Abstract
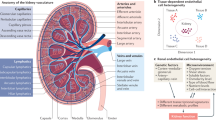
The kidney harbours different types of endothelia, each with specific structural and functional characteristics. The glomerular endothelium, which is highly fenestrated and covered by a rich glycocalyx, participates in the sieving properties of the glomerular filtration barrier and in the maintenance of podocyte structure. The microvascular endothelium in peritubular capillaries, which is also fenestrated, transports reabsorbed components and participates in epithelial cell function. The endothelium of large and small vessels supports the renal vasculature. These renal endothelia are protected by regulators of thrombosis, inflammation and complement, but endothelial injury (for example, induced by toxins, antibodies, immune cells or inflammatory cytokines) or defects in factors that provide endothelial protection (for example, regulators of complement or angiogenesis) can lead to acute or chronic renal injury. Moreover, renal endothelial cells can transition towards a mesenchymal phenotype, favouring renal fibrosis and the development of chronic kidney disease. Thus, the renal endothelium is both a target and a driver of kidney and systemic cardiovascular complications. Emerging therapeutic strategies that target the renal endothelium may lead to improved outcomes for both rare and common renal diseases.
Key points
-
The kidney contains diverse populations of endothelial cells, including the glomerular endothelium, microvascular endothelium in peritubular capillaries and the endothelium of large and small vessels, and each of these populations has specific characteristics and functions.
-
Homeostasis of renal endothelial cells is crucial for the preservation of glomerular structure and function, the preservation of an anti-inflammatory and an antithrombotic environment and the prevention of renal fibrosis.
-
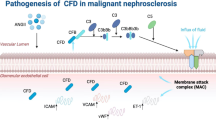
Glomerular endothelial cells, in particular, are susceptible to injury in typical and atypical haemolytic uraemic syndrome, lupus nephritis, antineutrophil cytoplasmic antibody vasculitides and antibody-mediated rejection as well as in situations of vascular endothelial growth factor (VEGF) depletion.
-
Common forms of chronic kidney disease (CKD) — diabetic kidney disease and arteriolar nephrosclerosis — are also characterized by renal endothelial dysfunction.
-
Alterations in endothelial repair capacity, endothelial-to-mesenchymal transition and capillary rarefaction contribute to the fibrogenic processes that lead to CKD.
-
Therapeutic strategies aimed at preserving and/or restoring the integrity of the endothelial glycocalyx, reversing the procoagulant and pro-inflammatory phenotype of injured endothelial cells and slowing renal fibrosis hold promise for the treatment of renal disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Verma, S. K. & Molitoris, B. A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 35, 96–107 (2015).
Roumenina, L. T., Rayes, J., Frimat, M. & Fremeaux-Bacchi, V. Endothelial cells: source, barrier, and target of defensive mediators. Immunol. Rev. 274, 307–329 (2016).
Molitoris, B. A. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J. Clin. Invest. 124, 2355–2363 (2014).
Jourde-Chiche, N., Dou, L., Cerini, C., Dignat-George, F. & Brunet, P. Vascular incompetence in dialysis patients—protein-bound uremic toxins and endothelial dysfunction. Semin. Dial. 24, 327–337 (2011).
Zoccali, C. et al. The systemic nature of CKD. Nat. Rev. Nephrol. 13, 344–358 (2017).
Tarbell, J. M., Simon, S. I. & Curry, F. R. Mechanosensing at the vascular interface. Annu. Rev. Biomed. Eng. 16, 505–532 (2014).
Aird, W. C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2, a006429 (2012).
Chi, J. T. et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl Acad. Sci. USA 100, 10623–10628 (2003).
Satchell, S. C. & Braet, F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 296, F947–F956 (2009).
Satchell, S. The role of the glomerular endothelium in albumin handling. Nat. Rev. Nephrol. 9, 717–725 (2013).
Rabelink, T. J. & de Zeeuw, D. The glycocalyx—linking albuminuria with renal and cardiovascular disease. Nat. Rev. Nephrol. 11, 667–676 (2015).
Stan, R. V., Kubitza, M. & Palade, G. E. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc. Natl Acad. Sci. USA 96, 13203–13207 (1999).
Rabelink, T. J., Wijewickrama, D. C. & de Koning, E. J. Peritubular endothelium: the Achilles heel of the kidney? Kidney Int. 72, 926–930 (2007).
Shaw, I., Rider, S., Mullins, J., Hughes, J. & Peault, B. Pericytes in the renal vasculature: roles in health and disease. Nat. Rev. Nephrol. 14, 521–534 (2018).
Kramann, R. & Humphreys, B. D. Kidney pericytes: roles in regeneration and fibrosis. Semin. Nephrol. 34, 374–383 (2014).
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Pober, J. S. & Sessa, W. C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 (2007).
Sartain, S. E., Turner, N. A. & Moake, J. L. TNF regulates essential alternative complement pathway components and impairs activation of protein C in human glomerular endothelial cells. J. Immunol. 196, 832–845 (2016).
Rezaie, A. R. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb. Haemost. 112, 876–882 (2014).
Frank, R. D. et al. The synthetic pentasaccharide fondaparinux reduces coagulation, inflammation and neutrophil accumulation in kidney ischemia-reperfusion injury. J. Thromb. Haemost. 3, 531–540 (2005).
Nomura, K. et al. Roles of coagulation pathway and factor Xa in rat mesangioproliferative glomerulonephritis. Lab Invest. 87, 150–160 (2007).
Moussa, L., Apostolopoulos, J., Davenport, P., Tchongue, J. & Tipping, P. G. Protease-activated receptor-2 augments experimental crescentic glomerulonephritis. Am. J. Pathol. 171, 800–808 (2007).
Chung, H., Ramachandran, R., Hollenberg, M. D. & Muruve, D. A. Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-beta receptor signaling pathways contributes to renal fibrosis. J. Biol. Chem. 288, 37319–37331 (2013).
Oe, Y. et al. Coagulation factor Xa and protease-activated receptor 2 as novel therapeutic targets for diabetic nephropathy. Arterioscler Thromb. Vasc. Biol. 36, 1525–1533 (2016).
Kohan, D. E., Inscho, E. W., Wesson, D. & Pollock, D. M. Physiology of endothelin and the kidney. Compr. Physiol. 1, 883–919 (2011).
