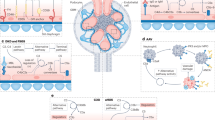
Abstract
Increasing evidence indicates an integral role for the complement system in the deleterious inflammatory reactions that occur during critical phases of the transplantation process, such as brain or cardiac death of the donor, surgical trauma, organ preservation and ischaemia–reperfusion injury, as well as in humoral and cellular immune responses to the allograft. Ischaemia is the most common cause of complement activation in kidney transplantation and in combination with reperfusion is a major cause of inflammation and graft damage. Complement also has a prominent role in antibody-mediated rejection (ABMR) owing to ABO and HLA incompatibility, which leads to devastating damage to the transplanted kidney. Emerging drugs and treatment modalities that inhibit complement activation at various stages in the complement cascade are being developed to ameliorate the damage caused by complement activation in transplantation. These promising new therapies have various potential applications at different stages in the process of transplantation, including inhibiting the destructive effects of ischaemia and/or reperfusion injury, treating ABMR, inducing accommodation and modulating the adaptive immune response.
Key points
-
Complement activation in the donor, the graft and the recipient before, during and after transplantation is a major cause of damage to the kidney transplant.
-
Ischaemia and subsequent reperfusion of the graft is the most important mechanism that triggers complement activation; reperfusion is generally regarded as the most detrimental phase of the transplantation process.
-
Following transplantation, complement has a role in innate immunological and inflammatory processes that further damage the graft and result in a gradual decrease in its functional mass.
-
Complement-targeted strategies might have a role in optimizing graft quality as well as in the treatment of antibody-mediated rejection, induction of accommodation and modulation of the adaptive immune response.
-
Promising data from preclinical and clinical studies suggest that complement-targeted therapies could potentially become part of the standard of care for kidney transplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rana, A. et al. Survival outcomes following pediatric liver transplantation (Pedi-SOFT) score: a novel predictive index. Am. J. Transplant. 15, 1855–1863 (2015).
Vautmans, H. & Jakovc˘ic´, I. Organ donation and transplant in the EU – progress but much more to do. European Commision http://ec.europa.eu/health/newsletter/183/focus_newsletter_en.htm (2016).
Colvin, R. B. & Smith, R. N. Antibody-mediated organ-allograft rejection. Nat. Rev. Immunol. 5, 807–817 (2005).
Ekberg, H. et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl. J. Med. 357, 2562–2575 (2007).
Halloran, P. F. et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J. Am. Soc. Nephrol. 26, 1711–1720 (2015).
Halloran, P. F., Famulski, K. S. & Reeve, J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat. Rev. Nephrol. 12, 534–548 (2016).
D’Alessandro, A. M. et al. Living unrelated renal donation: the University of Wisconsin experience. Surgery 124, 604–610; discussion 610–611 (1998).
Terasaki, P. I., Cecka, J. M., Gjertson, D. W. & Takemoto, S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 333, 333–336 (1995).
Voiculescu, A. et al. Kidney transplantation from related and unrelated living donors in a single German centre. Nephrol. Dial. Transplant. 18, 418–425 (2003).
Yarlagadda, S. G., Coca, S. G., Formica, R. N., Poggio, E. D. & Parikh, C. R. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol. Dial. Transplant. 24, 1039–1047 (2009).
Farrar, C. A., Kupiec-Weglinski, J. W. & Sacks, S. H. The innate immune system and transplantation. Cold Spring Harb. Perspect. Med. 3, a015479 (2013).
Baldwin, W. M., Ota, H. & Rodriguez, E. R. Complement in transplant rejection: diagnostic and mechanistic considerations. Springer Semin. Immunopathol. 25, 181–197 (2003).
Damman, J. et al. Targeting complement activation in brain-dead donors improves renal function after transplantation. Transpl. Immunol. 24, 233–237 (2011).
Lin, T., Zhou, W. & Sacks, S. H. The role of complement and Toll-like receptors in organ transplantation. Transpl. Int. 20, 481–489 (2007).
Sacks, S. H. & Zhou, W. The role of complement in the early immune response to transplantation. Nat. Rev. Immunol. 12, 431–442 (2012).
Cravedi, P. & Heeger, P. S. Complement as a multifaceted modulator of kidney transplant injury. J. Clin. Invest. 124, 2348–2354 (2014).
