Abstract
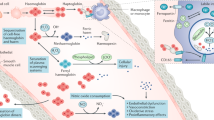
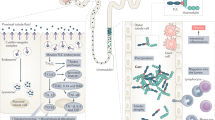
Acute kidney injury (AKI), a major public health problem associated with high mortality and increased risk of progression towards end-stage renal disease, is characterized by the activation of intra-renal haemostatic and inflammatory processes. Platelets, which are present in high numbers in the circulation and can rapidly release a broad spectrum of bioactive mediators, are important acute modulators of inflammation and haemostasis, as they are the first cells to arrive at sites of acute injury, where they interact with endothelial cells and leukocytes. Diminished control of platelet reactivity by endothelial cells and/or an increased release of platelet-activating mediators can lead to uncontrolled platelet activation in AKI. As increased platelet sequestration and increased expression levels of the markers P-selectin, thromboxane A2, CC-chemokine ligand 5 and platelet factor 4 on platelets have been reported in kidneys following AKI, platelet activation likely plays a part in AKI pathology. Results from animal models and some clinical studies highlight the potential of antiplatelet therapies in the preservation of renal function in the context of AKI, but as current strategies also affect other cell types and non-platelet-derived mediators, additional studies are required to further elucidate the extent of platelet contribution to the pathology of AKI and to determine the best therapeutic approach by which to specifically target related pathogenic pathways.
Key points
-
Both ischaemia–reperfusion and systemic inflammation lead to alterations in the renal macrocirculation and microcirculation that often result in poorly controlled inflammatory and haemostatic responses, thereby causing irreversible renal tissue damage.
-
Platelets are the first cells to arrive at sites of acute injury, where they interact with endothelial cells and leukocytes.
-
Activation of platelets during acute kidney injury (AKI) may be exaggerated owing to an excess of platelet stimuli in combination with diminished antiplatelet regulation.
-
Platelets are important acute modulators of haemostasis and likely disturb renal haemodynamic processes during AKI, which leads to sustained hypoxaemic renal tissue injury.
-
Platelets facilitate inflammation during the pathophysiology of AKI, mainly by stimulating endothelial cells as well as by recruiting and activating leukocytes during the inflammatory reaction.
-
Early data from animal and human studies and from randomized clinical trials suggest that antiplatelet therapies will reduce the risk of AKI.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bellomo, R., Kellum, J. A. & Ronco, C. Acute kidney injury. Lancet 380, 756–766 (2012).
Hsu, R. K., McCulloch, C. E., Dudley, R. A., Lo, L. J. & Hsu, C. Y. Temporal changes in incidence of dialysis-requiring AKI. J. Am. Soc. Nephrol. 24, 37–42 (2013).
Hsu, C. Y. et al. Community-based incidence of acute renal failure. Kidney Int. 72, 208–212 (2007).
Jefferson, J. A., Thurman, J. M. & Schier, R. W. Pathophysiology and etiology of acute kidney injury. Ann. Clin. Biochem. 52, 797–812 (2010).
Zarbock, A., Gomez, H. & Kellum, J. A. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 20, 588–595 (2014).
Basile, D. P. & Yoder, M. C. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc. Hematol. Disord. Drug Targets 14, 3–14 (2014).
Souza, A. C., Yuen, P. S. & Star, R. A. Microparticles: markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and AKI. Kidney Int. 87, 1100–1108 (2015).
Basile, D. P., Anderson, M. D. & Sutton, T. A. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303–1353 (2012).
Vogel, S. et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Invest. 125, 4638–4654 (2015).
Dole, V. S., Bergmeier, W., Mitchell, H. A., Eichenberger, S. C. & Wagner, D. D. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood 106, 2334–2339 (2005).
Gawaz, M., Dickfeld, T., Bogner, C., Fateh-Moghadam, S. & Neumann, F. J. Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med. 23, 379–385 (1997).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Caudrillier, A. et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 122, 2661–2671 (2012).
Lievens, D. et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 116, 4317–4327 (2010).
