. Hum. Reprod. 21, 296–308 (2015).
Caniggia, I. et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J. Clin. Invest. 105, 577–587 (2000).
Rajakumar, A., Doty, K., Daftary, A., Harger, G. & Conrad, K. P. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta 24, 199–208 (2003).
Tal, R. et al. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 177, 2950–2962 (2010).
Nevo, O. et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1085–R1093 (2006).
Kanasaki, K. et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453, 1117–1121 (2008).
Barnea, E. R., MacLusky, N. J., DeCherney, A. H. & Naftolin, F. Catechol-o-methyl transferase activity in the human term placenta. Am. J. Perinatol. 5, 121–127 (1988).
Palmer, K. et al. Severe early-onset preeclampsia is not associated with a change in placental catechol O-methyltransferase (COMT) expression. Am. J. Pathol. 178, 2484–2488 (2011).
Burton, G. J. & Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 287–299 (2011).
Hung, T. H., Skepper, J. N. & Burton, G. J. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am. J. Pathol. 159, 1031–1043 (2001).
Sedeek, M. et al. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am. J. Hypertens. 21, 1152–1156 (2008).
Wu, L. & Wang, R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 57, 585–630 (2005).
Neuzil, J. & Stocker, R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J. Biol. Chem. 269, 16712–16719 (1994).
Ahmed, A. et al. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol. Med. 6, 391–409 (2000).
Cudmore, M. et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 115, 1789–1797 (2007).
George, E. M. et al. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57, 941–948 (2011).
Zhao, H., Wong, R. J., Kalish, F. S., Nayak, N. R. & Stevenson, D. K. Effect of heme oxygenase-1 deficiency on placental development. Placenta 30, 861–868 (2009).
Lian, I. A. et al. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta 32, 823–829 (2011).
Fu, J., Zhao, L., Wang, L. & Zhu, X. Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwan. J. Obstet. Gynecol. 54, 19–23 (2015).
Kaitu’u-Lino, T. J. et al. Activating transcription factor 3 is reduced in preeclamptic placentas and negatively regulates sFlt-1 (soluble fms-like tyrosine kinase 1), soluble endoglin, and proinflammatory cytokines in placenta. Hypertension 70, 1014–1024 (2017).
Ratsep, M. T. et al. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction 149, R91–R102 (2015).
Cavalli, R. C. et al. Induced human decidual NK-like cells improve utero-placental perfusion in mice. PLOS ONE 11, e0164353 (2016).
Hiby, S. E. et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120, 4102–4110 (2010). This manuscript provides evidence that mismatch between maternal natural killer cell receptors and HLA-C haplotypes in the placenta may be a fundamental cause of pre-eclampsia.
Chazara, O., Xiong, S. & Moffett, A. Maternal KIR and fetal HLA-C: a fine balance. J. Leukoc. Biol. 90, 703–716 (2011).
Robillard, P. Y., Dekker, G. A. & Hulsey, T. C. Revisiting the epidemiological standard of preeclampsia: primigravidity or primipaternity? Eur. J. Obstet. Gynecol. Reprod. Biol. 84, 37–41 (1999).
Deen, M. E., Ruurda, L. G., Wang, J. & Dekker, G. A. Risk factors for preeclampsia in multiparous women: primipaternity versus the birth interval hypothesis. J. Matern. Fetal Neonatal Med. 19, 79–84 (2006).
Saito, S. & Sakai, M. Th1/Th2 balance in preeclampsia. J. Reprod. Immunol. 59, 161–173 (2003).
Sasaki, Y. et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 149, 139–145 (2007).
Girardi, G. Complement activation, a threat to pregnancy. Semin. Immunopathol. 40, 103–111 (2018).
Girardi, G., Yarilin, D., Thurman, J. M., Holers, V. M. & Salmon, J. E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 203, 2165–2175 (2006).
Lynch, A. M. et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am. J. Obstet. Gynecol. 198, 385 (2008).
Sones, J. L. et al. Angiogenic factor imbalance precedes complement deposition in placentae of the BPH/5 model of preeclampsia. FASEB J. 32, 2574–2586 (2018).
Vaught, A. J. et al. Germline mutations in the alternative pathway of complement predispose to HELLP syndrome. JCI Insight 3, 99128 (2018).