Fuquay, R. et al. Renal ischemia-reperfusion injury amplifies the humoral immune response. J. Am. Soc. Nephrol. 24, 1063–1072 (2013).
Wu, W. K., Famure, O., Li, Y. & Kim, S. J. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 88, 851–858 (2015).
Mizuno, M., Suzuki, Y. & Ito, Y. Complement regulation and kidney diseases: recent knowledge of the double-edged roles of complement activation in nephrology. Clin. Exp. Nephrol. 22, 3–14 (2018).
Reis, E. S. et al. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology 220, 476–482 (2015).
Ekdahl, K. N., Soveri, I., Hilborn, J., Fellstrom, B. & Nilsson, B. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat. Rev. Nephrol. 13, 285–296 (2017).
Mares, J. et al. Proteomic profiling of blood-dialyzer interactome reveals involvement of lectin complement pathway in hemodialysis-induced inflammatory response. Proteomics Clin. Appl. 4, 829–838 (2010).
Huang, Z., Gao, D., Letteri, J. J. & Clark, W. R. Blood-membrane interactions during dialysis. Semin. Dial. 22, 623–628 (2009).
Nilsson, B., Ekdahl, K. N., Mollnes, T. E. & Lambris, J. D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 44, 82–94 (2007).
Andersson, J., Ekdahl, K. N., Larsson, R., Nilsson, U. R. & Nilsson, B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J. Immunol. 168, 5786–5791 (2002).
Tengvall, P., Askendal, A. & Lundström, I. Complement activation by IgG immobilized on methylated silicon. J. Biomed. Mater. Res. 31, 305–312 (1996).
Van Biesen, W., Veys, N., Vanholder, R. & Lameire, N. The impact of the pre-transplant renal replacement modality on outcome after cadaveric kidney transplantation: the ghent experience. Contrib. Nephrol. 150, 254–258 (2006).
Fehrman-Ekholm, I., Elinder, C. G., Stenbeck, M., Tydén, G. & Groth, C. G. Kidney donors live longer. Transplantation 64, 976–978 (1997).
Ibrahim, H. N. et al. Long-term consequences of kidney donation. N. Engl. J. Med. 360, 459–469 (2009).
Kiberd, B. A. & Tennankore, K. K. Lifetime risks of kidney donation: a medical decision analysis. BMJ Open 7, e016490 (2017).
Damman, J. et al. Hypoxia and complement-and-coagulation pathways in the deceased organ donor as the major target for intervention to improve renal allograft outcome. Transplantation 99, 1293–1300 (2015).
Blogowski, W. et al. Clinical analysis of perioperative complement activity during ischemia/reperfusion injury following renal transplantation. Clin. J. Am. Soc. Nephrol. 7, 1843–1851 (2012).
Damman, J. et al. Systemic complement activation in deceased donors is associated with acute rejection after renal transplantation in the recipient. Transplantation 92, 163–169 (2011).
Burk, A.-M. et al. Early complementopathy after multiple injuries in humans. Shock 37, 348–354 (2012).
Halbgebauer, R. et al. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J. Crit. Care 44, 229–237 (2017).
Huber-Lang, M., Lambris, J. D. & Ward, P. A. Innate immune responses to trauma. Nat. Immunol. 19, 327–341 (2018).
van Griensven, M. et al. Protective effects of the complement inhibitor compstatin CP40 in hemorrhagic shock. Shock https://doi.org/10.1097/SHK.0000000000001127 (2018).
Brown, K. M. et al. Influence of donor C3 allotype on late renal-transplantation outcome. N. Engl. J. Med. 354, 2014–2023 (2006).
Damman, J. et al. Association of complement C3 gene variants with renal transplant outcome of deceased cardiac dead donor kidneys. Am. J. Transplant. 12, 660–668 (2012).
Sim, E. & Sim, R. B. Enzymic assay of C3b receptor on intact cells and solubilized cells. Biochem. J. 210, 567–576 (1983).
Denk, S. et al. Complement C5a functions as a master switch for the pH balance in neutrophils exerting fundamental immunometabolic effects. J. Immunol. 198, 4846–4854 (2017).
Farrar, C. A. et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J. Clin. Invest. 126, 1911–1925 (2016).
Kolár˘ová, H., Ambru˚zová, B., Svihálková Šindlerová, L., Klinke, A. & Kubala, L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm. 2014, 694312–694317 (2014).