Hu, H. et al. Clopidogrel protects from cell apoptosis and oxidative damage in a mouse model of renal ischaemia-reperfusion injury. J. Pathol. 225, 265–275 (2011).
Jansen, M. P. et al. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney Int. (2016).
Saboor, M., Ayub, Q., Ilyas, S. & Moinuddin, S. Platelet receptors; an instrumental of platelet physiology. Pak.J. Med. Sci. 29, 891–896 (2013).
Maynard, D. M., Heijnen, H. F., Horne, M. K., White, J. G. & Gahl, W. A. Proteomic analysis of platelet a-granules using mass spectrometry. J. Thromb. Haemost 5, 1945–1955 (2007).
Wijten, P. et al. High precision platelet releasate definition by quantitative reversed protein profiling — brief report. Arterioscler Thromb. Vasc. Biol. 33, 1635–1638 (2013).
Gidlöf, O. et al. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 21, 3908–3917 (2013).
Kirschbaum, M. et al. Horizontal RNA transfer mediates platelet-induced hepatocyte proliferation. Blood 126, 798–806 (2015).
Chappell, D. et al. [Expedition glycocalyx. A newly discovered “Great Barrier Reef]. Anaesthesist 57, 959–969 (2008).
de Melo Bezerra Cavalcante, C. T. et al. Syndecan-1 improves severe acute kidney injury prediction after pediatric cardiac surgery. J. Thorac Cardiovasc. Surg. 152, 178–186 (2016).
Chelazzi, C., Villa, G., Mancinelli, P., De Gaudio, A. R. & Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 19, 26 (2015).
Adembri, C. et al. Sepsis induces albuminuria and alterations in the glomerular filtration barrier- a morphofunctional study in the rat. Crit. Care 15, R277 (2011).
Chappell, D. et al. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur. J. Anaesthesiol 31, 474–481 (2014).
Chappell, D. et al. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc. Res. 83, 388–396 (2009).
Chappell, D. et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 115, 482–491 (2011).
Rex, S. & Freedman, J. E. in Platelets Second Edition (ed. Michelson, A.) 251–267 (Elsevier, 2006).
Noiri, E., Peresleni, T., Miller, F. & Goligorsky, M. S. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J. Clin. Invest. 97, 2377–2383 (1996).
Goligorsky, M. S., Brodsky, S. V. & Noiri, E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 61, 855–861 (2002).
Horn, P. et al. Circulating microparticles carry a functional endothelial nitric oxide synthase that is decreased in patients with endothelial dysfunction. J. Am. Heart Assoc. 2, e003764 (2012).
Yamasowa, H., Shimizu, S., Inoue, T., Takaoka, M. & Matsumura, Y. Endothelial nitric oxide contributes to the renal protective effects of ischemic preconditioning. J. Pharmacol. Exp. Ther. 312, 153–159 (2005).
Piepot, H. A., Boer, C., Groeneveld, A. B., Van Lambalgen, A. A. & Sipkema, P. Lipopolysaccharide impairs endothelial nitric oxide synthesis in rat renal arteries. Kidney Int. 57, 2502–2510 (2000).
Wang, W. et al. Endothelial nitric oxide synthase-deficient mice exhibit increased susceptibility to endotoxin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 287, F1044–F1048 (2004).
Kwon, O., Hong, S. M. & Ramesh, G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 296, F25–F33 (2009).
Kawabe, J., Ushikubi, F. & Hasebe, N. Prostacyclin in vascular diseases. Circ. J. 74, 836–843 (2010).
Bonventre, J. V. & Nemenoff, R. Renal tubular arachidonic acid metabolism. Kidney Int. 39, 438–449 (1991).
Yokoyama, C. et al. Prostacyclin-deficient mice develop ischemic renal disorders, including nephrosclerosis and renal infarction. Circulation 106, 2397–2403 (2002).
Wang, W. et al. Prostacyclin in endotoxemia-induced acute kidney injury: cyclooxygenase inhibition and renal prostacyclin synthase transgenic mice. Am. J. Physiol. Renal Physiol. 293, F1131–F1136 (2007).