Rossi, G. P. et al. Endothelial factors in the pathogenesis and treatment of chronic kidney disease Part I: General mechanisms: a joint consensus statement from the European Society of Hypertension Working Group on Endothelin and Endothelial Factors and The Japanese Society of Hypertension. J. Hypertens. 36, 451–461 (2018).
Daehn, I. et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Invest. 124, 1608–1621 (2014).
Kohan, D. E. & Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 86, 896–904 (2014).
Siragy, H. M. & Carey, R. M. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am. J. Nephrol. 31, 541–550 (2010).
Clark, S. J. et al. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J. Immunol. 190, 2049–2057 (2013).
Louise, C. B. & Obrig, T. G. Human renal microvascular endothelial cells as a potential target in the development of the hemolytic uremic syndrome as related to fibrinolysis factor expression, in vitro. Microvasc. Res. 47, 377–387 (1994).
Roumenina, L. T. et al. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood 119, 4182–4191 (2012).
Du, L. et al. Interleukin-1β increases permeability and upregulates the expression of vascular endothelial-cadherin in human renal glomerular endothelial cells. Mol. Med. Rep. 11, 3708–3714 (2015).
Murakami, S. et al. Expression of adhesion molecules by cultured human glomerular endothelial cells in response to cytokines: comparison to human umbilical vein and dermal microvascular endothelial cells. Microvasc. Res. 62, 383–391 (2001).
Betzen, C. et al. Shiga toxin 2a-induced endothelial injury in hemolytic uremic syndrome: a metabolomic analysis. J. Infect. Dis. 213, 1031–1040 (2016).
Gomez, S. A. et al. The oxidative stress induced in vivo by Shiga toxin-2 contributes to the pathogenicity of haemolytic uraemic syndrome. Clin. Exp. Immunol. 173, 463–472 (2013).
Merle, N. S. et al. Characterization of renal injury and inflammation in an experimental model of intravascular hemolysis. Front. Immunol. 9, 179 (2018).
Merle, N. et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 3, 96910 (2018).
Frimat, M. et al. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 122, 282–292 (2013).
Roumenina, L. T., Rayes, J., Lacroix-Desmazes, S. & Dimitrov, J. D. Heme: modulator of plasma systems in hemolytic diseases. Trends Mol. Med. 22, 200–213 (2016).
Dejana, E., Hirschi, K. K. & Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun. 8, 14361 (2017).
Li, J., Qu, X. & Bertram, J. F. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol. 175, 1380–1388 (2009).
Egorova, A. D. et al. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ. Res. 108, 1093–1101 (2011).
Camenisch, T. D. et al. Temporal and distinct TGFβ ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 248, 170–181 (2002).
Basile, D. P. et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Renal Physiol. 300, F721–F733 (2011).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Xavier, S. et al. Curtailing endothelial TGFβ signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J. Am. Soc. Nephrol. 26, 817–829 (2015).
Huang, X. et al. Loss of caveolin-1 promotes endothelial-mesenchymal transition during sepsis: a membrane proteomic study. Int. J. Mol. Med. 32, 585–592 (2013).
Stasi, A. et al. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol. Dial. Transplant. 32, 24–31 (2017).
Xu-Dubois, Y. C. et al. Markers of endothelial-to-mesenchymal transition: evidence for antibody-endothelium interaction during antibody-mediated rejection in kidney recipients. J. Am. Soc. Nephrol. 27, 324–332 (2016).
Goligorsky, M. S. Endothelial progenitor cells: from senescence to rejuvenation. Semin. Nephrol. 34, 365–373 (2014).
Sabatier, F., Camoin-Jau, L., Anfosso, F., Sampol, J. & Dignat-George, F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J. Cell. Mol. Med. 13, 454–471 (2009).
Ridger, V. C. et al. Microvesicles in vascular homeostasis and diseases. Position paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb. Haemost. 117, 1296–1316 (2017).
Woywodt, A. et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J. Thromb. Haemost. 4, 671–677 (2006).
Yao, G. et al. Evaluation of renal vascular lesions using circulating endothelial cells in patients with lupus nephritis. Rheumatology (Oxford) 47, 432–436 (2008).
Koc, M. et al. Circulating endothelial cells are associated with future vascular events in hemodialysis patients. Kidney Int. 67, 1078–1083 (2005).
Faure, V. et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J. Thromb. Haemost. 4, 566–573 (2006).
Amabile, N. et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 16, 3381–3388 (2005).
Karpman, D., Stahl, A. L. & Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 13, 545–562 (2017).
Kirsch, T. et al. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood 109, 2854–2862 (2007).
Haubitz, M., Dhaygude, A. & Woywodt, A. Mechanisms and markers of vascular damage in ANCA-associated vasculitis. Autoimmunity 42, 605–614 (2009).
Erdbruegger, U. et al. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology (Oxford) 47, 1820–1825 (2008).
Gao, C. et al. Thrombotic role of blood and endothelial cells in uremia through phosphatidylserine exposure and microparticle release. PLOS ONE 10, e0142835 (2015).
Gondouin, B. et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 84, 733–744 (2013).
Combes, V. et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Invest. 104, 93–102 (1999).
Dignat-George, F. et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb. Haemost. 91, 667–673 (2004).
Karpman, D. et al. Complement interactions with blood cells, endothelial cells and microvesicles in thrombotic and inflammatory conditions. Adv. Exp. Med. Biol. 865, 19–42 (2015).
Renner, B. et al. Cyclosporine induces endothelial cell release of complement-activating microparticles. J. Am. Soc. Nephrol. 24, 1849–1862 (2013).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Ito, T., Suzuki, A., Imai, E., Okabe, M. & Hori, M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J. Am. Soc. Nephrol. 12, 2625–2635 (2001).
Rookmaaker, M. B. et al. Bone-marrow-derived cells contribute to glomerular endothelial repair in experimental glomerulonephritis. Am. J. Pathol. 163, 553–562 (2003).
Schirutschke, H. et al. Injured kidney endothelium is only marginally repopulated by cells of extrarenal origin. Am. J. Physiol. Renal Physiol. 305, F1042–F1052 (2013).
Sangidorj, O. et al. Bone marrow-derived endothelial progenitor cells confer renal protection in a murine chronic renal failure model. Am. J. Physiol. Renal Physiol. 299, F325–F335 (2010).
Hillebrands, J. L., Klatter, F. A., van Dijk, W. D. & Rozing, J. Bone marrow does not contribute substantially to endothelial-cell replacement in transplant arteriosclerosis. Nat. Med. 8, 194–195 (2002).