Brocklebank, V., Wood, K. M. & Kavanagh, D. Thrombotic microangiopathy and the kidney. Clin. J. Am. Soc. Nephrol. 13, 300–317 (2018).
Hecht, J. L. et al. The pathology of eclampsia: an autopsy series. Hypertens. Pregnancy 36, 259–268 (2017).
Gaber, L. W., Spargo, B. H. & Lindheimer, M. D. Renal pathology in pre-eclampsia. Baillieres Clin. Obstet. Gynaecol. 8, 443–468 (1994).
Stillman, I. E. & Karumanchi, S. A. The glomerular injury of preeclampsia. J. Am. Soc. Nephrol. 18, 2281–2284 (2007).
Deen, W. M. What determines glomerular capillary permeability? J. Clin. Invest. 114, 1412–1414 (2004).
Garovic, V. D. et al. Urinary podocyte excretion as a marker for preeclampsia. Am. J. Obstet. Gynecol. 196, 320 (2007).
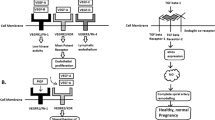
Powe, C. E., Levine, R. J. & Karumanchi, S. A. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123, 2856–2869 (2011).
Ahmad, S. & Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 95, 884–891 (2004).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 (2003). This key paper demonstrates that excess sFLT1 is sufficient to induce pre-eclampsia.
Venkatesha, S. et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649 (2006).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 (2004). This paper highlights the utility of angiogenic factors for use as biomarkers in the early diagnosis of pre-eclampsia.
Levine, R. J. et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 355, 992–1005 (2006).
Noori, M., Donald, A. E., Angelakopoulou, A., Hingorani, A. D. & Williams, D. J. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 122, 478–487 (2010).
Park, J. E., Chen, H. H., Winer, J., Houck, K. A. & Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 269, 25646–25654 (1994).
Maynard, S., Epstein, F. H. & Karumanchi, S. A. Preeclampsia and angiogenic imbalance. Annu. Rev. Med. 59, 61–78 (2008).
Kendall, R. L. & Thomas, K. A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl Acad. Sci. USA 90, 10705–10709 (1993).
Lu, F. et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am. J. Obstet. Gynecol. 196, 396; discussion 396 (2007).
Li, Z. et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50, 686–692 (2007).
Szalai, G. et al. Full-length human placental sFlt-1-e15a isoform induces distinct maternal phenotypes of preeclampsia in mice. PLOS ONE 10, e0119547 (2015).
Verlohren, S., Stepan, H. & Dechend, R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin. Sci. 122, 43–52 (2012).
Young, B. C., Levine, R. J. & Karumanchi, S. A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. 5, 173–192 (2010).
March, M. I. et al. Circulating angiogenic factors and the risk of adverse outcomes among haitian women with preeclampsia. PLOS ONE 10, e0126815 (2015).
Chaiworapongsa, T. et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J. Matern. Fetal. Neonatal Med. 27, 132–144 (2014).
Rana, S. et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 125, 911–919 (2012).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Patel, T. V. et al. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J. Natl Cancer Inst. 100, 282–284 (2008).
Vigneau, C. et al. All anti-vascular endothelial growth factor drugs can induce ‘pre-eclampsia-like syndrome’: a RARe study. Nephrol. Dial. Transplant. 29, 325–332 (2014).
Launay-Vacher, V. & Deray, G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs 20, 81–82 (2009).
Sela, S. et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ. Res. 102, 1566–1574 (2008).
Tannetta, D. S., Dragovic, R. A., Gardiner, C., Redman, C. W. & Sargent, I. L. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLOS ONE 8, e56754 (2013).
Rajakumar, A. et al. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension 59, 256–264 (2012).
Redman, C. W. et al. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 33 (Suppl.), S48–S54 (2012).
Vaisbuch, E. et al. Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am. J. Obstet. Gynecol. 204, 152 (2011).
Romero, R. et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J. Matern. Fetal Neonatal Med. 21, 9–23 (2008).
Wallace, K. et al. Hypertension, inflammation and T lymphocytes are increased in a rat model of HELLP syndrome. Hypertens. Pregnancy 33, 41–54 (2014).