Eltzschig, H. K. et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 198, 783–796 (2003).
Paul, S., Feoktistov, I., Hollister, A. S., Robertson, D. & Biaggioni, I. Adenosine inhibits the rise in intracellular calcium and platelet aggregation produced by thrombin: evidence that both effects are coupled to adenylate cyclase. Mol. Pharmacol. 37, 870–875 (1990).
Koziak, K., Sévigny, J., Robson, S. C., Siegel, J. B. & Kaczmarek, E. Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb. Haemost. 82, 1583–1544 (1999).
Candinas, D. et al. Loss of rat glomerular ATP diphosphohydrolase activity during reperfusion injury is associated with oxidative stress reactions. Thromb. Haemost. 76, 807–812 (1996).
Takahashi-Sato, K., Murakawa, M., Kimura, J., Ito, M. A. & Matsuoka, I. Loss of ectonucleotidases from the coronary vascular bed after ischemia-reperfusion in isolated rat heart. BMC Cardiovasc. Disord. 13, 53 (2013).
Robson, S. C. et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J. Exp. Med. 185, 153–164 (1997).
Kohler, D. et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116, 1784–1794 (2007).
Sun, X. et al. Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic Signal. 7, 427–434 (2011).
Guckelberger, O. et al. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb. Haemost. 91, 576–586 (2004).
Grenz, A. et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 21, 2863–2873 (2007).
Crikis, S. et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am. J. Transplant 10, 2586–2595 (2010).
Sutton, T. A., Fisher, C. J. & Molitoris, B. A. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 62, 1539–1549 (2002).
Basile, D. P. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 72, 151–156 (2007).
Vischer, U. M., Jornot, L., Wollheim, C. B. & Theler, J. M. Reactive oxygen intermediates induce regulated secretion of von willebrand factor from cultured human vascular endothelial cells. Blood 85, 3164–3172 (1995).
Hechler, B. et al. Arterial thrombosis: relevance of a model with two levels of severity assessed by histologic, ultrastructural and functional characterization. J. Thromb. Haemost. 8, 173–184 (2010).
Miller, D. L., Yaron, R. & Yellin, M. J. CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J. Leukoc. Biol. 63, 373–379 (1998).
Szotowski, B., Antoniak, S., Poller, W., Schultheiss, H. P. & Rauch, U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ. Res. 96, 1233–1239 (2005).
Andrews, R. K., Berndt, M. C. & Lopez, J. A. in Platelets Second Edition (ed. Michelson, A.) 145–163 (Elsevier, 2006).
Kim, M. G. et al. The ADAMTS13-von Willebrand factor axis is involved in the pathophysiology of kidney ischemia-reperfusion injury. Nephrology 22, 913–920 (2016).
Litvinov, R. I., Farrell, D. H., Weisel, J. W. & Bennett, J. S. The platelet integrin alphaIIbbeta3 differentially interacts with fibrin versus fibrinogen. J. Biol. Chem. 291, 7858–7867 (2016).
Alshehri, O. M. et al. Fibrin activates GPVI in human and mouse platelets. Blood 126, 1601–1608 (2015).
Mangin, P. H. et al. Immobilized fibrinogen activates human platelets through GPVI. Haematologica https://doi.org/10.3324/haematol.2017.182972 (2018).
Kahn, M. L., Nakanishi-Matsui, M., Shapiro, M. J., Ishihara, H. & Coughlin, S. R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 103, 879–887 (1999).
Ushigome, H. et al. The role of tissue factor in renal ischemic reperfusion injury of the rat. Journal of surgical research 102, 102–109 (2002).
Erlich, J., Fearns, C., Mathison, J., Ulevitch, R. J. & Mackman, N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect. Immun. 67, 2540–2546 (1999).
Sevastos, J. et al. Tissue factor deficiency and PAR-1 deficiency are protective against renal ischemia reperfusion injury. Blood 109, 577–583 (2007).