Lekakis, J. et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur. J. Cardiovasc. Prev. Rehabil. 18, 775–789 (2011).
Rabelink, T. J., de Boer, H. C. & van Zonneveld, A. J. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat. Rev. Nephrol. 6, 404–414 (2010).
Noël, L.-H. Atlas de Pathologie Rénale (Médecine Sciences Flammarion, 2008).
Babickova, J. et al. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 91, 70–85 (2017).
Lachmann, P. et al. Interference with Gsα-coupled receptor signaling in renin-producing cells leads to renal endothelial damage. J. Am. Soc. Nephrol. 28, 3479–3489 (2017).
Lee, C. J. et al. The clinicopathologic significance of endothelial tubuloreticular inclusions in glomerular diseases. Ultrastruct. Pathol. 37, 386–394 (2013).
Schiessl, I. M., Hammer, A., Riquier-Brison, A. & Peti-Peterdi, J. Just look! Intravital microscopy as the best means to study kidney cell death dynamics. Semin. Nephrol. 36, 220–236 (2016).
Hackl, M. J. et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat. Med. 19, 1661–1666 (2013).
Diaspro, A. et al. Multi-photon excitation microscopy. Biomed. Eng. Online 5, 36 (2006).
Salmon, A. H. et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 23, 1339–1350 (2012).
Chrobak, K. M., Potter, D. R. & Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 71, 185–196 (2006).
Onoe, H. et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 12, 584–590 (2013).
Huling, J., Ko, I. K., Atala, A. & Yoo, J. J. Fabrication of biomimetic vascular scaffolds for 3D tissue constructs using vascular corrosion casts. Acta Biomater. 32, 190–197 (2016).
Soo, J. Y., Jansen, J., Masereeuw, R. & Little, M. H. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat. Rev. Nephrol. 14, 378–393 (2018).
Xinaris, C. et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J. Am. Soc. Nephrol. 23, 1857–1868 (2012).
Fakhouri, F., Zuber, J., Fremeaux-Bacchi, V. & Loirat, C. Haemolytic uraemic syndrome. Lancet 390, 681–696 (2017).
Keepers, T. R., Gross, L. K. & Obrig, T. G. Monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and RANTES recruit macrophages to the kidney in a mouse model of hemolytic-uremic syndrome. Infect. Immun. 75, 1229–1236 (2007).
Mallick, E. M. et al. A novel murine infection model for Shiga toxin-producing Escherichia coli. J. Clin. Invest. 122, 4012–4024 (2012).
Warnier, M. et al. Trafficking of Shiga toxin/Shiga-like toxin-1 in human glomerular microvascular endothelial cells and human mesangial cells. Kidney Int. 70, 2085–2091 (2006).
Obrig, T. G. et al. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268, 15484–15488 (1993).
Basu, D. & Tumer, N. E. Do the A subunits contribute to the differences in the toxicity of Shiga toxin 1 and Shiga toxin 2? Toxins (Basel) 7, (1467–1485 (2015).
Petruzziello-Pellegrini, T. N. et al. The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice. J. Clin. Invest. 122, 759–776 (2012).
Morigi, M. et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J. Immunol. 187, 172–180 (2011).
Nolasco, L. H. et al. Hemolytic uremic syndrome-associated Shiga toxins promote endothelial-cell secretion and impair ADAMTS13 cleavage of unusually large von Willebrand factor multimers. Blood 106, 4199–4209 (2005).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Belizna, C., Duijvestijn, A., Hamidou, M. & Tervaert, J. W. Antiendothelial cell antibodies in vasculitis and connective tissue disease. Ann. Rheum. Dis. 65, 1545–1550 (2006).
van Paassen, P. et al. Induction of endothelial cell apoptosis by IgG antibodies from SLE patients with nephropathy: a potential role for anti-endothelial cell antibodies. Ann. NY Acad. Sci. 1108, 147–156 (2007).
Perry, G. J. et al. Antiendothelial cell antibodies in lupus: correlations with renal injury and circulating markers of endothelial damage. Q. J. Med. 86, 727–734 (1993).
D’Cruz, D. P. et al. Antibodies to endothelial cells in systemic lupus erythematosus: a potential marker for nephritis and vasculitis. Clin. Exp. Immunol. 85, 254–261 (1991).
Kondo, A. et al. The level of IgA antibodies to endothelial cells correlates with histological evidence of disease activity in patients with lupus nephritis. PLOS ONE 11, e0163085 (2016).
Skaggs, B. J., Hahn, B. H. & McMahon, M. Accelerated atherosclerosis in patients with SLE—mechanisms and management. Nat. Rev. Rheumatol 8, 214–223 (2012).
Vasilev, V. V. et al. Functional characterization of autoantibodies against complement component C3 in patients with lupus nephritis. J. Biol. Chem. 290, 25343–25355 (2015).
Denny, M. F. et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110, 2907–2915 (2007).
Kahlenberg, J. M. et al. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J. Immunol. 187, 6143–6156 (2011).
Miyakis, S. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 4, 295–306 (2006).
Bienaime, F., Legendre, C., Terzi, F. & Canaud, G. Antiphospholipid syndrome and kidney disease. Kidney Int. 91, 34–44 (2017).
de Groot, P. G. & Urbanus, R. T. The significance of autoantibodies against β2-glycoprotein I. Blood 120, 266–274 (2012).
Du, V. X., Kelchtermans, H., de Groot, P. G. & de Laat, B. From antibody to clinical phenotype, the black box of the antiphospholipid syndrome: pathogenic mechanisms of the antiphospholipid syndrome. Thromb. Res. 132, 319–326 (2013).
Steinkasserer, A., Estaller, C., Weiss, E. H., Sim, R. B. & Day, A. J. Complete nucleotide and deduced amino acid sequence of human beta 2-glycoprotein I. Biochem. J. 277, 387–391 (1991).
Nonaka, M., Matsuda, Y., Shiroishi, T., Moriwaki, K. & Natsuume-Sakai, S. Molecular cloning of mouse beta 2-glycoprotein I and mapping of the gene to chromosome 11. Genomics 13, 1082–1087 (1992).
Giannakopoulos, B. & Krilis, S. A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 368, 1033–1044 (2013).
Corban, M. T. et al. Antiphospholipid syndrome: role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J. Am. Coll. Cardiol. 69, 2317–2330 (2017).