Maharaj, A. S. et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J. Exp. Med. 205, 491–501 (2008).
Saleh, L., Verdonk, K., Visser, W., van den Meiracker, A. H. & Danser, A. H. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther. Adv. Cardiovasc. Dis. 10, 282–293 (2016).
Verdonk, K., Visser, W., Van Den Meiracker, A. H. & Danser, A. H. The renin-angiotensin-aldosterone system in pre-eclampsia: the delicate balance between good and bad. Clin. Sci. 126, 537–544 (2014).
Burke, S. D. et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Invest. 126, 2561–2574 (2016).
Wallukat, G. et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Invest. 103, 945–952 (1999). This paper is the first to demonstrate a biological role for autoantibodies against the angiotensin AT1 receptor.
Zhou, C. C. et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 14, 855–862 (2008).
Hubel, C. A. et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 49, 612–617 (2007).
Xia, Y. & Kellems, R. E. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ. Res. 113, 78–87 (2013).
Quitterer, U. et al. Beta-arrestin1 prevents preeclampsia by downregulation of mechanosensitive AT1-B2 receptor heteromers. Cell 176, 318–333 (2018).
Osol, G. et al. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 294, H1381–H1387 (2008).
Zhang, H. H., Chen, J. C., Sheibani, L., Lechuga, T. J. & Chen, D. B. Pregnancy augments VEGF-stimulated in vitro angiogenesis and vasodilator (NO and H2S) production in human uterine artery endothelial cells. J. Clin. Endocrinol. Metab. 102, 2382–2393 (2017).
Pimentel, A. M. et al. L-Arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertens. Res. 36, 783–788 (2013).
Sandrim, V. C. et al. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension 52, 402–407 (2008).
Zeng, Y., Li, M., Chen, Y. & Wang, S. Homocysteine, endothelin-1 and nitric oxide in patients with hypertensive disorders complicating pregnancy. Int. J. Clin. Exp. Pathol. 8, 15275–15279 (2015).
Goncalves-Rizzi, V. H., Possomato-Vieira, J. S., Sales Graca, T. U., Nascimento, R. A. & Dias-Junior, C. A. Sodium nitrite attenuates hypertension-in-pregnancy and blunts increases in soluble fms-like tyrosine kinase-1 and in vascular endothelial growth factor. Nitric Oxide 57, 71–78 (2016).
Osol, G., Ko, N. L. & Mandala, M. Altered endothelial nitric oxide signaling as a paradigm for maternal vascular maladaptation in preeclampsia. Curr. Hypertens. Rep. 19, 82 (2017).
Lankhorst, S., Danser, A. H. & van den Meiracker, A. H. Endothelin-1 and antiangiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R230–R234 (2016).
George, E. M. & Granger, J. P. Endothelin: key mediator of hypertension in preeclampsia. Am. J. Hypertens. 24, 964–969 (2011).
Kingman, M., Ruggiero, R. & Torres, F. Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin. Pharmacother. 10, 1847–1858 (2009).
Wang, K. et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 127, 2514–2522 (2013).
Holwerda, K. M. et al. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J. Am. Soc. Nephrol. 25, 717–725 (2014).
Snijder, P. M. et al. Sodium thiosulfate attenuates angiotensin II-induced hypertension, proteinuria and renal damage. Nitric Oxide 42, 87–98 (2014).
Gant, N. F., Daley, G. L., Chand, S., Whalley, P. J. & MacDonald, P. C. A study of angiotensin II pressor response throughout primigravid pregnancy. J. Clin. Invest. 52, 2682–2689 (1973).
Saxena, A. R. et al. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 55, 1239–1245 (2010).
Germain, A. M. et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 49, 90–95 (2007).
Gammill, H. S., Lin, C. & Hubel, C. A. Endothelial progenitor cells and preeclampsia. Front. Biosci. 12, 2383–2394 (2007).
O’Brien, T. E., Ray, J. G. & Chan, W. S. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology 14, 368–374 (2003).
Catalano, P. M., Tyzbir, E. D., Roman, N. M., Amini, S. B. & Sims, E. A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 165, 1667–1672 (1991).