Welty-Wolf, K. E. et al. Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am. J. Respir. Crit. Care Med. 10, 1988–1996 (2001).
Scrascia, G. et al. Acute kidney injury in high-risk cardiac surgery patients: roles of inflammation and coagulation. J. Cardiovasc. Med. 18, 359–365 (2015).
Allam, R. et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J. Am. Soc. Nephrol. 23, 1375–1388 (2012).
Tsuji, N. et al. Role of mitochondrial DNA in septic AKI via toll-like receptor 9. J. Am. Soc. Nephrol. 27, 2009–2020 (2016).
Zakiyanov, O. et al. Placental growth factor, pregnancy-associated plasma protein-A, soluble receptor for advanced glycation end products, extracellular newly identified receptor for receptor for advanced glycation end products binding protein and high mobility group box 1 levels in patients with acute kidney injury: a cross sectional study. BMC Nephrol. 14, 245 (2013).
Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014).
Beutler, B. A. TLRs and innate immunity. Blood 113, 1399–1407 (2009).
Aslam, R. et al. Platelet toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 107, 637–641 (2006).
Andonegui, G. et al. Platelets express functional Toll-like receptor-4. Blood 106, 2417–2423 (2005).
Zhang, G. et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 182, 7997–8004 (2009).
Thurman, J. M., Lucia, M. S., Ljubanovic, D. & Holers, V. M. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 67, 524–530 (2005).
Ricklin, D., Reis, E. S. & Lambris, J. D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12, 383–401 (2016).
Ricklin, D., Mastellos, D. C., Reis, E. S. & Lambris, J. D. The renaissance of complement therapeutics. Nat Rev Nephrol. 1, 26–47 (2018).
Hajishengallis, G., Reis, E. S., Mastellos, D. C., Ricklin, D. & Lambris, J. D. Novel mechanisms and functions of complement. Nat. Immunol. 18, 1288–1298 (2017).
Verschoor, A. & Langer, H. F. Crosstalk between platelets and the complement system in immune protection and disease. Thromb. Haemost. 110, 910–919 (2013).
Cosgrove, L. J., d’Apice, A. J., Haddad, A., Pedersen, J. & McKenzie, I. F. CR3 receptor on platelets and its role in the prostaglandin metabolic pathway. Immunol. Cell Biol. 65, 453–460 (1987).
Martel, C. et al. Requirements for membrane attack complex formation and anaphylatoxins binding to collagen-activated platelets. PLoS ONE 6, e18812 (2011).
Peerschke, E. I. & Ghebrehiwet, B. Platelet receptors for the complement component C1q: implications for hemostasis and thrombosis. Immunobiology 199, 239–249 (1998).
Peerschke, E. I. & Ghebrehiwet, B. Human blood platelets possess specific binding sites for C1q. J. Immunol. 138, 1537–1541 (1987).
Peerschke, E. I. & Ghebrehiwet, B. Platelet membrane receptors for the complement component C1q. Semin. Hematol. 31, 320–328 (1994).
Ando, B., Wiedmer, T., Hamilton, K. K. & Sims, P. J. Complement proteins C5b-9 initiate secretion of platelet storage granules without increased binding of fibrinogen or von Willebrand factor to newly expressed cell surface GPIIb-IIIa. J. Biol. Chem. 263, 11907–11914 (1988).
Wiedmer, T., Esmon, C. T. & Sims, P. J. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood 68, 875–880 (1986).
Yamamoto, T. et al. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am. J. Physiol. Renal Physiol. 282, F1105–1105 (2002).
Stokes, K. Y. & Granger, D. N. Platelets: a critical link between inflammation and microvascular dysfunction. J. Physiol. 590, 1023–1034 (2012).
Möhle, R., Green, D., Moore, M. A., Nachman, R. L. & Rafii, S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl Acad. Sci. USA 94, 663–668 (1997).
Knezevic, I. I. et al. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J. Biol. Chem. 284, 5381–5394 (2009).
