Abstract
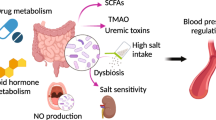
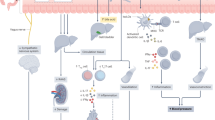
Crosstalk between the gut microbiota and the host has attracted considerable attention owing to its involvement in diverse diseases. Chronic kidney disease (CKD) is commonly associated with hypertension and is characterized by immune dysregulation, metabolic disorder and sympathetic activation, which are all linked to gut dysbiosis and altered host–microbiota crosstalk. In this Review, we discuss the complex interplay between the brain, the gut, the microbiota and the kidney in CKD and hypertension and explain our brain–gut–kidney axis hypothesis for the pathogenesis of these diseases. Consideration of the role of the brain–gut–kidney axis in the maintenance of normal homeostasis and of dysregulation of this axis in CKD and hypertension could lead to the identification of novel therapeutic targets. In addition, the discovery of unique microbial communities and their associated metabolites and the elucidation of brain–gut–kidney signalling are likely to fill fundamental knowledge gaps leading to innovative research, clinical trials and treatments for CKD and hypertension.
Key points
-
The gut microbiota has crucial roles in a variety of diseases, including hypertension and chronic kidney disease (CKD).
-
The gut microbiota communicates with the endocrine, nervous and immune systems to regulate host homeostasis, including blood pressure and kidney functions.
-
The gut–kidney axis is mediated through metabolism-dependent and immune pathways.
-
The brain–gut–kidney axis involves connections between these organs that are mediated by descending autonomic regulation from the brain and signals from the gut and the kidney, such as immune products and microbial metabolites.
-
Potential therapeutic strategies for CKD and hypertension that target the gut microbiota include dietary interventions, probiotics, prebiotics, synbiotics, faecal microbiota transplant and metabolome modulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Rao, M. V., Qiu, Y., Wang, C. & Bakris, G. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999–2004. Am. J. Kidney Dis. 51, S30–S37 (2008).
Taler, S. J. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am. J. Kidney Dis. 62, 201–213 (2013).
Inker, L. A. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 63, 713–735 (2014).
Andrassy, K. M. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 84, 622–623 (2013).
Dinan, T. G. & Cryan, J. F. Gut-brain axis in 2016: Brain-gut-microbiota axis — mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 14, 69–70 (2017).
Josefsdottir, K. S., Baldridge, M. T., Kadmon, C. S. & King, K. Y. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 129, 729–739 (2017).
Karbach, S. H. et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J. Am. Heart Assoc. 5, e003698 (2016).
Evenepoel, P., Poesen, R. & Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 32, 2005–2014 (2017).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Bercik, P. et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 23, 1132–1139 (2011).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011).
Akchurin, O. M. & Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 39, 84–92 (2015).
Shankland, S. J. & Jefferson, J. A. A bone marrow factor contributes to kidney disease. Nat. Med. 23, 13–14 (2017).
Santisteban, M. M. et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ. Res. 117, 178–191 (2015).
Carabotti, M., Scirocco, A., Maselli, M. A. & Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Cigarran Guldris, S., González Parra, E. & Cases Amenós, A. Gut microbiota in chronic kidney disease. Nefrologia 37, 9–19 (2017).
Konturek, P. C. et al. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. 66, 483–491 (2015).
Rodríguez, J. M. et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050 (2015).
Mueller, N. T., Bakacs, E., Combellick, J., Grigoryan, Z. & Dominguez-Bello, M. G. The infant microbiome development: mom matters. Trends Mol. Med. 21, 109–117 (2015).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Ou, J. et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 98, 111–120 (2013).
Walker, W. A. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr. Res. 82, 387–395 (2017).
Francavilla, R. et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr. Allergy Immunol. 23, 420–427 (2012).
Ulluwishewa, D. et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141, 769–776 (2011).
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Claesson, M. J. et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4586–4591 (2011).
Qi, Y. et al. Intestinal permeability biomarker zonulin is elevated in healthy aging. J. Am. Med. Dir. Assoc. 18, 810.e1–810.e4 (2017).
Matsumoto, M., Kurihara, S., Kibe, R., Ashida, H. & Benno, Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 6, e23652 (2011).