Chisholm, K. M. & Folkins, A. K. Placental and clinical characteristics of term small-for-gestational-age neonates: a case-control study. Pediatr. Dev. Pathol. 19, 37–46 (2016).
Fuh, M. M. et al. Resistance to insulin-mediated glucose uptake and hyperinsulinemia in women who had preeclampsia during pregnancy. Am. J. Hypertens. 8, 768–771 (1995).
Martinez Abundis, E., Gonzalez Ortiz, M., Quinones Galvan, A. & Ferrannini, E. Hyperinsulinemia in glucose-tolerant women with preeclampsia. A controlled study. Am. J. Hypertens. 9, 610–614 (1996).
Arkwright, P. D., Rademacher, T. W., Dwek, R. A. & Redman, C. W. Pre-eclampsia is associated with an increase in trophoblast glycogen content and glycogen synthase activity, similar to that found in hydatidiform moles. J. Clin. Invest. 91, 2744–2753 (1993).
Scioscia, M. et al. Insulin resistance in human preeclamptic placenta is mediated by serine phosphorylation of insulin receptor substrate-1 and -2. J. Clin. Endocrinol. Metab. 91, 709–717 (2006).
Thadhani, R. et al. Insulin resistance and alterations in angiogenesis: additive insults that may lead to preeclampsia. Hypertension 43, 988–992 (2004).
Sandgren, J. A. et al. Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight 3, e99403 (2018).
Rana, S., Karumanchi, S. A. & Lindheimer, M. D. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension 63, 198–202 (2014).
Baltajian, K. et al. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. Am. J. Obstet. Gynecol. 215, 89.e1–89.e10 (2016).
Rana, S. et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hypertens. 13, 100–106 (2018).
Rana, S. et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens. Pregnancy 32, 189–201 (2013).
Kleinrouweler, C. E. et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG 119, 778–787 (2012).
Kusanovic, J. P. et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J. Matern. Fetal. Neonatal Med. 22, 1021–1038 (2009).
Moore Simas, T. A. et al. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. J. Matern. Fetal. Neonatal Med. 27, 1038–1048 (2014).
Chappell, L. C. et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 128, 2121–2131 (2013).
Erez, O. et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J. Matern. Fetal. Neonatal Med. 21, 279–287 (2008).
Chaiworapongsa, T. et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am. J. Obstet. Gynecol. 208, 287.e1–287.e15 (2013).
Chaiworapongsa, T. et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am. J. Obstet. Gynecol. 190, 1541–1547; discussion 1547–1550 (2004).
Leanos-Miranda, A. et al. Changes in circulating concentrations of soluble fms-like tyrosine kinase-1 and placental growth factor measured by automated electrochemiluminescence immunoassays methods are predictors of preeclampsia. J. Hypertens. 30, 2173–2181 (2012).
Chaiworapongsa, T. et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J. Matern. Fetal. Neonatal Med. 17, 3–18 (2005).
Moore, A. G. et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J. Matern. Fetal. Neonatal Med. 25, 2651–2657 (2012).
Zeisler, H. et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 374, 13–22 (2016). This large prospective clinical study demonstrates a utility for serum angiogenic biomarkers in women with suspected pre-eclampsia.
Zeisler, H. et al. The sFlt-1/PlGF Ratio: ruling out pre-eclampsia for up to 4 weeks and the value of retesting. Ultrasound Obstet. Gynecol. https://doi.org/10.1002/uog.19178 (2018).
Schnettler, W. T. et al. Cost and resource implications with serum angiogenic factor estimation in the triage of pre-eclampsia. BJOG 120, 1224–1232 (2013).
Hadker, N. et al. Financial impact of a novel pre-eclampsia diagnostic test versus standard practice: a decision-analytic modeling analysis from a UK healthcare payer perspective. J. Med. Econ. 13, 728–737 (2010).
Rolfo, A. et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 83, 177–181 (2013).
Verdonk, K. et al. Differential diagnosis of preeclampsia: remember the soluble fms-like tyrosine kinase 1/placental growth factor ratio. Hypertension 60, 884–890 (2012).
Verlohren, S. et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 206, 58 (2012).
Young, B. et al. The use of angiogenic biomarkers to differentiate non-HELLP related thrombocytopenia from HELLP syndrome. J. Matern. Fetal. Neonatal Med. 23, 366–370 (2010).