Holers, V. M. et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 195, 211–220 (2002).
Pierangeli, S. S. et al. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 52, 2120–2124 (2005).
Girardi, G. et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 112, 1644–1654 (2003).
Oku, K. et al. Complement and thrombosis in the antiphospholipid syndrome. Autoimmun Rev. 15, 1001–1004 (2016).
Loupy, A. et al. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transplant. 17, 28–41 (2017).
Everly, M. J. et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 95, 410–417 (2013).
Wiebe, C. et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am. J. Transplant. 15, 2921–2930 (2015).
Lefaucheur, C. et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J. Am. Soc. Nephrol. 21, 1398–1406 (2010).
Stegall, M. D., Chedid, M. F. & Cornell, L. D. The role of complement in antibody-mediated rejection in kidney transplantation. Nat. Rev. Nephrol. 8, 670–678 (2012).
Jindra, P. T., Jin, Y. P., Rozengurt, E. & Reed, E. F. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J. Immunol. 180, 2357–2366 (2008).
Tible, M. et al. Pathologic classification of antibody-mediated rejection correlates with donor-specific antibodies and endothelial cell activation. J. Heart Lung Transplant. 32, 769–776 (2013).
Blogowski, W. et al. Clinical analysis of perioperative complement activity during ischemia/reperfusion injury following renal transplantation. Clin. J. Am. Soc. Nephrol. 7, 1843–1851 (2012).
Roumenina, L. T., Zuber, J. & Fremeaux-Bacchi, V. Physiological and therapeutic complement regulators in kidney transplantation. Curr. Opin. Organ Transplant. 18, 421–429 (2013).
Jane-Wit, D. et al. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-κB signaling in endothelial cells. Circulation 128, 2504–2516 (2013).
Stites, E., Le Quintrec, M. & Thurman, J. M. The complement system and antibody-mediated transplant rejection. J. Immunol. 195, 5525–5531 (2015).
Hirohashi, T. et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am. J. Transplant. 12, 313–321 (2012).
Roda, J. M. et al. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 66, 517–526 (2006).
Hidalgo, L. G. et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am. J. Transplant. 10, 1812–1822 (2010).
Loupy, A. et al. Gene expression profiling for the identification and classification of antibody-mediated heart rejection. Circulation 135, 917–935 (2017).
Legris, T. et al. Antibody-dependent NK cell activation is associated with late kidney allograft dysfunction and the complement-independent alloreactive potential of donor-specific antibodies. Front. Immunol. 7, 288 (2016).
Cardinal, H., Dieude, M. & Hebert, M. J. The emerging importance of non-HLA autoantibodies in kidney transplant complications. J. Am. Soc. Nephrol. 28, 400–406 (2017).
Cross, A. R., Glotz, D. & Mooney, N. The role of the endothelium during antibody-mediated rejection: from victim to accomplice. Front. Immunol. 9, 106 (2018).
Lahat, N., Bitterman, H., Weiss-Cerem, L. & Rahat, M. A. Hypoxia increases membranal and secreted HLA-DR in endothelial cells, rendering them T cell activators. Transpl. Int. 24, 1018–1026 (2011).
Pober, J. S., Merola, J., Liu, R. & Manes, T. D. Antigen presentation by vascular cells. Front. Immunol. 8, 1907 (2017).
Taflin, C. et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc. Natl Acad. Sci. USA 108, 2891–2896 (2011).
Jennette, J. C. & Falk, R. J. Small-vessel vasculitis. N. Engl. J. Med. 337, 1512–1523 (1997).
Lee, K. H. et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev. 16, 1160–1173 (2017).
Williams, J. M. et al. Activation of the Gi heterotrimeric G protein by ANCA IgG F(ab’)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J. Am. Soc. Nephrol. 14, 661–669 (2003).
Reumaux, D., Hordijk, P. L., Duthilleul, P. & Roos, D. Priming by tumor necrosis factor-alpha of human neutrophil NADPH-oxidase activity induced by anti-proteinase-3 or anti-myeloperoxidase antibodies. J. Leukoc. Biol. 80, 1424–1433 (2006).
Choi, M. et al. Endothelial NF-κB blockade abrogates ANCA-induced GN. J. Am. Soc. Nephrol. 28, 3191–3204 (2017).
Schreiber, A. et al. Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J. Am. Soc. Nephrol. 23, 470–482 (2012).
Jarrot, P. A. & Kaplanski, G. Pathogenesis of ANCA-associated vasculitis: an update. Autoimmun Rev. 15, 704–713 (2016).
Schreiber, A., Luft, F. C. & Kettritz, R. Phagocyte NADPH oxidase restrains the inflammasome in ANCA-induced GN. J. Am. Soc. Nephrol. 26, 411–424 (2015).
Sorensen, O. E. & Borregaard, N. Neutrophil extracellular traps - the dark side of neutrophils. J. Clin. Invest. 126, 1612–1620 (2016).
Xiao, H., Schreiber, A., Heeringa, P., Falk, R. J. & Jennette, J. C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170, 52–64 (2007).
Wang, H., Wang, C., Zhao, M. H. & Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 181, 518–527 (2015).
Katayama, S. et al. Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit. Care 21, 229 (2017).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part I: molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015).
Merle, N. S., Noe, R., Halbwachs-Mecarelli, L., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part II: role in immunity. Front. Immunol. 6, 257 (2015).
Blanc, C. et al. Overall neutralization of complement factor H by autoantibodies in the acute phase of the autoimmune form of atypical hemolytic uremic syndrome. J. Immunol. 189, 3528–3537 (2012).
Jozsi, M. et al. Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood 110, 1516–1518 (2007).
Merinero, H. M. et al. Complete functional characterization of disease-associated genetic variants in the complement factor H gene. Kidney Int. 93, 470–481 (2018).
Liszewski, M. K. & Atkinson, J. P. Complement regulator CD46: genetic variants and disease associations. Hum. Genom. 9, 7 (2015).
Bienaime, F. et al. Mutations in components of complement influence the outcome of Factor I-associated atypical hemolytic uremic syndrome. Kidney Int. 77, 339–349 (2010).
Fremeaux-Bacchi, V. et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112, 4948–4952 (2008).
Schramm, E. C. et al. Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood 125, 2359–2369 (2015).
Goicoechea de Jorge, E. et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc. Natl Acad. Sci. USA 104, 240–245 (2007).