Minot, S. et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011).
Minot, S., Grunberg, S., Wu, G. D., Lewis, J. D. & Bushman, F. D. Hypervariable loci in the human gut virome. Proc. Natl Acad. Sci. USA 109, 3962–3966 (2012).
Minot, S. et al. Rapid evolution of the human gut virome. Proc. Natl Acad. Sci. USA 110, 12450–12455 (2013).
Zhao, G. et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl Acad. Sci. USA 114, E6166–E6175 (2017).
Iliev, I. D. & Leonardi, I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 17, 635–646 (2017).
Nguyen, T. L., Vieira-Silva, S., Liston, A. & Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 8, 1–16 (2015).
Atuma, C., Strugala, V., Allen, A. & Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G922–G929 (2001).
McDermott, A. J. & Huffnagle, G. B. The microbiome and regulation of mucosal immunity. Immunology 142, 24–31 (2014).
Furness, J. B., Callaghan, B. P., Rivera, L. R. & Cho, H. J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71 (2014).
Zheng, L., Kelly, C. J. & Colgan, S. P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 309, C350–C360 (2015).
Worthington, J. J., Reimann, F. & Gribble, F. M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 11, 3–20 (2018).
Psichas, A. et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39, 424–429 (2015).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Samuel, B. S. et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl Acad. Sci. USA 105, 16767–16772 (2008).
Donohoe, D. R. et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48, 612–626 (2012).
Raqib, R. et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl Acad. Sci. USA 103, 9178–9183 (2006).
Zeng, X. et al. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS ONE 8, e72922 (2013).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Kelly, C. J. et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF Augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015).
Berthoud, H. R., Blackshaw, L. A., Brookes, S. J. & Grundy, D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol. Motil. 16 (Suppl. 1), 28–33 (2004).
Costa, M., Brookes, S. J. & Hennig, G. W. Anatomy and physiology of the enteric nervous system. Gut 47 (Suppl. 4), iv15–iv19 (2000).
McVey Neufeld, K. A., Perez-Burgos, A., Mao, Y. K., Bienenstock, J. & Kunze, W. A. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol. Motil. 27, 627–636 (2015).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Vaughn, A. C. et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. 77, 18–30 (2017).
de Lartigue, G., de La Serre, C. B. & Raybould, H. E. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 105, 100–105 (2011).
Lal, S., Kirkup, A. J., Brunsden, A. M., Thompson, D. G. & Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G907–G915 (2001).
Zadeh-Tahmasebi, M. et al. Activation of short and long chain fatty acid sensing machinery in the ileum lowers glucose production in vivo. J. Biol. Chem. 291, 8816–8824 (2016).
Chow, J., Lee, S. M., Shen, Y., Khosravi, A. & Mazmanian, S. K. Host-bacterial symbiosis in health and disease. Adv. Immunol. 107, 243–274 (2010).
Eberl, G. & Lochner, M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2, 478–485 (2009).
Macpherson, A. J. & Harris, N. L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4, 478–485 (2004).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Bunker, J. J. et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 358, eaan6619 (2017).
Crabbé, P. A., Bazin, H., Eyssen, H. & Heremans, J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int. Arch. Allergy Appl. Immunol. 34, 362–375 (1968).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Ostman, S., Rask, C., Wold, A. E., Hultkrantz, S. & Telemo, E. Impaired regulatory T cell function in germ-free mice. Eur. J. Immunol. 36, 2336–2346 (2006).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Thaiss, C. A., Zmora, N., Levy, M. & Elinav, E. The microbiome and innate immunity. Nature 535, 65–74 (2016).
Kandori, H., Hirayama, K., Takeda, M. & Doi, K. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp. Anim. 45, 155–160 (1996).
Nowacki, M. R. Cell proliferation in colonic crypts of germ-free and conventional mice — preliminary report. Folia Histochem. Cytobiol. 31, 77–81 (1993).
Johansson, M. E. et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18, 582–592 (2015).
Kozakova, H. et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell. Mol. Immunol. 13, 251–262 (2016).
Braniste, V. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl Med. 6, 263ra158 (2014).
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004).