Sieve, I., Münster-Kühnel, A. K. & Hilfiker-Kleiner, D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul. Pharmacol. 100, 26–33 (2018).
Yang, G. et al. Novel mechanisms of endothelial dysfunction in diabetes. J. Cardiovasc. Dis. Res. 1, 59–63 (2010).
Nguyen, H. X., Galvan, M. D. & Anderson, A. J. Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal cord injury. J. Neuroinflamm. 5, 26 (2008).
Triantafilou, K., Hughes, T. R., Triantafilou, M. & Morgan, B. P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell. Sci. 126, 2903–2913 (2013).
Danobeitia, J. et al. Complement blockade prevents delayed graft function in a non-human primate model of kidney allo-transplantation [abstract]. Am. J Transplant. 13 (Suppl. 5), 119 (2013).
Mathern, D. R. & Heeger, P. S. Molecules great and small: the complement system. Clin. J. Am. Soc. Nephrol. 10, 1636–1650 (2015).
Pratt, J. R., Basheer, S. A. & Sacks, S. H. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat. Med. 8, 582–587 (2002).
Farrar, C. A., Zhou, W., Lin, T. & Sacks, S. H. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 20, 217–226 (2006).
Damman, J. et al. Local renal complement C3 induction by donor brain death is associated with reduced renal allograft function after transplantation. Nephrol. Dial. Transplant. 26, 2345–2354 (2011).
Siedlecki, A., Irish, W. & Brennan, D. C. Delayed graft function in the kidney transplant. Am. J. Transplant. 11, 2279–2296 (2011).
Kapitsinou, P. P. & Haase, V. H. Molecular mechanisms of ischemic preconditioning in the kidney. Am. J. Physiol. Renal Physiol. 309, F821–F834 (2015).
de Vries, D. K. et al. Acute but transient release of terminal complement complex after reperfusion in clinical kidney transplantation. Transplantation 95, 816–820 (2013).
Castellano, G. et al. Complement modulation of anti-aging factor klotho in ischemia/reperfusion injury and delayed graft function. Am. J. Transplant. 16, 325–333 (2015).
Delpech, P.-O. et al. Inhibition of complement improves graft outcome in a pig model of kidney autotransplantation. J. Transl Med. 14, 701–713 (2016).
Thurman, J. M. et al. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 17, 707–715 (2006).
Asgari, E. et al. Mannan-binding lectin-associated serine protease 2 is critical for the development of renal ischemia reperfusion injury and mediates tissue injury in the absence of complement C4. FASEB J. 28, 3996–4003 (2014).
Walsh, M. C. et al. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J. Immunol. 175, 541–546 (2005).
Orsini, F. et al. Mannan binding lectin-associated serine protease-2 (MASP-2) critically contributes to post-ischemic brain injury independent of MASP-1. J. Neuroinflamm. 13, 213 (2016).
Einecke, G. et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am. J. Transplant. 9, 2520–2531 (2009).
Haas, M. et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transplant. 14, 272–283 (2014).
Wang, H., Ricklin, D. & Lambris, J. D. Complement-activation fragment C4a mediates effector functions by binding as untethered agonist to protease-activated receptors 1 and 4. Proc. Natl Acad. Sci. USA 114, 10948–10953 (2017).
Laumonnier, Y., Karsten, C. M. & Köhl, J. Novel insights into the expression pattern of anaphylatoxin receptors in mice and men. Mol. Immunol. 89, 44–58 (2017).
Valenzuela, N. M., Mulder, A. & Reed, E. F. HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcγRs. J. Immunol. 190, 6635–6650 (2013).
Tedesco, F. et al. The cytolytically inactive terminal complement component complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J. Exp. Med. 185, 1619–1627 (1997).
Brunn, G. J. Differential regulation of endothelial cell activation by complement and interleukin 1. Circ. Res. 98, 793–800 (2006).
Foreman, K. E. et al. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Invest. 94, 1147–1155 (1994).
Ikeda, K. et al. C5a induces tissue factor activity on endothelial cells. Thromb. Haemost. 77, 394–398 (1997).
Jane-wit, D. et al. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-B signaling in endothelial cells. Circulation 128, 2504–2516 (2013).
Stegall, M. D., Chedid, M. F. & Cornell, L. D. The role of complement in antibody-mediated rejection in kidney transplantation. Nat. Rev. Nephrol. 8, 670–678 (2012).