Cloutier, N. et al. Platelets can enhance vascular permeability. Blood 120, 1334–1343 (2012).
Sutton, T. A. et al. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am. J. Physiol. Renal Physiol. 285, F191–198 (2003).
Mariano, F. et al. Production of platelet-activating factor in patients with sepsis-associated acute renal failure. Nephrol. Dial. Transplant. 14, 1150–1157 (1999).
Mercado, C. P. & Kilic, F. The molecular mechanism of SERT in platelets: regulation of plasma serotonin levels. Mol. Interv. 10, 231–241 (2010).
Li, Y. et al. Sepsis-induced elevation in plasma serotonin facilitates endothelial hyperpermeability. Sci. Rep. 6, 22747 (2016).
Löwenberg, E. C., Meijers, J. C. M. & Levi, M. Platelet-vessel wall interaction in health and disease. J. Med. 68, 242–251 (2010).
Slupsky, J. R. et al. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb. Haemost. 6, 1008–1014 (1998).
Muller, F. et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 139, 1143–1156 (2009).
Ruiz, F. A., Lea, C. R., Oldfield, E. & Docampo, R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J. Biol. Chem. 279, 44250–44257 (2004).
Hou, Y. et al. Platelets in hemostasis and thrombosis: novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J. Biomed. Res. 29, 437–444 (2015).
Bevers, E. M., Comfurius, P., van Rijn, J. L., Hemker, H. C. & Zwaal, R. F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur. J. Biochem. 122, 429–436 (1982).
Wu, H. et al. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. 21, 1878–1890 (2010).
Bonventre, J. V. & Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121, 4210–4221 (2011).
Zuchtriegel, G. et al. Platelets guide leukocytes to their sites of extravasation. PLoS Biol. 14, e1002459 (2016).
Massberg, S. et al. Platelet-endothelial cell interactions during ischemia reperfusion, the role of P-selectin. Blood 92, 507–515 (1998).
Massberg, S. et al. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood 94, 3829–3838 (1999).
Semple, J. W., Italiano, J. E. Jr & & Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 (2011).
Gleissner, C. A., von Hundelshausen, P. & Ley, K. Platelet chemokines in vascular disease. Arterioscler Thromb. Vasc. Biol. 11, 1920–1927 (2008).
Inwald, D. P., McDowall, A., Peters, M. J., Callard, R. E. & Klein, N. J. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ. Res. 92, 1041–1048 (2003).
Chakrabarti, S., Varghese, S., Vitseva, O., Tanriverdi, K. & Freedman, J. E. CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler Thromb. Vasc. Biol. 25, 2428–2434 (2005).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
Nagasawa, M. et al. Analysis of serum soluble CD40 ligand (sCD40L) in the patients undergoing allogeneic stem cell transplantation: platelet is a major source of serum sCD40L. Eur. J. Haematol. 74, 54–60 (2004).
de Ramon, L. et al. CD154-CD40 T cell co-stimulation pathway is a key mechanism in kidney ischemia-reperfusion injury. Kidney Int. 88, 538–549 (2015).
Lapchak, P. H. et al. Platelet-associated CD40/CD154 mediates remote tissue damage after mesenteric ischemia/reperfusion injury. PLoS ONE 7, e32260 (2012).
Gawaz, M. et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis 148, 75–85 (2000).
Lindemann, S. et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 154, 485–490 (2001).
Grommes, J. et al. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am. J. Respir. Crit. Care Med. 185, 628–636 (2012).
Thurman, J. M., Ljubanovic, D., Edelstein, C. L., Gilkeson, G. S. & Holers, V. M. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J. Immunol. 170, 1517–1523 (2003).
Mulligan, M. S. et al. C5a-dependent up-regulation in vivo of lung vascular P-selectin. J. Immunol. 158, 1857–1861 (1997).
Del Conde, I., Crúz, M. A., Zhang, H., López, J. A. & Afshar-Kharghan, V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 201, 871–879 (2005).