Marinozzi, M. C. et al. Complement factor B mutations in atypical hemolytic uremic syndrome-disease-relevant or benign? J. Am. Soc. Nephrol. 25, 2053–2065 (2014).
Lemaire, M. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat. Genet. 45, 531–536 (2013).
Delvaeye, M. et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 345–357 (2009).
Bruneau, S. et al. Loss of DGKepsilon induces endothelial cell activation and death independently of complement activation. Blood 125, 1038–1046 (2015).
Ueda, Y., Gullipalli, D. & Song, W. C. Modeling complement-driven diseases in transgenic mice: values and limitations. Immunobiology 221, 1080–1090 (2016).
Pickering, M. C. et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 31, 424–428 (2002).
de Jorge, E. G. et al. The development of atypical hemolytic uremic syndrome depends on complement C5. J. Am. Soc. Nephrol. 22, 137–145 (2011).
Vernon, K. A. et al. Partial complement factor H deficiency associates with C3 glomerulopathy and thrombotic microangiopathy. J. Am. Soc. Nephrol. 27, 1334–1342 (2016).
Pickering, M. C. et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J. Exp. Med. 204, 1249–1256 (2007).
Ueda, Y. et al. Murine systemic thrombophilia and hemolytic uremic syndrome from a factor H point mutation. Blood 129, 1184–1196 (2017).
Ueda, Y. et al. Blocking properdin prevents complement-mediated hemolytic uremic syndrome and systemic thrombophilia. J. Am. Soc. Nephrol. 29, 1928–1937 (2018).
Bekassy, Z. D. et al. Aliskiren inhibits renin-mediated complement activation. Kidney Int. 94, 689–700 (2018).
Sansbury, F. H. et al. Factors determining penetrance in familial atypical haemolytic uraemic syndrome. J. Med. Genet. 51, 756–764 (2014).
Rodriguez de Cordoba, S., Hidalgo, M. S., Pinto, S. & Tortajada, A. Genetics of atypical hemolytic uremic syndrome (aHUS). Semin. Thromb. Hemost. 40, 422–430 (2014).
Roumenina, L. T. et al. Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood 114, 2837–2845 (2009).
Noris, M. et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood 124, 1715–1726 (2014).
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Shalaby, F. et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 (1995).
Fong, G. H., Rossant, J., Gertsenstein, M. & Breitman, M. L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376, 66–70 (1995).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Jin, J. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151, 384–399 (2012).
Venkatesha, S. et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649 (2006).
Levine, R. J. et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 355, 992–1005 (2006).
Izzedine, H. et al. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine (Baltimore) 93, 333–339 (2014).
Thadhani, R. et al. Removal of soluble Fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J. Am. Soc. Nephrol. 27, 903–913 (2016).
Le Roux, S. et al. Elevated soluble Flt1 inhibits endothelial repair in PR3-ANCA-associated vasculitis. J. Am. Soc. Nephrol. 23, 155–164 (2012).
Ritz, E. & Orth, S. R. Nephropathy in patients with type 2 diabetes mellitus. N. Engl. J. Med. 341, 1127–1133 (1999).
Thomas, M. C., Cooper, M. E. & Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 12, 73–81 (2016).
Tervaert, T. W. et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 21, 556–563 (2010).
Najafian, B., Alpers, C. E. & Fogo, A. B. Pathology of human diabetic nephropathy. Contrib. Nephrol. 170, 36–47 (2011).
Gilbert, R. E. The endothelium in diabetic nephropathy. Curr. Atheroscler. Rep. 16, 410 (2014).
Cheng, H., Wang, H., Fan, X., Paueksakon, P. & Harris, R. C. Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int. 82, 1176–1183 (2012).
Toyoda, M., Najafian, B., Kim, Y., Caramori, M. L. & Mauer, M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 56, 2155–2160 (2007).
Weil, E. J. et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 82, 1010–1017 (2012).
Peng, H. et al. High glucose induces activation of the local reninangiotensin system in glomerular endothelial cells. Mol. Med. Rep. 9, 450–456 (2014).
Ho, F. M., Liu, S. H., Liau, C. S., Huang, P. J. & Lin-Shiau, S. Y. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH2-terminal kinase and caspase-3. Circulation 101, 2618–2624 (2000).
Isermann, B. et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 13, 1349–1358 (2007).
Singh, A. et al. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am. J. Physiol. Renal Physiol. 300, F40–F48 (2011).
van den Born, J. et al. Reduction of heparan sulphate-associated anionic sites in the glomerular basement membrane of rats with streptozotocin-induced diabetic nephropathy. Diabetologia 38, 1169–1175 (1995).
van den Hoven, M. J. et al. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 70, 2100–2108 (2006).
Rabelink, T. J. et al. Heparanase: roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat. Rev. Nephrol. 13, 201–212 (2017).
van den Hoven, M. J. et al. Regulation of glomerular heparanase expression by aldosterone, angiotensin II and reactive oxygen species. Nephrol. Dial. Transplant. 24, 2637–2645 (2009).
An, X. et al. The receptor for advanced glycation endproducts mediates podocyte heparanase expression through NF-κB signaling pathway. Mol. Cell Endocrinol. (2017).
Goldberg, R. et al. Role of heparanase-driven inflammatory cascade in pathogenesis of diabetic nephropathy. Diabetes 63, 4302–4313 (2014).
Xu, G. et al. Heparanase-driven inflammation from the AGEs-stimulated macrophages changes the functions of glomerular endothelial cells. Diabetes Res. Clin. Pract. 124, 30–40 (2017).
Sward, P. & Rippe, B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol. (Oxf.) 204, 294–307 (2012).
Kelly, D. J. et al. Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 52, 512–518 (2003).
Majumder, S. & Advani, A. VEGF and the diabetic kidney: more than too much of a good thing. J. Diabetes Complicat. 31, 273–279 (2017).
Hohenstein, B. et al. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 69, 1654–1661 (2006).
Cheng, H. & Harris, R. C. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc. Hematol. Disord. Drug Targets 14, 22–33 (2014).
Rajah, T. T., Olson, A. L. & Grammas, P. Differential glucose uptake in retina- and brain-derived endothelial cells. Microvasc. Res. 62, 236–242 (2001).
Alpert, E. et al. Delayed autoregulation of glucose transport in vascular endothelial cells. Diabetologia 48, 752–755 (2005).
Mapanga, R. F. & Essop, M. F. Damaging effects of hyperglycemia on cardiovascular function: spotlight on glucose metabolic pathways. Am. J. Physiol. Heart Circ. Physiol. 310, H153–H173 (2016).