Panda, S. & Ding, J. L. Natural antibodies bridge innate and adaptive immunity. J. Immunol. 194, 13–20 (2015).
Garcia de Mattos Barbosa, M., Cascalho, M. & Platt, J. L. Accommodation in ABO-incompatible organ transplants. Xenotransplantation 25, e12418 (2018).
Sheil, A. G., Stewart, J. H., Tiller, D. J. & May, J. ABO blood group incompatibility in renal transplantation. Transplantation 8, 299–300 (1969).
Ugurlar, D. et al. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 359, 794–797 (2018).
De Clippel, D. et al. Screening for HLA antibodies in plateletpheresis donors with a history of transfusion or pregnancy. Transfusion 54, 3036–3042 (2014).
Saadi, S., Takahashi, T., Holzknecht, R. A. & Platt, J. L. Pathways to acute humoral rejection. Am. J. Pathol. 164, 1073–1080 (2004).
Valenzuela, N. M., McNamara, J. T. & Reed, E. F. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr. Opin. Organ Transplant. 19, 33–40 (2014).
Dahlbäck, B. & Hildebrand, B. Degradation of human complement component C4b in the presence of the C4b-binding protein-protein S complex. Biochem. J. 209, 857–863 (1983).
Hamer, R. et al. Human leukocyte antigen-specific antibodies and gamma-interferon stimulate human microvascular and glomerular endothelial cells to produce complement factor C4. Transplantation 93, 867–873 (2012).
Loupy, A. et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N. Engl. J. Med. 369, 1215–1226 (2013).
Sicard, A. et al. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J. Am. Soc. Nephrol. 26, 457–467 (2015).
Lefaucheur, C. et al. Complement-activating anti-HLA antibodies in kidney transplantation: allograft gene expression profiling and response to treatment. J. Am. Soc. Nephrol. 29, 620–635 (2018).
Zipfel, P. F. et al. The role of complement in C3 glomerulopathy. Mol. Immunol. 67, 21–30 (2015).
Sethi, S. & Fervenza, F. C. Membranoproliferative glomerulonephritis—a new look at an old entity. N. Engl. J. Med. 366, 1119–1131 (2012).
Le Quintrec, M. et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am. J. Transplant. 13, 663–675 (2013).
Salvadori, M. & Bertoni, E. Complement related kidney diseases: recurrence after transplantation. World J. Transplant. 6, 632–645 (2016).
Poppelaars, F. et al. C1-inhibitor treatment decreases renal injury in an established brain-dead rat model. Transplantation 102, 79–87 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02435732 (2017).
Lewis, A. G., Kohl, G., Ma, Q., Devarajan, P. & Kohl, J. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin. Exp. Immunol. 153, 117–126 (2008).
Rich, M. C. et al. Site-targeted complement inhibition by a complement receptor 2-conjugated inhibitor (mTT30) ameliorates post-injury neuropathology in mouse brains. Neurosci. Lett. 617, 188–194 (2016).
Ruseva, M. M., Ramaglia, V., Morgan, B. P. & Harris, C. L. An anticomplement agent that homes to the damaged brain and promotes recovery after traumatic brain injury in mice. Proc. Natl Acad. Sci. USA 112, 14319–14324 (2015).
Yu, Z. X. et al. Targeting complement pathways during cold ischemia and reperfusion prevents delayed graft function. Am. J. Transplant. 16, 2589–2597 (2016).
Emlen, W., Li, W. & Kirschfink, M. Therapeutic complement inhibition: new developments. Semin. Thromb. Hemost. 36, 660–668 (2010).
Ricklin, D., Mastellos, D. C., Reis, E. S. & Lambris, J. D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 14, 26–47 (2018).
Parker, C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet 373, 759–767 (2009).
Zuber, J. et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat. Rev. Nephrol. 8, 643–657 (2012).
Howard, J. F. et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve 48, 76–84 (2013).
Burbach, M. et al. Report of the inefficacy of eculizumab in two cases of severe antibody-mediated rejection of renal grafts. Transplantation 98, 1056–1059 (2014).
Cornell, L. D., Schinstock, C. A., Gandhi, M. J., Kremers, W. K. & Stegall, M. D. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am. J. Transplant. 15, 1293–1302 (2015).