Crumeyrolle-Arias, M. et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42, 207–217 (2014).
Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F. & Tillisch, K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496 (2014).
Sewell, D. L., Wostmann, B. S., Gairola, C. & Aleem, M. I. Oxidative energy metabolism in germ-free and conventional rat liver mitochondria. Am. J. Physiol. 228, 526–529 (1975).
Hallman, T. M. et al. The mitochondrial and kidney disease phenotypes of kd/kd mice under germfree conditions. J. Autoimmun 26, 1–6 (2006).
Yang, T. & Zubcevic, J. Gut-brain axis in regulation of blood pressure. Front. Physiol. 8, 845 (2017).
Aroor, A. R. et al. The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front. Endocrinol. 4, 161 (2013).
Young, C. N. & Davisson, R. L. Angiotensin-II, the brain, and hypertension: an update. Hypertension 66, 920–926 (2015).
Mancia, G. & Grassi, G. The autonomic nervous system and hypertension. Circ. Res. 114, 1804–1814 (2014).
Harrison, D. G. The immune system in hypertension. Trans. Am. Clin. Climatol Assoc. 125, 130–140 (2014).
Wise, I. A. & Charchar, F. J. Epigenetic modifications in essential hypertension. Int. J. Mol. Sci. 17, 451 (2016).
Ahn, S. Y. & Gupta, C. Genetic programming of hypertension. Front. Pediatr. 5, 285 (2017).
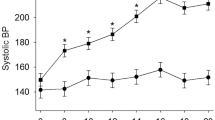
Yang, T. et al. Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340 (2015). This study demonstrates a clear association between gut dysbiosis and hypertension in rats and a small cohort of human patients with hypertension.
Mell, B. et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 47, 187–197 (2015).
Durgan, D. J. et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension 67, 469–474 (2016).
Li, J. et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5, 14 (2017).
Santisteban, M. M. et al. Hypertension-linked pathophysiological alterations in the gut. Circ. Res. 120, 312–323 (2017).
Wilck, N. et al. Salt-responsive gut commensal modulates T. Nature 551, 585–589 (2017).
Pluznick, J. L. et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl Acad. Sci. USA 110, 4410–4415 (2013).
Natarajan, N. et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 48, 826–834 (2016).
Marques, F. Z. et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135, 964–977 (2017).
Aleixandre, A. & Miguel, M. Dietary fiber and blood pressure control. Food Funct. 7, 1864–1871 (2016).
Whelton, S. P. et al. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J. Hypertens. 23, 475–481 (2005).
Khalesi, S., Sun, J., Buys, N. & Jayasinghe, R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64, 897–903 (2014).
Qi, Y., Aranda, J. M., Rodriguez, V., Raizada, M. K. & Pepine, C. J. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension — a case report. Int. J. Cardiol. 201, 157–158 (2015).
Werder, A. A., Amos, M. A., Nielsen, A. H. & Wolfe, G. H. Comparative effects of germfree and ambient environments on the development of cystic kidney disease in CFWwd mice. J. Lab Clin. Med. 103, 399–407 (1984).
Vaziri, N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 83, 308–315 (2013). This is a comprehensive study demonstrating that the gut microbiota is linked to CKD in rats and humans. Uraemia significantly altered gut microbial composition.
Felizardo, R. J., Castoldi, A., Andrade-Oliveira, V. & Câmara, N. O. The microbiota and chronic kidney diseases: a double-edged sword. Clin. Transl Immunol. 5, e86 (2016).
Ranganathan, N. et al. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 25, 1919–1930 (2009).
Fukuuchi, F. et al. Intestinal bacteria-derived putrefactants in chronic renal failure. Clin. Exp. Nephr. 6, 99–104 (2002).
Wang, F. et al. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 17, 733–738 (2012).
Kikuchi, M., Ueno, M., Itoh, Y., Suda, W. & Hattori, M. Uremic toxin-producing gut microbiota in rats with chronic kidney disease. Nephron 135, 51–60 (2017).
Jiang, S. et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 7, 2870 (2017).