Perni, U. et al. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension 59, 740–746 (2012).
Leanos-Miranda, A. et al. Circulating angiogenic factors and the risk of preeclampsia in systemic lupus erythematosus pregnancies. J. Rheumatol. 42, 1141–1149 (2015).
Kim, M. Y. et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am. J. Obstet. Gynecol. 214, 108.e1–108.e14 (2016).
Chaiworapongsa, T. et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J. Matern. Fetal. Neonatal Med. 29, 1214–1228 (2016).
Buyon, J. P. et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann. Intern. Med. 163, 153–163 (2015).
Hagmann, H., Thadhani, R., Benzing, T., Karumanchi, S. A. & Stepan, H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin. Chem. 58, 837–845 (2012).
Sovio, U. et al. Prediction of preeclampsia using the soluble fms-like tyrosine kinase 1 to placental growth factor ratio: a prospective cohort study of unselected nulliparous women. Hypertension 69, 731–738 (2017).
Poon, L. C., Kametas, N. A., Maiz, N., Akolekar, R. & Nicolaides, K. H. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension 53, 812–818 (2009).
Rolnik, D. L. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 377, 613–622 (2017). This prospective clinical trial demonstrates that aspirin, when given to high-risk women early in pregnancy, can prevent preterm pre-eclampsia.
Charkiewicz, K., Jasinska, E. & Laudanski, P. Identification of proteomic biomarkers of preeclampsia using protein microarray and tandem mass spectrometry [Polish]. Postepy Hig. Med. Dosw. 69, 562–570 (2015).
Law, K. P., Han, T. L., Tong, C. & Baker, P. N. Mass spectrometry-based proteomics for pre-eclampsia and preterm birth. Int. J. Mol. Sci. 16, 10952–10985 (2015).
Kenny, L. C. et al. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension 56, 741–749 (2010).
Spaans, F., de Vos, P., Bakker, W. W., van Goor, H. & Faas, M. M. Danger signals from ATP and adenosine in pregnancy and preeclampsia. Hypertension 63, 1154–1160 (2014).
Bahado-Singh, R. O. et al. First-trimester metabolomic detection of late-onset preeclampsia. Am. J. Obstet. Gynecol. 208, 58 (2013).
Kuc, S. et al. Metabolomics profiling for identification of novel potential markers in early prediction of preeclampsia. PLOS ONE 9, e98540 (2014).
Hahn, S., Rusterholz, C., Hosli, I. & Lapaire, O. Cell-free nucleic acids as potential markers for preeclampsia. Placenta 32 (Suppl.), S17–S20 (2011).
Purwosunu, Y. et al. Prediction of preeclampsia by analysis of cell-free messenger RNA in maternal plasma. Am. J. Obstet. Gynecol. 200, 386 (2009).
Sekizawa, A. et al. Prediction of pre-eclampsia by an analysis of placenta-derived cellular mRNA in the blood of pregnant women at 15–20 weeks of gestation. BJOG 117, 557–564 (2010).
Farina, A. et al. Performance of messenger RNAs circulating in maternal blood in the prediction of preeclampsia at 10–14 weeks. Am. J. Obstet. Gynecol. 203, 575 (2010).
Bergmann, A. et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J. Cell. Mol. Med. 14, 1857–1867 (2010).
Gilbert, J. S. et al. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55, 380–385 (2010).
Siddiqui, A. H. et al. Recombinant vascular endothelial growth factor 121 attenuates autoantibody-induced features of pre-eclampsia in pregnant mice. Am. J. Hypertens. 24, 606–612 (2011).
Makris, A. et al. Placental growth factor reduces blood pressure in a uteroplacental ischemia model of preeclampsia in nonhuman primates. Hypertension 67, 1263–1272 (2016).
Spradley, F. T. et al. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension 67, 740–747 (2016).
Santiago-Font, J. A. et al. Serelaxin improves the pathophysiology of placental ischemia in the reduced uterine perfusion pressure rat model of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R1158–R1163 (2016).
Ashar-Patel, A. et al. FLT1 and transcriptome-wide polyadenylation site (PAS) analysis in preeclampsia. Sci. Rep. 7, 12139 (2017).