González-Roncero, F. et al. Eculizumab treatment of acute antibody-mediated rejection in renal transplantation: case reports. Transplant. Proc. 44, 2690–2694 (2012).
Locke, J. E. et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am. J. Transplant. 9, 231–235 (2009).
Stegall, M. D. et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am. J. Transplant. 11, 2405–2413 (2011).
Yelken, B. et al. Eculizumab for treatment of refractory antibody-mediated rejection in kidney transplant patients: a single-center experience. Transplant. Proc. 47, 1754–1759 (2015).
Orandi, B. J. et al. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 98, 857–863 (2014).
Biglarnia, A.-R. et al. Prompt reversal of a severe complement activation by eculizumab in a patient undergoing intentional ABO-incompatible pancreas and kidney transplantation. Transplant Int. 24, e61–e66 (2011).
West-Thielke, P. et al. Eculizumab for prevention of antibody-mediated rejection in blood group-incompatible renal transplantation. Transplant. Proc. 50, 66–69 (2018).
Bentall, A. et al. Antibody-mediated rejection despite inhibition of terminal complement. Transpl. Int. 27, 1235–1243 (2014).
Alexion. Alexion provides update on phase 2 clinical trial with eculizumab in antibody mediated rejection (AMR) in living-donor kidney transplant recipients. AlexionPharma https://news.alexionpharma.com/press-release/company-news/alexion-provides-update-phase-2-clinical-trial-eculizumab-antibody-mediat (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01399593 (2018).
Harder, M. J. et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood 129, 970–980 (2017).
Kirschfink, M. C1-inhibitor and transplantation. Immunobiology 205, 534–541 (2002).
Tillou, X. et al. Recombinant human C1-inhibitor prevents acute antibody-mediated rejection in alloimmunized baboons. Kidney Int. 78, 152–159 (2010).
Vo, A. A. et al. A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients. Transplantation 99, 299–308 (2015).
Viglietti, D. et al. C1 inhibitor in acute antibody-mediated rejection nonresponsive to conventional therapy in kidney transplant recipients: a pilot study. Am. J. Transplant. 16, 1596–1603 (2016).
Montgomery, R. A. et al. Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: results of a randomized double-blind placebo-controlled pilot study. Am. J. Transplant. 16, 3468–3478 (2016).
Halloran, P. F., Reeve, J. P., Pereira, A. B., Hidalgo, L. G. & Famulski, K. S. Antibody-mediated rejection, T cell–mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 85, 258–264 (2014).
Eskandary, F. et al. Anti-C1s monoclonal antibody BIVV009 in late antibody-mediated kidney allograft rejection-results from a first-in-patient phase 1 trial. Am. J. Transplant. 8, 670–926 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03347396 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03316521 (2018).
Mastellos, D. C. et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur. J. Clin. Invest. 45, 423–440 (2015).
Qu, H. et al. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology 218, 496–505 (2013).
Pawel-Rammingen, von, U. & Björck, L. IdeS and SpeB: immunoglobulin-degrading cysteine proteinases of Streptococcus pyogenes. Curr. Opin. Microbiol. 6, 50–55 (2003).
Brezski, R. J. et al. Tumor-associated and microbial proteases compromise host IgG effector functions by a single cleavage proximal to the hinge. Proc. Natl Acad. Sci. USA 106, 17864–17869 (2009).
Jordan, S. C. et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N. Engl. J. Med. 377, 442–453 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02224820 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02426684 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02475551 (2018).
Platt, J. L. et al. Transplantation of discordant xenografts: a review of progress. Immunol. Today 11, 450–456 (1990).
Park, W. D. et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am. J. Transplant. 3, 952–960 (2003).
Zhong, S. et al. Complement inhibition enables renal allograft accommodation and long-term engraftment in presensitized nonhuman primates. Am. J. Transplant. 11, 2057–2066 (2011).
Narayanan, K., Jendrisak, M. D., Phelan, D. L. & Mohanakumar, T. HLA class I antibody mediated accommodation of endothelial cells via the activation of PI3K/cAMP dependent PKA pathway. Transpl. Immunol. 15, 187–197 (2006).
Dijke, E. I. et al. B cells in transplantation. J. Heart Lung Transplant. 35, 704–710 (2016).
Chen Song, S. et al. Complement inhibition enables renal allograft accommodation and long-term engraftment in presensitized nonhuman primates. Am. J. Transplant. 11, 2057–2066 (2011).