Ekdahl, K. N. & Nilsson, B. Phosphorylation of complement component C3 and C3 fragments by a human platelet protein kinase. Inhibition of factor I-mediated cleavage of C3b. J. Immunol. 154, 6502–6510 (1995).
Ekdahl, K. N. & Nilsson, B. Alterations in C3 activation and binding caused by phosphorylation by a casein kinase released from activated human platelets. J. Immunol. 162, 7426–7433 (1999).
Lapchak, P. H. et al. Platelets orchestrate remote tissue damage after mesenteric ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G888–G897 (2012).
Chintala, M. S., Bernardino, V. & Chiu, P. J. Cyclic GMP but not cyclic AMP prevents renal platelet accumulation after ischemia-reperfusion in anesthetized rats. J. Pharmacol. Exp. Ther. 271, 1203–1208 (1995).
Ed Rainger, G. et al. The role of platelets in the recruitment of leukocytes during vascular disease. Platelets 26, 507–520 (2015).
Kelly, K. J. et al. Intercellular adhesion molecule-1–deficient mice are protected against ischemic renal injury. J. Clin. Invest. 97, 1056–1063 (1996).
Day, Y. J., Huang, L., Ye, H., Linden, J. & Okusa, M. D. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am. J. Physiol. Renal Physiol. 288, F722–F731 (2005).
Kuckleburg, C. J. et al. Endothelial cell-borne platelet bridges selectively recruit monocytes in human and mouse models of vascular inflammation. Cardiovasc. Res. 91, 134–141 (2011).
Slaba, I. et al. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology 62, 1593–1605 (2015).
Salter, J. W., Krieglstein, C. F., Issekutz, A. C. & Granger, D. N. Platelets modulate ischemia:reperfusion-induced leukocyte recruitment in the mesenteric circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 28, G1432–1439 (2001).
Zarbock, A., Singbartl, K. & Ley, K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Invest. 116, 3211–3219 (2006).
Schwarzenberger, C. et al. Platelets are relevant mediators of renal injury induced by primary endothelial lesions. Am. J. Physiol. Renal Physiol. 308, F1238–F1246 (2015).
Page, C. & Pitchford, S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment and activation. Int. Immunopharmacol. 17, 1176–1184 (2013).
Weber, C. & Springer, T. A. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to IIb 3 and stimulated by platelet-activating factor. J. Clin. Invest. 100, 2085–2093 (1997).
Fernandes, L. S. et al. Platelet–monocyte complex formation: effect of blocking PSGL-1 alone, and in combination with αIIbβ3 and αMβ2, in coronary stenting. Thromb. Res. 111, 171–177 (2003).
Simon, D. I. et al. Platelet glycoprotein Ib is a counterreceptor for the leukocyte integrin Mac-1 (CD11b:CD18). J. Exp. Med. 192, 193–204 (2000).
Chen, C. et al. Platelet glycoprotein receptor Ib blockade ameliorates experimental cerebral ischemia-reperfusion injury by strengthening the blood-brain barrier function and anti-thrombo-inflammatory property. Brain Behav. Immun. 1591, 30520–30522 (2017).
Herter, J. M., Rossaint, J., Spieker, T. & Zarbock, A. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J. Innate Immun. 6, 597–606 (2014).
Gerdes, N. et al. Platelet CD40 exacerbates atherosclerosis by transcellular activation of endothelial cells and leukocytes. Arterioscler Thromb. Vasc. Biol. 36, 482–490 (2016).
Lax, S. et al. Platelet CLEC-2 protects against lung injury via effects of its ligand podoplanin on inflammatory alveolar macrophages in the mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L1016–L1029 (2017).
Rainger, G. E., Buckley, C. D., Simmons, D. L. & Nash, G. B. Neutrophils sense flow-generated stress and direct their migration through alphaVbeta3-integrin. Am. J. Physiol. 276, H858–H864 (1999).
Rainger, G. E., Buckley, C., Simmons, D. L. & Nash, G. B. Cross-talk between cell adhesion molecules regulates the migration velocity of neutrophils. Curr. Biol. 7, 316–325 (1997).