Duranton, F. et al. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 23, 1258–1270 (2012).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Xu, K. Y. et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 7, 1445 (2017). This functional analysis of gut microbial communities in CKD identifies several altered genes responsible for TMAO production. Transplantation of faecal samples from patients with CKD induced an increased TMAO levels in the mouse recipients.
Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 35, S35–38 (1994).
Sirich, T. L., Plummer, N. S., Gardner, C. D., Hostetter, T. H. & Meyer, T. W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 9, 1603–1610 (2014).
Aronov, P. A. et al. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol. 22, 1769–1776 (2011).
Stephen, A. M., Wiggins, H. S. & Cummings, J. H. Effect of changing transit time on colonic microbial metabolism in man. Gut 28, 601–609 (1987).
Hatch, M. & Vaziri, N. D. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin. Sci. 86, 511–516 (1994).
Vaziri, N. D., Yuan, J. & Norris, K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol. 37, 1–6 (2013).
Vaziri, N. D. et al. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 27, 2686–2693 (2012).
Al Khodor, S. & Shatat, I. F. Gut microbiome and kidney disease: a bidirectional relationship. Pediatr. Nephrol. 32, 921–931 (2017).
Shi, K. et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig. Dis. Sci. 59, 2109–2117 (2014).
Yan, J. et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl Acad. Sci. USA 113, E7554–E7563 (2016).
Callen, I. R. & Limarzi, L. R. Blood and bone marrow studies in renal disease. Am. J. Clin. Pathol. 20, 3–23 (1950).
Hingorani, S., Guthrie, K. A., Schoch, G., Weiss, N. S. & McDonald, G. B. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 39, 223–229 (2007).
Hingorani, S., Gooley, T., Pao, E., Sandmaier, B. & McDonald, G. Urinary cytokines after HCT: evidence for renal inflammation in the pathogenesis of proteinuria and kidney disease. Bone Marrow Transplant. 49, 403–409 (2014).
Hahm, E. et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat. Med. 23, 100–106 (2017).
Hayek, S. S., Quyyumi, A. A. & Reiser, J. Soluble urokinase receptor and chronic kidney disease. N. Engl. J. Med. 374, 891 (2016).
Napoli, C., Maione, C., Schiano, C., Fiorito, C. & Ignarro, L. J. Bone marrow cell-mediated cardiovascular repair: potential of combined therapies. Trends Mol. Med. 13, 278–286 (2007).
Sugimoto, H. et al. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc. Natl Acad. Sci. USA 103, 7321–7326 (2006).
Huls, M., Russel, F. G. & Masereeuw, R. Insights into the role of bone marrow-derived stem cells in renal repair. Kidney Blood Press Res. 31, 104–110 (2008).
Jung, C., Hugot, J. P. & Barreau, F. Peyer’s patches: the immune sensors of the intestine. Int. J. Inflam. 2010, 823710 (2010).
Pedrinelli, R. et al. Low-grade inflammation and microalbuminuria in hypertension. Arterioscler Thromb. Vasc. Biol. 24, 2414–2419 (2004).
Costello-White, R., Ryff, C. D. & Coe, C. L. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age 37, 9808 (2015).
Wenzel, P. et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124, 1370–1381 (2011).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Chan, C. T. et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66, 1023–1033 (2015).
Moghadamrad, S. et al. Attenuated portal hypertension in germ-free mice: function of bacterial flora on the development of mesenteric lymphatic and blood vessels. Hepatology 61, 1685–1695 (2015).
Chassaing, B. & Gewirtz, A. T. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol. Pathol. 42, 49–53 (2014).
Cani, P. D., Osto, M., Geurts, L. & Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3, 279–288 (2012).
Mishima, E. et al. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 92, 634–645 (2017). This paper examines metabolite profiles of plasma, faeces and urine in germ-free animals compared with SPF controls and outlines the contributions of gut microbiota to the production of uraemic solutes.
Meijers, B. K., Bammens, B., Verbeke, K. & Evenepoel, P. A review of albumin binding in CKD. Am. J. Kidney Dis. 51, 839–850 (2008).
Sirich, T. L., Aronov, P. A., Plummer, N. S., Hostetter, T. H. & Meyer, T. W. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int. 84, 585–590 (2013).
Wu, I. W. et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 26, 938–947 (2011).