Dehoux, J.-P. & Gianello, P. Accommodation and antibodies. Transpl. Immunol. 21, 106–110 (2009).
Benson, B. A., Vercellotti, G. M. & Dalmasso, A. P. IL-4 and IL-13 induce protection from complement and melittin in endothelial cells despite initial loss of cytoplasmic proteins: membrane resealing impairs quantifying cytotoxicity with the lactate dehydrogenase permeability assay. Xenotransplantation 22, 295–301 (2015).
Suhr, B. D., Black, S. M., Guzman-Paz, M., Matas, A. J. & Dalmasso, A. P. Inhibition of the membrane attack complex of complement for induction of accommodation in the hamster-to-rat heart transplant model. Xenotransplantation 14, 572–579 (2007).
Tan, C. D. et al. Correlation of donor-specific antibodies, complement and its regulators with graft dysfunction in cardiac antibody-mediated rejection. Am. J. Transplant. 9, 2075–2084 (2009).
Griesemer, A. D. et al. Upregulation of CD59: potential mechanism of accommodation in a large animal model. Transplantation 87, 1308–1317 (2009).
Platt, J. L., Kaufman, C. L., Garcia de Mattos Barbosa, M. & Cascalho, M. Accommodation and related conditions in vascularized composite allografts. Curr. Opin. Organ Transplant. 22, 470–476 (2017).
Bannett, A. D., McAlack, R. F., Morris, M., Chopek, M. W. & Platt, J. L. ABO incompatible renal transplantation: a qualitative analysis of native endothelial tissue ABO antigens after transplantation. Transplant. Proc. 21, 783–785 (1989).
Chopek, M. W., Simmons, R. L. & Platt, J. L. ABO-incompatible kidney transplantation: initial immunopathologic evaluation. Transplant. Proc. 19, 4553–4557 (1987).
Wang, H. et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J. Immunol. 179, 4451–4463 (2007).
Wang, H. et al. Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation 79, 1121–1127 (2005).
Vogel, C.-W. & Fritzinger, D. C. Cobra venom factor: structure, function, and humanization for therapeutic complement depletion. Toxicon 56, 1198–1222 (2010).
Montero, R. M., Sacks, S. H. & Smith, R. A. Complement-here, there and everywhere, but what about the transplanted organ? Semin. Immunol. 28, 250–259 (2016).
Pepys, M. B. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J. Exp. Med. 140, 126–145 (1974).
Carroll, M. C. Complement and humoral immunity. Vaccine 26 (Suppl. 8), I28–133 (2008).
Heyman, B., Wiersma, E. J. & Kinoshita, T. In vivo inhibition of the antibody response by a complement receptor-specific monoclonal antibody. J. Exp. Med. 172, 665–668 (1990).
Carter, R. H. & Fearon, D. T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 256, 105–107 (1992).
Prodeus, A. P. et al. A critical role for complement in maintenance of self-tolerance. Immunity 9, 721–731 (1998).
Sacks, S., Lee, Q., Wong, W. & Zhou, W. The role of complement in regulating the alloresponse. Curr. Opin. Organ Transplant 14, 10–15 (2009).
Heeger, P. S. & Kemper, C. Novel roles of complement in T effector cell regulation. Immunobiology 217, 216–224 (2012).
Arbore, G. et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science 352, aad1210 (2016).
Quell, K. M. et al. Monitoring C3aR expression using a floxed tdTomato-C3aR reporter knock-in mouse. J. Immunol. 199, 688–706 (2017).
Karsten, C. M. et al. Monitoring C5aR2 expression using a floxed tdTomato-C5aR2 knock-in mouse. J. Immunol. 199, 3234–3248 (2017).
Kwan, W.-H., van der Touw, W., Paz-Artal, E., Li, M. O. & Heeger, P. S. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 210, 257–268 (2013).
Strainic, M. G. et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28, 425–435 (2008).
Le Friec, G., Köhl, J. & Kemper, C. A complement a day keeps the Fox(p3) away. Nat. Immunol. 14, 110–112 (2013).
Ellinghaus, U. et al. Dysregulated CD46 shedding interferes with Th1-contraction in systemic lupus erythematosus. Eur. J. Immunol. 47, 1200–1210 (2017).
Strainic, M. G., Shevach, E. M., An, F., Lin, F. & Medof, M. E. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat. Immunol. 14, 162–171 (2013).
van der Touw, W. et al. Cutting edge: receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J. Immunol. 190, 5921–5925 (2013).