Turanov, A. A. et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 36, 1164–1173 (2018).
Paauw, N. D. et al. Sildenafil during pregnancy: a preclinical meta-analysis on fetal growth and maternal blood pressure. Hypertension 70, 998–1006 (2017).
Mandala, M. & Osol, G. Physiological remodelling of the maternal uterine circulation during pregnancy. Bas. Clin. Pharmacol. Toxicol. 110, 12–18 (2012).
Trapani, A. Jr. et al. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: a randomized controlled trial. Obstet. Gynecol. 128, 253–259 (2016).
Pels, A. et al. STRIDER (Sildenafil TheRapy in dismal prognosis early onset fetal growth restriction): an international consortium of randomised placebo-controlled trials. BMC Pregnancy Childbirth 17, 440 (2017).
Hawkes, N. Trial of Viagra for fetal growth restriction is halted after baby deaths. BMJ 362, k3247 (2018).
Rana, S. et al. Ouabain inhibits placental sFlt1 production by repressing HSP27-dependent HIF-1alpha pathway. FASEB J. 28, 4324–4334 (2014).
Kalafat, E., Sukur, Y. E., Abdi, A., Thilaganathan, B. & Khalil, A. Metformin for the prevention of hypertensive disorders of pregnancy in women with gestational diabetes and obesity: a systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 52, 706–714 (2018).
Brownfoot, F. C. et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 214, 356.e1–356.e15 (2016).
Cluver, C. A. et al. Esomeprazole to treat women with preterm preeclampsia: a randomised placebo controlled trial. Am. J. Obstet. Gynecol. 219, 388.e1–388.e17 (2018).
Kaitu’u-Lino, T. J. et al. Combining metformin and esomeprazole is additive in reducing sFlt-1 secretion and decreasing endothelial dysfunction — implications for treating preeclampsia. PLOS ONE 13, e0188845 (2018).
Klingel, R., Gohlen, B., Schwarting, A., Himmelsbach, F. & Straube, R. Differential indication of lipoprotein apheresis during pregnancy. Ther. Apher. Dial. 7, 359–364 (2003).
Thadhani, R. et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124, 940–950 (2011). This proof-of-concept clinical study demonstrates that removal of sFLT1 was associated with improvement of pre-eclamptic signs and extension of pregnancy.
Thadhani, R. et al. Removal of soluble fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J. Am. Soc. Nephrol. 27, 903–913 (2016).
Roberts, J. M. et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N. Engl. J. Med. 362, 1282–1291 (2010).
Haddad, B. et al. Enoxaparin and aspirin compared with aspirin alone to prevent placenta-mediated pregnancy complications: a randomized controlled trial. Obstet. Gynecol. 128, 1053–1063 (2016).
Roberge, S., Bujold, E. & Nicolaides, K. H. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am. J. Obstet. Gynecol. 218, 287–293 (2018).
LeFevre, M. L. & U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 161, 819–826 (2014).
Poston, L. et al. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 367, 1145–1154 (2006).
Covarrubias, A. E. et al. AP39, a modulator of mitochondrial bioenergetics, reduces anti-angiogenic response and oxidative stress in hypoxia-exposed trophoblasts: relevance for preeclampsia pathogenesis. Am. J. Pathol. 189, 104–114 (2018).
Vaka, V. R. et al. Role of mitochondrial dysfunction and reactive oxygen species in mediating hypertension in the reduced uterine perfusion pressure rat model of preeclampsia. Hypertension 72, 703–711 (2018).
Girardi, G. Pravastatin to treat and prevent preeclampsia. Preclinical and clinical studies. J. Reprod. Immunol. 124, 15–20 (2017).
Ramma, W. & Ahmed, A. Therapeutic potential of statins and the induction of heme oxygenase-1 in preeclampsia. J. Reprod. Immunol. 101–102, 153–160 (2014).
Costantine, M. M. et al. Using pravastatin to improve the vascular reactivity in a mouse model of soluble fms-like tyrosine kinase-1-induced preeclampsia. Obstet. Gynecol. 116, 114–120 (2010).
Kumasawa, K. et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl Acad. Sci. USA 108, 1451–1455 (2011).