Lin, C. J. et al. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J. Clin. Lab. Anal. 25, 191–197 (2011).
Magnusson, M., Magnusson, K. E., Sundqvist, T. & Denneberg, T. Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: effects of low- and high-protein diets. Nephron 56, 306–311 (1990).
Magnusson, M., Magnusson, K. E., Sundqvist, T. & Denneberg, T. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 32, 754–759 (1991).
de Almeida Duarte, J. B., de Aguilar-Nascimento, J. E., Nascimento, M. & Nochi, R. J. Bacterial translocation in experimental uremia. Urol. Res. 32, 266–270 (2004).
Vaziri, N. D., Dure-Smith, B., Miller, R. & Mirahmadi, M. K. Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am. J. Gastroenterol. 80, 608–611 (1985).
Ito, S. & Yoshida, M. Protein-bound uremic toxins: new culprits of cardiovascular events in chronic kidney disease patients. Toxins 6, 665–678 (2014).
Koppe, L. et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J. Am. Soc. Nephrol. 24, 88–99 (2013).
Sun, C. Y., Chang, S. C. & Wu, M. S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 7, e34026 (2012).
Wong, J. et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 39, 230–237 (2014).
Jiang, S. et al. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 109, 1389–1396 (2016).
Corrêa-Oliveira, R., Fachi, J. L., Vieira, A., Sato, F. T. & Vinolo, M. A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl Immunol. 5, e73 (2016).
Wang, L. et al. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J. Hypertens. 35, 1899–1908 (2017).
Yang, T. et al. Shifts in the gut microbiota composition due to depleted bone marrow beta adrenergic signaling are associated with suppressed inflammatory transcriptional networks in the mouse colon. Front. Physiol. 8, 220 (2017).
Kim, S. et al. Angiotensin II regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension 67, 574–584 (2016).
Zubcevic, J. et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension 63, 542–550 (2014).
Zubcevic, J. et al. A single angiotensin II hypertensive stimulus is associated with prolonged neuronal and immune system activation in Wistar-Kyoto rats. Front. Physiol. 8, 592 (2017).
Kim, S. et al. Hypertensive patients exhibit gut microbial dysbiosis and an increase in TH17 cells [abstract]. J. Hypertension 33 (Suppl. 1), 6B.07 (2015).
Richards, E. M., Pepine, C. J., Raizada, M. K. & Kim, S. The gut, its microbiome, and hypertension. Curr. Hypertens. Rep. 19, 36 (2017).
Ramezani, A. et al. Role of the gut microbiome in uremia: a potential therapeutic target. Am. J. Kidney Dis. 67, 483–498 (2016).
Afsar, B. et al. Brain-kidney cross-talk: definition and emerging evidence. Eur. J. Intern. Med. 36, 7–12 (2016).
Kaur, J., Young, B. E. & Fadel, P. J. Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int. J. Mol. Sci. 18, 1682 (2017).
Johns, E. J., Kopp, U. C. & DiBona, G. F. Neural control of renal function. Compr. Physiol. 1, 731–767 (2011).
Bigazzi, R., Kogosov, E. & Campese, V. M. Altered norepinephrine turnover in the brain of rats with chronic renal failure. J. Am. Soc. Nephrol. 4, 1901–1907 (1994).
Amann, K. et al. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J. Am. Soc. Nephrol. 11, 1469–1478 (2000).
Hausberg, M. et al. Sympathetic nerve activity in end-stage renal disease. Circulation 106, 1974–1979 (2002).
Pongratz, G. & Straub, R. H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 16, 504 (2014).
Lorton, D. & Bellinger, D. L. Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int. J. Mol. Sci. 16, 5635–5665 (2015).
Singh, M. V., Chapleau, M. W., Harwani, S. C. & Abboud, F. M. The immune system and hypertension. Immunol. Res. 59, 243–253 (2014).
Grassi, G. et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 57, 846–851 (2011).
Fisher, J. P., Young, C. N. & Fadel, P. J. Central sympathetic overactivity: maladies and mechanisms. Auton. Neurosci. 148, 5–15 (2009).