Frimat, M. et al. Kidney, heart and brain: three organs targeted by ageing and glycation. Clin. Sci. (Lond.) 131, 1069–1092 (2017).
Wautier, M. P. et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 280, E685–E694 (2001).
Daroux, M. et al. Advanced glycation end-products: implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 36, 1–10 (2010).
Schaffer, S. W., Jong, C. J. & Mozaffari, M. Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascul. Pharmacol. 57, 139–149 (2012).
Jaimes, E. A., Hua, P., Tian, R. X. & Raij, L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am. J. Physiol. Renal Physiol. 298, F125–F132 (2010).
Kiritoshi, S. et al. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes 52, 2570–2577 (2003).
Toth, E. et al. Contribution of polyol pathway to arteriolar dysfunction in hyperglycemia. Role of oxidative stress, reduced NO, and enhanced PGH(2)/TXA(2) mediation. Am. J. Physiol. Heart Circ. Physiol. 293, H3096–H3104 (2007).
Ha, H. & Lee, H. B. Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and in diabetic kidney. Nephrology (Carlton) 10 (Suppl), S7–S10 (2005).
Sutariya, B., Jhonsa, D. & Saraf, M. N. TGF-β: the connecting link between nephropathy and fibrosis. Immunopharmacol. Immunotoxicol. 38, 39–49 (2016).
Buhl, E. M. et al. The role of PDGF-D in healthy and fibrotic kidneys. Kidney Int. 89, 848–861 (2016).
Chen, Y. T. et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80, 1170–1181 (2011).
Shi, S. et al. Interactions of DPP-4 and integrin β1 influences endothelial-to-mesenchymal transition. Kidney Int. 88, 479–489 (2015).
Kanasaki, K. et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 63, 2120–2131 (2014).
Makino, H. et al. Decreased circulating CD34+ cells are associated with progression of diabetic nephropathy. Diabet. Med. 26, 171–173 (2009).
Jarajapu, Y. P. et al. Blockade of NADPH oxidase restores vasoreparative function in diabetic CD34+ cells. Invest. Ophthalmol. Vis. Sci. 52, 5093–5104 (2011).
Shigiyama, F. et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 16, 84 (2017).
Klag, M. J. et al. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 334, 13–18 (1996).
Freedman, B. I. & Cohen, A. H. Hypertension-attributed nephropathy: what’s in a name? Nat. Rev. Nephrol. 12, 27–36 (2016).
Ehret, G. B. et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 48, 1171–1184 (2016).
Ehret, G. B. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011).
Freedman, B. I. et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J. Am. Soc. Nephrol. 21, 1422–1426 (2010).
Maruhashi, T. et al. Endothelial function is impaired in patients receiving antihypertensive drug treatment regardless of blood pressure level: FMD-J study (flow-mediated dilation Japan). Hypertension 70, 790–797 (2017).
Higashi, Y. et al. Effects of L-arginine infusion on renal hemodynamics in patients with mild essential hypertension. Hypertension 25, 898–902 (1995).
Panza, J. A., Quyyumi, A. A., Brush, J. E. Jr & Epstein, S. E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 323, 22–27 (1990).
Bellien, J. et al. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation 125, 1266–1275 (2012).
Zoccali, C. Endothelial dysfunction in subcutaneous small resistance arteries and cardiovascular events. J. Hypertens. 24, 1900–1901; author reply 1901–1902 (2006).
Boffa, J. J., Tharaux, P. L., Dussaule, J. C. & Chatziantoniou, C. Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 37, 490–496 (2001).
Ochodnický, P. et al. Renal vascular dysfunction precedes the development of renal damage in the hypertensive Fawn-Hooded rat. Am. J. Physiol. Renal Physiol. 298, F625–F633 (2010).
Caillon, A. et al. γδ T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation 135, 2155–2162 (2017).
Norlander, A. E. et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2, 92801 (2017).
Mennuni, S. et al. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 28, 74–79 (2014).
Puddu, P., Puddu, G. M., Galletti, L., Cravero, E. & Muscari, A. Mitochondrial dysfunction as an initiating event in atherogenesis: a plausible hypothesis. Cardiology 103, 137–141 (2005).
Dikalova, A. E. et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 107, 106–116 (2010).
Choi, H., Tostes, R. C. & Webb, R. C. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J. Am. Soc. Hypertens. 5, 154–160 (2011).
Landmesser, U. & Drexler, H. Endothelial function and hypertension. Curr. Opin. Cardiol. 22, 316–320 (2007).
Guerrot, D. et al. Progression of renal fibrosis: the underestimated role of endothelial alterations. Fibrogen. Tissue Repair 5(Suppl. 1), S15 (2012).
Ponnuchamy, B. & Khalil, R. A. Cellular mediators of renal vascular dysfunction in hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1001–R1018 (2009).
Seccia, T. M. et al. Endothelin-1 drives epithelial-mesenchymal transition in hypertensive nephroangiosclerosis. J. Am. Heart Assoc. 5, e003888 (2016).
Hirata, Y. et al. Endothelial function and cardiovascular events in chronic kidney disease. Int. J. Cardiol. 173, 481–486 (2014).
Chen, J. et al. Interrelationship of multiple endothelial dysfunction biomarkers with chronic kidney disease. PLOS ONE 10, e0132047 (2015).
Recio-Mayoral, A., Banerjee, D., Streather, C. & Kaski, J. C. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease—a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 216, 446–451 (2011).
Verbeke, F. H. et al. Flow-mediated vasodilation in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 6, 2009–2015 (2011).
Khandelwal, P. et al. Dyslipidemia, carotid intima-media thickness and endothelial dysfunction in children with chronic kidney disease. Pediatr. Nephrol. 31, 1313–1320 (2016).
Kari, J. A. et al. Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int. 52, 468–472 (1997).
Brunet, P. et al. Does uremia cause vascular dysfunction? Kidney Blood Press Res. 34, 284–290 (2011).
Duranton, F. et al. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 23, 1258–1270 (2012).
Rossi, M. et al. Uremic toxin development in living kidney donors: a longitudinal study. Transplantation 97, 548–554 (2014).
Vanholder, R., Schepers, E., Pletinck, A., Nagler, E. V. & Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J. Am. Soc. Nephrol. 25, 1897–1907 (2014).