Braza, F., Durand, M., Degauque, N. & Brouard, S. Regulatory T cells in kidney transplantation: new directions? Am. J. Transplant. 15, 2288–2300 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02129881 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02088931 (2016).
Jiménez-Reinoso, A. et al. Human plasma C3 is essential for the development of memory B, but not T, lymphocytes. J. Allergy Clin. Immunol. 141, 1151–1154.e14 (2017).
Gueler, F. et al. Complement 5a receptor inhibition improves renal allograft survival. J. Am. Soc. Nephrol. 19, 2302–2312 (2008).
Li, Q. et al. Deficiency of C5aR prolongs renal allograft survival. J. Am. Soc. Nephrol. 21, 1344–1353 (2010).
Farrar, C. A., Zhou, W. & Sacks, S. H. Role of the lectin complement pathway in kidney transplantation. Immunobiology 221, 1068–1072 (2016).
Wijkstrom, M. et al. Islet allograft survival in nonhuman primates immunosuppressed with basiliximab, RAD, and FTY7201. Transplantation 77, 827–835 (2004).
Atkinson, J. P., Oglesby, T. J., White, D., Adams, E. A. & Liszewski, M. K. Separation of self from non-self in the complement system: a role for membrane cofactor protein and decay accelerating factor. Clin. Exp. Immunol. 86 (Suppl. 1), 27–30 (1991).
Cooper, D. K. C., Ekser, B., Ramsoondar, J., Phelps, C. & Ayares, D. The role of genetically engineered pigs in xenotransplantation research. J. Pathol. 238, 288–299 (2016).
Yamanaka, K. et al. Depression of complement regulatory factors in rat and human renal grafts is associated with the progress of acute T-cell mediated rejection. PLOS ONE 11, e0148881 (2016).
Souza, D. G., Esser, D., Bradford, R., Vieira, A. T. & Teixeira, M. M. APT070 (Mirococept), a membrane-localised complement inhibitor, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br. J. Pharmacol. 145, 1027–1034 (2005).
Patel, H. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J. Am. Soc. Nephrol. 17, 1102–1111 (2006).
Kassimatis, T. et al. A double-blind randomised controlled investigation into the efficacy of Mirococept (APT070) for preventing ischaemia reperfusion injury in the kidney allograft (EMPIRIKAL): study protocol for a randomised controlled trial. Trials 18, 2279–2211 (2017).
Nilsson, P. H. et al. Autoregulation of thromboinflammation on biomaterial surfaces by a multicomponent therapeutic coating. Biomaterials 34, 985–994 (2013).
Hinglais, N. et al. Immunohistochemical study of the C5b-9 complex of complement in human kidneys. Kidney Int. 30, 399–410 (1986).
Okada, M. et al. Immunohistochemical localization of C3d fragment of complement and S-protein (vitronectin) in normal and diseased human kidneys: association with the C5b-9 complex and vitronectin receptor. Virchows Arch. A Pathol. Anat. Histopathol. 422, 367–373 (1993).
Sacks, S. H., Zhou, W., Pani, A., Campbell, R. D. & Martin, J. Complement C3 gene expression and regulation in human glomerular epithelial cells. Immunology 79, 348–354 (1993).
Mekori, Y. A., Steiner, P., Farkash, R., Moalem, T. & Klajman, A. Deposits of immunoglobulins and C3 in the walls of human renal arteries. Clin. Exp. Immunol. 43, 254–259 (1981).
Feucht, H. E. et al. Detection of both isotypes of complement C4, C4A and C4B, in normal human glomeruli. Kidney Int. 30, 932–936 (1986).
Zwirner, J., Felber, E., Herzog, V., Riethmüller, G. & Feucht, H. E. Classical pathway of complement activation in normal and diseased human glomeruli. Kidney Int. 36, 1069–1077 (1989).
Song, D., Zhou, W., Sheerin, S. H. & Sacks, S. H. Compartmental localization of complement component transcripts in the normal human kidney. Nephron 78, 15–22 (1998).
Cosio, F. G., Sedmak, D. D., Mahan, J. D. & Nahman, N. S. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 36, 100–107 (1989).
Nakanishi, I. et al. Identification and characterization of membrane cofactor protein (CD46) in the human kidneys. Eur. J. Immunol. 24, 1529–1535 (1994).