Shi, P. et al. Direct pro-inflammatory effects of prorenin on microglia. PLoS ONE 9, e92937 (2014).
Winklewski, P. J., Radkowski, M., Wszedybyl-Winklewska, M. & Demkow, U. Brain inflammation and hypertension: the chicken or the egg? J. Neuroinflamm. 12, 85 (2015).
de Kloet, A. D., Liu, M., Rodríguez, V., Krause, E. G. & Sumners, C. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R444–R458 (2015).
Adesso, S. et al. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front. Pharmacol. 8, 370 (2017).
Nishihara, M., Takesue, K. & Hirooka, Y. Renal denervation enhances GABA-ergic input into the PVN leading to blood pressure lowering in chronic kidney disease. Auton. Neurosci. 204, 88–97 (2017).
Kurella, M., Yaffe, K., Shlipak, M. G., Wenger, N. K. & Chertow, G. M. Chronic kidney disease and cognitive impairment in menopausal women. Am. J. Kidney Dis. 45, 66–76 (2005).
Kurella Tamura, M. et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am. J. Kidney Dis. 52, 227–234 (2008).
Jassal, S. K., Kritz-Silverstein, D. & Barrett-Connor, E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am. J. Epidemiol. 171, 277–286 (2010).
Helmer, C. et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 77, 2043–2051 (2011).
Kurella Tamura, M. et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am. J. Kidney Dis. 58, 756–763 (2011).
De Deyn, P. P., Vanholder, R., Eloot, S. & Glorieux, G. Guanidino compounds as uremic (neuro)toxins. Semin. Dial 22, 340–345 (2009).
Goek, O. N. et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol. Dial. Transplant. 28, 2131–2138 (2013).
Clarke, G. et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673 (2013).
Orhan, F. et al. Tryptophan metabolism along the kynurenine pathway downstream of Toll-like receptor stimulation in peripheral monocytes. Scand. J. Immunol. 84, 262–271 (2016).
Davis, I. & Liu, A. What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert Rev. Neurother 15, 719–721 (2015).
Kigerl, K. A., de Rivero Vaccari, J. P., Dietrich, W. D., Popovich, P. G. & Keane, R. W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 258, 5–16 (2014).
Maddison, D. C. & Giorgini, F. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 40, 134–141 (2015).
van Koppen, A. et al. Healthy bone marrow cells reduce progression of kidney failure better than CKD bone marrow cells in rats with established chronic kidney disease. Cell Transplant 21, 2299–2312 (2012).
Romano, K. A. et al. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 22, 279–290.e7 (2017).
Savidge, T. C. Epigenetic regulation of enteric neurotransmission by gut bacteria. Front. Cell Neurosci. 9, 503 (2015).
Li, L., Ma, L. & Fu, P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des. Devel Ther. 11, 3531–3542 S150825 (2017).
Paul, B. et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenet. 7, 112 (2015).
Yang, T., Owen, J. L., Lightfoot, Y. L., Kladde, M. P. & Mohamadzadeh, M. Microbiota impact on the epigenetic regulation of colorectal cancer. Trends Mol. Med. 19, 714–725 (2013).
Shiels, P. G., McGuinness, D., Eriksson, M., Kooman, J. P. & Stenvinkel, P. The role of epigenetics in renal ageing. Nat. Rev. Nephrol. 13, 471–482 (2017).
Shi, S. et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J. Am. Soc. Nephrol. 19, 2159–2169 (2008).
Ko, Y. A. et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 14, R108 (2013).
Mu, S. et al. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat. Med. 17, 573–580 (2011).
Lee, H. A. et al. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension 59, 621–626 (2012).
Hoban, A. E. et al. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 5, 102 (2017).
Semenkovich, N. P. et al. Impact of the gut microbiota on enhancer accessibility in gut intraepithelial lymphocytes. Proc. Natl Acad. Sci. USA 113, 14805–14810 (2016).
Mukerjee, S. et al. Perinatal exposure to Western diet programs autonomic dysfunction in the male offspring. Cell. Mol. Neurobiol. 38, 233–242 (2018).
Kim, S. et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 132, 701–718 (2018).
Ponticelli, C. & Campise, M. R. Neurological complications in kidney transplant recipients. J. Nephrol. 18, 521–528 (2005).