Jourde-Chiche, N. et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J. Thromb. Haemost. 7, 1576–1584 (2009).
Dou, L. et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 5, 1302–1308 (2007).
Dou, L. et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 26, 876–887 (2015).
Addi, T. et al. Mechanisms of tissue factor induction by the uremic toxin indole-3 acetic acid through aryl hydrocarbon receptor/nuclear factor-κ B signaling pathway in human endothelial cells. Arch. Toxicol. https://doi.org/10.1007/s00204-018-2328-3 (2018).
Sallee, M. et al. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 6, 934–949 (2014).
Yang, K. et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J. Am. Soc. Nephrol. 26, 2434–2446 (2015).
Barreto, F. C. et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 4, 1551–1558 (2009).
Six, I. et al. Deleterious vascular effects of indoxyl sulfate and reversal by oral adsorbent AST-120. Atherosclerosis 243, 248–256 (2015).
Yu, M., Kim, Y. J. & Kang, D. H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 6, 30–39 (2011).
Gil, N. et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes 61, 208–216 (2012).
Packham, D. K. et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 23, 123–130 (2012).
Lewis, E. J. et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am. J. Kidney Dis. 58, 729–736 (2011).
Boels, M. G. et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes 65, 2429–2439 (2016).
Heerspink, H. J. L. et al. Rationale and protocol of the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes. Metab. 20, 1369–1376 (2018).
Raghunathan, V. et al. Targeting renin-angiotensin system in malignant hypertension in atypical hemolytic uremic syndrome. Indian J. Nephrol. 27, 136–140 (2017).
Verhave, J. C., Wetzels, J. F. & van de Kar, N. C. Novel aspects of atypical haemolytic uraemic syndrome and the role of eculizumab. Nephrol. Dial. Transplant. 29(Suppl. 4), iv131–iv141 (2014).
Mortara, A. Lo studio RELAX-AHF [Italian]. G. Ital. Cardiol. (Rome) 16, 193–197 (2015).
Legendre, C. et al. Eculizumab in renal transplantation. Transplant Rev. (Orlando) 27, 90–92 (2013).
Noris, M., Mescia, F. & Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8, 622–633 (2012).
Loos, S., Oh, J. & Kemper, M. J. Eculizumab in STEC-HUS: need for a proper randomized controlled trial. Pediatr. Nephrol. 33, 1277–1281 (2018).
Le Quintrec, M. et al. Patterns of clinical response to eculizumab in patients with C3 glomerulopathy. Am. J. Kidney Dis. 72, 84–92 (2018).
Oosterveld, M. J. et al. Eculizumab in pediatric dense deposit disease. Clin. J. Am. Soc. Nephrol. 10, 1773–1782 (2015).
Jayne, D. R. W. et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 2756–2767 (2017).
Ka, S. M. et al. Kidney-targeting Smad7 gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear factor κB (NF-κB) signalling pathways, and improves diabetic nephropathy in mice. Diabetologia 55, 509–519 (2012).
Shen, W. C. et al. Indoxyl sulfate enhances IL-1β-induced E-selectin expression in endothelial cells in acute kidney injury by the ROS/MAPKs/NFκB/AP-1 pathway. Arch. Toxicol. 90, 2779–2792 (2016).
Borensztajn, K., Peppelenbosch, M. P. & Spek, C. A. Factor Xa: at the crossroads between coagulation and signaling in physiology and disease. Trends Mol. Med. 14, 429–440 (2008).
Tanaka, M. et al. Role of coagulation factor Xa and protease-activated receptor 2 in human mesangial cell proliferation. Kidney Int. 67, 2123–2133 (2005).
Shimosawa, M. et al. Lipopolysaccharide-triggered acute aggravation of mesangioproliferative glomerulonephritis through activation of coagulation in a high IgA strain of ddY mice. Nephron Exp. Nephrol. 112, e81–e91 (2009).
Sumi, A. et al. Roles of coagulation pathway and factor Xa in the progression of diabetic nephropathy in db/db mice. Biol. Pharm. Bull. 34, 824–830 (2011).
Tillet, S. et al. Kidney graft outcome using an anti-Xa therapeutic strategy in an experimental model of severe ischaemia-reperfusion injury. Br. J. Surg. 102, 132–142; discussion 142 (2015).
Annane, D. et al. Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am. J. Respir. Crit. Care Med. 187, 1091–1097 (2013).
Yamakawa, K. et al. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J. Thromb. Haemost. 13, 508–519 (2015).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Lin, J. R. et al. Suppression of endothelial-to-mesenchymal transition by SIRT (Sirtuin) 3 alleviated the development of hypertensive renal injury. Hypertension 72, 350–360 (2018).
Cooley, B. C. et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl Med. 6, 227ra34 (2014).
Mahmoud, M. M. et al. TWIST1 integrates endothelial responses to flow in vascular dysfunction and atherosclerosis. Circ. Res. 119, 450–462 (2016).
Mahmoud, M. M. et al. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci. Rep. 7, 3375 (2017).
Acknowledgements
The authors are grateful to all members of the French Renal Endothelial Society (FRENDS) for fruitful discussions. FRENDS is a collaborative group of nephrologists and researchers working on the endothelium in different renal diseases. The research was supported by grants from the Agence Nationale de la Recherche (ANR) JCJC–INFLACOMP 2015–2018 (ANR-15-CE15-0001; to L.T.R.); INSERM (to L.T.R.); EU FP7 grant 2012–305608 (EURenOmics; to V.F.-B.); Assistance Publique-Hôpitaux de Paris (AP-HP)-Programme Hospitaliser de Recherche Clinique (PHRC) AOM08198 (to V.F.-B.); Association pour l'Information et la Recherche sur les maladies rénales Génétiques (AIRG) (to V.F.-B.); the French Society of Nephrology (to V.F.-B.); a grant from Appel d'Offre de Recherche Clinique (AORC) 2011-A01347 (to G.K.); and Assistance Publique-Hôpitaux de Marseille (AP-HM)-PHRC grant 2012-A00217-36 (to G.K.).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussing the article’s content and writing, reviewing and editing the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
F.F. has received consultancy fees and/or speaker honoraria from Alexion and Roche. M.L.Q. has received consultancy fees and/or speaker honoraria from Alexion. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Glossary
- Uraemic toxins
-
Blood compounds that are normally eliminated in the urine but accumulate in patients with chronic kidney disease or acute kidney injury and exert deleterious effects.