Saad, A. F. et al. Pravastatin effects on placental prosurvival molecular pathways in a mouse model of preeclampsia. Reprod. Sci. 23, 1593–1599 (2016).
Saad, A. F. et al. Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod. Sci. 21, 138–145 (2014).
Brownfoot, F. C. et al. Effects of simvastatin, rosuvastatin and pravastatin on soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sENG) secretion from human umbilical vein endothelial cells, primary trophoblast cells and placenta. BMC Pregnancy Childbirth 16, 117 (2016).
Chaiworapongsa, T. et al. Pravastatin for the prevention of adverse pregnancy outcome: preeclampsia and more? J. Matern. Fetal Neonatal Med. 30, 3 (2017).
Brownfoot, F. C. et al. Effects of pravastatin on human placenta, endothelium, and women with severe preeclampsia. Hypertension 66, 687–697; discussion 445 (2015).
Lefkou, E. et al. Pravastatin improves pregnancy outcomes in obstetric antiphospholipid syndrome refractory to antithrombotic therapy. J. Clin. Invest. 126, 2933–2940 (2016).
Costantine, M. M. et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am. J. Obstet. Gynecol. 214, 720.e1–720.e17 (2016).
Chen, C. W., Jaffe, I. Z. & Karumanchi, S. A. Pre-eclampsia and cardiovascular disease. Cardiovasc. Res. 101, 579–586 (2014).
Ahmed, R., Dunford, J., Mehran, R., Robson, S. & Kunadian, V. Pre-eclampsia and future cardiovascular risk among women: a review. J. Am. Coll. Cardiol. 63, 1815–1822 (2014).
Mosca, L. et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women — 2011 update: a guideline from the American Heart Association. J. Am. Coll. Cardiol. 57, 1404–1423 (2011).
Bellamy, L., Casas, J. P., Hingorani, A. D. & Williams, D. J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335, 974 (2007). This paper provides a systematic review on the relationship between pre-eclampsia and long-term CVD.
Leslie, M. S. & Briggs, L. A. Preeclampsia and the risk of future vascular disease and mortality: a review. J. Midwifery Womens Health 61, 315–324 (2016).
Veerbeek, J. H. et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 65, 600–606 (2015).
Al-Nasiry, S. et al. Metabolic syndrome after pregnancies complicated by pre-eclampsia or small-for-gestational-age: a retrospective cohort. BJOG 122, 1818–1823 (2015).
Bello, N., Rendon, I. S. H. & Arany, Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 62, 1715–1723 (2013).
Patten, I. S. et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485, 333–338 (2012).
Shahul, S. et al. Circulating antiangiogenic factors and myocardial dysfunction in hypertensive disorders of pregnancy. Hypertension 67, 1273–1280 (2016).
Vikse, B. E., Irgens, L. M., Leivestad, T., Skjaerven, R. & Iversen, B. M. Preeclampsia and the risk of end-stage renal disease. N. Engl. J. Med. 359, 800–809 (2008). This paper is the first to link pre-eclampsia with future ESRD.
McDonald, S. D., Han, Z., Walsh, M. W., Gerstein, H. C. & Devereaux, P. J. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am. J. Kidney Dis. 55, 1026–1039 (2010).
Tangren, J. S. et al. Pregnancy outcomes after clinical recovery from AKI. J. Am. Soc. Nephrol. 28, 1566–1574 (2017). This paper reports that a prior history of acute kidney injury is a major risk factor for pre-eclampsia.
Piccoli, G. B. et al. Risk of adverse pregnancy outcomes in women with CKD. J. Am. Soc. Nephrol. 26, 2011–2022 (2015).
Pruthi, D. et al. Exposure to experimental preeclampsia in mice enhances the vascular response to future injury. Hypertension 65, 863–870 (2015).
Bytautiene, E. et al. Long-term alterations in maternal plasma proteome after sFlt1-induced preeclampsia in mice. Am. J. Obstet. Gynecol. 208, 388.e1–388.e10 (2013).
Wang, A. et al. Circulating anti-angiogenic factors during hypertensive pregnancy and increased risk of respiratory distress syndrome in preterm neonates. J. Matern. Fetal Neonatal Med. 25, 1447–1452 (2012).