Shi, P. et al. Brain microglial cytokines in neurogenic hypertension. Hypertension 56, 297–303 (2010).
Hering, D. et al. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int. J. Cardiol. 232, 93–97 (2017).
Ott, C. et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J. Hypertens. 33, 1261–1266 (2015).
Clark, A. & Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J. Int. Soc. Sports Nutr. 13, 43 (2016).
Steinberg, D., Bennett, G. G. & Svetkey, L. The DASH diet, 20 years later. JAMA 317, 1529–1530 (2017).
Jenkins, D. J. et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am. J. Clin. Nutr. 75, 834–839 (2002).
Pins, J. J. et al. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? J. Fam. Pract. 51, 353–359 (2002).
Chiavaroli, L., Mirrahimi, A., Sievenpiper, J. L., Jenkins, D. J. & Darling, P. B. Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 69, 761–768 (2015).
Lu, L. et al. Dietary fiber intake is associated with chronic kidney disease (CKD) progression and cardiovascular risk, but not protein nutritional status, in adults with CKD. Asia Pac. J. Clin. Nutr. 26, 598–605 (2017).
Rossi, M. et al. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): a randomized trial. Clin. J. Am. Soc. Nephrol. 11, 223–231 (2016).
Vaziri, N. D. et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 9, e114881 (2014).
Koppe, L., Mafra, D. & Fouque, D. Probiotics and chronic kidney disease. Kidney Int. 88, 958–966 (2015). This review introduces basic concepts of gut and kidney communication, summarizes the current available probiotic treatments in animals and human patients with CKD and highlights the potential mechanisms of probiotics in the treatment of CKD.
Kieffer, D. A. et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol. Renal Physiol. 310, F857–F871 (2016).
Hida, M. et al. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74, 349–355 (1996).
Eyler, R. F. & Mueller, B. A. Antibiotic pharmacokinetic and pharmacodynamic considerations in patients with kidney disease. Adv. Chron. Kidney Dis. 17, 392–403 (2010).
Kim, G. J., Je, N. K., Kim, D. S. & Lee, S. Adherence with renal dosing recommendations in outpatients undergoing haemodialysis. J. Clin. Pharm. Ther. 41, 26–33 (2016).
Smith, K. E. et al. Antibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, Minnesota. Pediatr. Infect. Dis. J. 31, 37–41 (2012).
Xu, Y., Liu, Y., Pei, J., Yao, S. & Cheng, C. Bacteriophage therapy against Enterobacteriaceae. Virol. Sin. 30, 11–18 (2015).
Hamdi, S. et al. Characterization of two polyvalent phages infecting Enterobacteriaceae. Sci. Rep. 7, 40349 (2017).
Thongprayoon, C., Cheungpasitporn, W., Phatharacharukul, P., Mahaparn, P. & Bruminhent, J. High mortality risk in chronic kidney disease and end stage kidney disease patients with Clostridium difficile infection: a systematic review and meta-analysis. J. Nat. Sci. 1, e85 (2015).
Youngster, I. et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312, 1772–1778 (2014).
Ott, S. J. et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile Infection. Gastroenterology 152, 799–811.e7 (2017).
Itoh, Y., Ezawa, A., Kikuchi, K., Tsuruta, Y. & Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 403, 1841–1850 (2012).
Yamaguchi, J., Tanaka, T. & Inagi, R. Effect of AST-120 in chronic kidney disease treatment: still a controversy? Nephron 135, 201–206 (2017).
Nikolic, S. B., Sharman, J. E., Adams, M. J. & Edwards, L. M. Metabolomics in hypertension. J. Hypertens. 32, 1159–1169 (2014).
Goek, O. N. et al. Serum metabolite concentrations and decreased GFR in the general population. Am. J. Kidney Dis. 60, 197–206 (2012).
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G. & Cryan, J. F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48 (2015).
Madeo, F., Eisenberg, T., Pietrocola, F. & Kroemer, G. Spermidine in health and disease. Science 359, eaan2788 (2018).