- Flow-mediated dilation
-
Noninvasive, ultrasonography-based test of endothelial function that measures brachial arterial diameter in response to a substantial increase in arterial flow after the release of brachial constriction with a cuff. Flow-mediated dilation reflects the ability of endothelial cells to secrete nitric oxide (NO) and induce arterial vasodilation in response to shear stress. Alterations in flow-mediated dilation represent an early marker of endothelial dysfunction and can be reversed with endothelial recovery.
- Tubulo-reticular inclusions
-
Cytoplasmic clusters of tubule-like structures arising from the membrane of the endoplasmic reticulum, thought to result from activation of type I interferons in endothelial cells and lymphocytes.
- Intravital multiphoton microscopy
-
(IVM). A form of microscopy that enables the observation of biological processes in living animals (owing to its low energy and phototoxicity, which allow the prolonged exposure of tissues) with high resolution owing to the deep penetration of tissues by photons.
- Oxidative burst
-
Also known as respiratory burst, oxidative burst is the rapid release of reactive oxygen species (ROS) from phagocytes (such as neutrophils or macrophages) to degrade internalized particles or pathogens within the phagosome. Primed neutrophils can also degranulate and undergo an oxidative burst in the absence of pathogens, as in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, leading to surrounding tissue damage.
- Neutrophil extracellular traps
-
(NETs). NETs are composed of DNA in association with histones and granular proteins such as neutrophil elastase and myeloperoxidase (MPO). They are released by neutrophils in a process called NETosis, which allows pathogens to be captured in a bactericidal net and destroyed. NETs can also cause tissue damage, such as in ANCA-associated vasculitis, or increase the exposure of autoantigens to the immune system, such as in lupus.
- Cell priming
-
Cell priming of neutrophils is the transition from a steady state with limited antimicrobial activity to an activated state, allowing the rapid enhancement of phagocytic activity and oxidative burst upon a second stimulation. Neutrophils can be primed by microbial products, chemo-attractants and inflammatory cytokines.
- Azurophilic granules
-
Azurophilic granules of neutrophils are intracytoplasmic vesicles containing peptides with antimicrobial activity, such as MPO, proteinase 3 (PR3), α-defensins, elastase, cathepsin G and bactericidal/permeability increasing protein (BPI).
- Histone-dependent cytotoxicity
-
Pro-inflammatory and cytotoxic signalling induced by extracellular histones. Histones and DNA normally reside in the nucleus and form nucleosomes. Extracellular histones are damage-associated molecular patterns (DAMPs), which elicit pro-inflammatory signalling through Toll-like receptors (TLRs) and promote cell death.
- Opsonization
-
Tagging of a pathogen, a cell or an apoptotic body, with opsonins, which increases their interaction with phagocytes and natural killer (NK) cells. These opsonins can be antibodies (immunoglobulin G (IgG) or IgE) or complement fractions (C3b or C4b).
- Heparanase
-
An enzyme that degrades polymeric heparan sulfate molecules into shorter-chain-length oligosaccharides. The action of heparanase disrupts the endothelial glycocalyx.
- The polyol pathway
-
Pathway that converts hexose sugars such as glucose into sugar alcohols (polyols). In this metabolic pathway, glucose is reduced to sorbitol (by the aldose reductase), which is then converted to fructose (by sorbitol dehydrogenase). The polyol pathway is activated in hyperglycaemia, owing to the saturation of physiological glucose metabolism, and leads to a cellular oxidative stress.
- Sorbitol
-
Sugar alcohol derived from glucose through the action of aldose reductase. Sorbitol is a component of the polyol pathway.
- eNOS uncoupling
-
Occurs when endothelial NO synthase (eNOS) is not coupled with its substrate (mainly l-arginine) or cofactors. eNOS uncoupling results in the production of the pro-oxidative superoxide anion (O2−) instead of NO, which characterizes endothelial dysfunction.
- Prostanoids
-
Physiologically active lipid compounds that are metabolites of the fatty acid arachidonic acid. Prostanoids comprise prostaglandins (which have vasodilator and anti-aggregant properties) and thromboxanes (which are potent vasoconstrictors that also activate platelet aggregation).
- Incretins
-
Glucose-lowering hormones secreted by the stomach during a meal. Incretins increase the release of insulin from pancreatic β-cells, slow gastric emptying and inhibit glucagon release from pancreatic α-cells. The two main incretins are glucagon-like peptide 1 (GLP1) and gastric inhibitory peptide (GIP), which are both rapidly inactivated by the enzyme dipeptidyl peptidase 4 (DPP4).
- Autoregulation
-
Autoregulation of renal blood is a homeostatic mechanism, relying both on myogenic response of afferent arterioles (vasoconstriction in case of transmural pressure elevation) and tubulo-glomerular feedback (depending on the sensing of sodium chloride delivery to the macula densa). Autoregulation protects the glomerular capillaries from elevations in arterial pressure and allows the kidney to maintain a fairly constant blood flow and glomerular filtration rate.
- Indolic compounds
-
Chemical compounds comprising an aromatic bicyclic structure resembling that of indole (C8H7N). Indolic uraemic compounds result from tryptophan metabolism by the gut and include indoxyl sulfate, indole-3 acetic acid and indoxyl-β-d-glucuronide.
- Dioxin
-
Also known as 2,3,7,8-tetrachlorodibenzodioxin (TCDD), dioxin and dioxin-like compounds are chemical pollutants of the environment, resulting from industrial practices (mostly incineration processes) and from forest fires and volcanic eruptions. Dioxin is the exogenous ligand of the transcription factor aryl hydrocarbon receptor (AhR). Exposure to dioxins, mostly through ingestion of contaminated food, is mutagenic and increases the risks of cardiovascular diseases.
Rights and permissions
About this article
Cite this article
Jourde-Chiche, N., Fakhouri, F., Dou, L. et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol 15, 87–108 (2019). https://doi.org/10.1038/s41581-018-0098-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0098-z
This article is cited by
-
Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review
Cell Communication and Signaling (2024)
-
Role of endothelial hyaluronan in peritoneal membrane transport and disease conditions during peritoneal dialysis
Scientific Reports (2024)
-
Entry and exit of extracellular vesicles to and from the blood circulation
Nature Nanotechnology (2024)
-
A guide to complement biology, pathology and therapeutic opportunity
Nature Reviews Immunology (2024)
-
Damage to endothelial barriers and its contribution to long COVID
Angiogenesis (2024)