Hansen, A. R., Barnes, C. M., Folkman, J. & McElrath, T. F. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J. Pediatr. 156, 532–536 (2010).
Thebaud, B. & Abman, S. H. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. 175, 978–985 (2007).
Vuorela, P. et al. Amniotic fluid—soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet. Gynecol. 95, 353–357 (2000).
Tang, J. R., Karumanchi, S. A., Seedorf, G., Markham, N. & Abman, S. H. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L36–L46 (2012).
Yu, X. D., Branch, D. W., Karumanchi, S. A. & Zhang, J. Preeclampsia and retinopathy of prematurity in preterm births. Pediatrics 130, e101–e107 (2012).
Sibai, B. M. et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am. J. Obstet. Gynecol. 177, 1003–1010 (1997).
Acknowledgements
The authors thank I. Stillman at the Department of Pathology, Beth Israel Deaconess Medical Center, USA, for providing the histology image used in figure 3.
Reviewer information
Nature Reviews Nephrology thanks A. Hennessy and the other anonymous reviewers for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
E.A.P and S.A.K. researched the data for and wrote the article. All authors made substantial contributions to discussions of the content and reviewed or edited the text before submission.
Corresponding author
Ethics declarations
Competing interests
S.A.K. is co-inventor on multiple patents (US Patent and Trademark Office (USPTO) #7,740,849, #7,407,658, #7,335,362, #7,344,892 and #8969322B2) related to the use of angiogenic markers for the diagnosis, prediction and therapy of pre-eclampsia. R.T. is a co-inventor on a patent (USPTO #7,344,892) related to the use of angiogenic proteins for the prediction of pre-eclampsia. These patents are held at Harvard Hospitals (Beth Israel Deaconess Medical Center and Massachusetts General Hospital). S.A.K and R.T. have financial interests in Aggamin Therapeutics LLC and have previously served as consultants for Roche Diagnostics and ThermoFisher. S.A.K. has received a research grant from Siemens. R.T. and T.B. have received a research grant from Kaneka Pharmaceuticals. The other authors report no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hydatidiform mole
-
A gestational, trophoblastic disease that occurs after aberrant fertilization, originates in the placenta and has potential to invade the uterus and metastasize.
- Trisomy 13
-
A severe chromosomal disorder caused by an extra copy of chromosome 13 that is characterized by multiple congenital abnormalities with a classic triad of abnormally small or missing eyes, cleft lip and/or palate and extra digits.
- Genome-wide association study
-
An analysis of markers (usually single-nucleotide polymorphisms) across the entire genome to identify those that are statistically more or less common in one population (often patients with a specific disease) than in another population (typically people who are unaffected by the specific disease).
- Spiral arteries
-
Small arteries derived from uterine arteries that supply blood to the endometrium of the uterus during the luteal phase of the menstrual cycle. These arteries are remodelled into highly dilated vessels by the action of invading trophoblasts during normal pregnancy to support the growing demands of the fetus.
- Foam cells
-
Cells that contain vacuoles or fat-laden macrophages seen in atherosclerosis.
- HELLP syndrome
-
A complication of pregnancy that is characterized by a syndrome of haemolysis, elevated liver enzymes and low platelet count.
- Haemosiderin
-
An insoluble form of tissue storage iron.
Rights and permissions
About this article
Cite this article
Phipps, E.A., Thadhani, R., Benzing, T. et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 15, 275–289 (2019). https://doi.org/10.1038/s41581-019-0119-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0119-6
This article is cited by
-
Factors associated with neonatal jaundice among neonates admitted at referral hospitals in northeast Ethiopia: a facility-based unmatched case-control study
BMC Pregnancy and Childbirth (2024)
-
The influence of Nrf2 gene promoter methylation on gene expression and oxidative stress parameters in preeclampsia
BMC Medical Genomics (2024)
-
The association between diet quality index-international and dietary diversity score with preeclampsia: a case–control study
BMC Women's Health (2024)
-
The relationship between severe hypertensive diseases of pregnancy and moderate-severe bronchopulmonary dysplasia
Journal of Perinatology (2024)
-
sFlt-1/PIGF ratio positive associated with non-dipper type change in ambulatory blood pressure monitoring(ABPM) for preeclampsia development
Hypertension Research (2024)