Mazumder, M. K., Giri, A., Kumar, S. & Borah, A. A highly reproducible mice model of chronic kidney disease: evidences of behavioural abnormalities and blood-brain barrier disruption. Life Sci. 161, 27–36 (2016).
Lau, W. L., Kalantar-Zadeh, K. & Vaziri, N. D. The gut as a source of inflammation in chronic kidney disease. Nephron 130, 92–98 (2015).
Vaziri, N. D., Zhao, Y. Y. & Pahl, M. V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 31, 737–746 (2016).
Wester, A. L., Vatn, M. H. & Fausa, O. Secondary amyloidosis in inflammatory bowel disease: a study of 18 patients admitted to Rikshospitalet University Hospital, Oslo, from 1962 to 1998. Inflamm. Bowel Dis. 7, 295–300 (2001).
McBryde, F. D., Guild, S. J., Barrett, C. J., Osborn, J. W. & Malpas, S. C. Angiotensin II-based hypertension and the sympathetic nervous system: the role of dose and increased dietary salt in rabbits. Exp. Physiol. 92, 831–840 (2007).
Author information
Authors and Affiliations
Contributions
T.Y. researched the data and wrote the article. M.K.R., T.Y. and E.M.R. made substantial contributions to discussions of the content. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Low-grade inflammation
-
A chronic systemic immune response that occurs without acute clinical symptoms.
- Probiotics
-
A group of microorganisms with beneficial effects on human health.
- Nucleus of the solitary tract
-
(NTS). A brainstem region that receives and integrates peripheral afferent inputs from the baroreceptors, chemoreceptors and subdiaphragmatic organs of the gastrointestinal tract. The NTS projects selectively to the paraventricular nucleus of hypothalamus or caudal ventrolateral medulla to modulate sympathetic outflow.
- TH1 and TH2 responses
-
CD4+ T cells can be divided into two subsets on the basis of their pattern of cytokine production. The TH1 response is characterized by the production of IFNγ and is generally more effective against intracellular pathogens, whereas the TH2 response is characterized by the production of IL-4 and is generally more effective against extracellular bacteria and parasites.
- Uraemic toxins
-
Various compounds, mainly derived from the gut microbiota, that accumulate in the blood and tissue with progression of renal failure. Some compounds exhibit high affinity for albumin and are difficult to remove by haemodialysis.
- Paraventricular nucleus of hypothalamus
-
(PVN). An important region in the central nervous system that contributes to sympathetic nervous system efferent transmission. Stimulation of the PVN with inflammatory cytokines or angiotensin II increases sympathetic outflow.
- Rostral ventrolateral medulla
-
(RVLM). The RVLM receives projections from the paraventricular nucleus of hypothalamus and caudal ventrolateral medulla to control sympathetic activity associated with cardiovascular functions.
- Kynurenine pathway
-
The kynurenine pathway catabolizes approximately 95–99% of ingested tryptophan that is not utilized for protein synthesis in mammalian cells. Dysregulation of the kynurenine pathway results in overproduction of quinolinic acid, which has been implicated in inflammatory neurological diseases, such as Alzheimer and Huntington diseases.
- Excitotoxin
-
A collection of chemical compounds that overactivate and exhaust neurons by binding to their receptors.
- Prebiotics
-
Food ingredients that promote growth of beneficial microorganisms.
- Synbiotics
-
Combinations of prebiotics and probiotics.
Rights and permissions
About this article
Cite this article
Yang, T., Richards, E.M., Pepine, C.J. et al. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol 14, 442–456 (2018). https://doi.org/10.1038/s41581-018-0018-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0018-2
This article is cited by
-
Intestinal microbiota and metabolome perturbations in ischemic and idiopathic dilated cardiomyopathy
Journal of Translational Medicine (2024)
-
Interorgan communication networks in the kidney–lung axis
Nature Reviews Nephrology (2024)
-
A new era in the science and care of kidney diseases
Nature Reviews Nephrology (2024)
-
Stroke and Distal Organ Damage: Exploring Brain-Kidney Crosstalk
Neurochemical Research (2024)
-
Analysis of intestinal flora and cognitive function in maintenance hemodialysis patients using combined 16S ribosome DNA and shotgun metagenome sequencing
Aging Clinical and Experimental Research (2024)