Endoh, M. et al. Immunohistochemical demonstration of membrane cofactor protein (MCP) of complement in normal and diseased kidney tissues. Clin. Exp. Immunol. 94, 182–188 (1993).
Ichida, S., Yuzawa, Y., Okada, H., Yoshioka, K. & Matsuo, S. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 46, 89–96 (1994).
Jokiranta, T. S. et al. Binding of complement factor H to endothelial cells is mediated by the carboxy-terminal glycosaminoglycan binding site. Am. J. Pathol. 167, 1173–1181 (2005).
Lesher, A. M. & Song, W.-C. Review: complement and its regulatory proteins in kidney diseases. Nephrology (Carlton) 15, 663–675 (2010).
Appay, M. D., Kazatchkine, M. D., Levi-Strauss, M., Hinglais, N. & Bariety, J. Expression of CR1 (CD35) mRNA in podocytes from adult and fetal human kidneys. Kidney Int. 38, 289–293 (1990).
Fayyazi, A. et al. The C5a receptor is expressed in normal renal proximal tubular but not in normal pulmonary or hepatic epithelial cells. Immunology 99, 38–45 (2000).
Zahedi, R. et al. The C5a receptor is expressed by human renal proximal tubular epithelial cells. Clin. Exp. Immunol. 121, 226–233 (2000).
Braun, M. C. et al. Renal expression of the C3a receptor and functional responses of primary human proximal tubular epithelial cells. J. Immunol. 173, 4190–4196 (2004).
Li, X., Ding, F., Zhang, X., Li, B. & Ding, J. The expression profile of complement components in podocytes. Int. J. Mol. Sci. 17, 471 (2016).
Liu, L. et al. C3a, C5a renal expression and their receptors are correlated to severity of IgA nephropathy. J. Clin. Immunol. 34, 224–232 (2014).
Acknowledgements
The authors thank Deborah McClellan for excellent editorial assistance before the manuscript was submitted. The European Community’s Seventh Framework Programme under the grant agreement n°602699 (DIREKT) has been a major contributor to the authors’ work, which was further supported by grant 2016-2075-5.1 and 2016–04519 from the Swedish Research Council (VR), and by the Deutsche Forschungsgemeinschaft (DFG) grant CRC1149 A01.
Reviewer information
Nature Reviews Nephrology thanks S. Jordan, D. Ricklin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors researched the data, made substantial contributions to discussions of the content, wrote the text and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Endotheliopathy
-
Disorder of the endothelial layer leading to morphological changes of the glycocalyx, exposure of intercellular adhesion molecules and changes in the global function of the endothelium.
- Glycocalyx
-
A glycoprotein and glycolipid shield that protects the membranes of endothelial cells and other cell types.
- Anaphylatoxin
-
A complement activation product that can induce a substantial inflammatory response. C3a, C4a and C5a are anaphylatoxins.
- Nucleophile
-
A molecule that donates an electron pair to form a new covalent bond.
- Inflammasome
-
A intracellular protein complex that upon activation induces the generation of IL-1β and inflammation.
- Alloresponse
-
An immune response resulting from the recognition of antigens expressed on the surface of cells of non-self origin.
- Endopeptidase
-
A proteolytic enzyme that cleaves peptide non-terminal bonds within a protein substrate.
Rights and permissions
About this article
Cite this article
Biglarnia, AR., Huber-Lang, M., Mohlin, C. et al. The multifaceted role of complement in kidney transplantation. Nat Rev Nephrol 14, 767–781 (2018). https://doi.org/10.1038/s41581-018-0071-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0071-x
This article is cited by
-
Complement networks in gene-edited pig xenotransplantation: enhancing transplant success and addressing organ shortage
Journal of Translational Medicine (2024)
-
Peripheral blood transcriptomic analysis identifies potential inflammation and immune signatures for central retinal artery occlusion
Scientific Reports (2024)
-
Immunological risk and complement genetic evaluations in early onset de novo thrombotic microangiopathy after living donor kidney transplantation: A Japanese multicenter registry
Clinical and Experimental Nephrology (2023)
-
Activation and regulation of alloreactive T cell immunity in solid organ transplantation
Nature Reviews Nephrology (2022)
-
A role for complement blockade in kidney transplantation
Cellular & Molecular Immunology (2022)