Suzuki, J. et al. Cytokine secretion from human monocytes potentiated by P-selectin-mediated cell adhesion. Int. Arch. Allergy Immunol. 160, 152–160 (2013).
Maugeri, N. et al. Polymorphonuclear leukocyte-platelet interaction: role of P-selectin in thromboxane B2 and leukotriene C4 cooperative synthesis. Thromb. Haemost. 72, 450–456 (1994).
Lammermann, T. et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 (2013).
Deng, B. et al. The leukotriene B4–leukotriene B4 receptor axis promotes cisplatin-induced acute kidney injury by modulating neutrophil recruitment. Kidney Int. 92, 89–100 (2017).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Chow, O. A. et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8, 445–454 (2010).
Kumar, S. V. et al. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J. Am. Soc. Nephrol. 26, 2399–2413 (2015).
Thomas, G. M. et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 119, 6335–6343 (2012).
Oklu, R., Albadawi, H., Jones, J. E., Yoo, H. J. & Watkins, M. T. Reduced hind limb ischemia-reperfusion injury in Toll-like receptor-4 mutant mice is associated with decreased neutrophil extracellular traps. J. Vasc. Surg. 58, 1627–1636 (2013).
de Boer, O. J. et al. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb. Haemost. 109, 290–297 (2013).
Brill, A. et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 10, 136–144 (2012).
Carestia, A. et al. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J. Leukoc. Biol. 99, 153–162 (2016).
Etulain, J. et al. P-Selectin promotes neutrophil extracellular trap formation in mice. Blood 126, 242–246 (2015).
Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238 (2014).
Maugeri, N. et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 12, 2074–2088 (2014).
Rossaint, J. et al. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 123, 2573–2584 (2014).
Whitaker, R. M. et al. Urinary mitochondrial DNA is a biomarker of mitochondrial disruption and renal dysfunction in acute kidney injury. Kidney Int. 88, 1336–1344 (2015).
Jansen, M. P. B. et al. Mitochondrial DNA is released in urine of Sirs patients with acute kidney injury and correlates with severity of renal dysfunction. Shock (2017).
Okubo, K. et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat. Med. 24, 232–238 (2018).
Agah, R., Plow, E. F. & Topol, E. J. in Platelets Second Edition (ed. Michelson, A.) 1145–1163 (Elsevier, 2006).
Taylor, F. B. et al. 7E3 F(ab’)2, a monoclonal antibody to the platelet GPIIb/IIIa receptor, protects against microangiopathic hemolytic anemia and microvascular thrombotic renal failure in baboons treated with C4b binding protein and a sublethal infusion of Escherichia coli. Blood 89, 4078–4084 (1997).
Guan, W. et al. Protective effects of tirofiban on ischemia/reperfusion-induced renal injury in vivo and in vitro. Eur. J. Pharmacol. 761, 144–152 (2015).
Cattaneo, M. in Platelets Second Edition (ed. Michelson, A.) 1127–1144 (Elsevier, 2006).
Mederle, K., Meurer, M., Castrop, H. & Höcherl, K. Inhibition of COX-1 attenuates the formation of thromboxane A2and ameliorates the acute decrease in glomerular filtration rate in endotoxemic mice. Am. J. Physiol. Renal Physiol. 309, F332–F340 (2015).
Ikeda, Y., Sudo, T. & Kimura, Y. in Platelets Second Edition (ed. Michelson, A.) 1181–1191 (Elsevier, 2006).
Ragab, D., Abdallah, D. M. & El-Abhar, H. S. Cilostazol renoprotective effect: modulation of PPAR-gamma, NGAL, KIM-1 and IL-18 underlies its novel effect in a model of ischemia-reperfusion. PLoS ONE 9, e95313 (2014).
Abdelrahman, M., Sivarajah, A. & Thiemermann, C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc. Res. 65, 772–781 (2005).
Bischoff, A., Bucher, M., Gekle, M. & Sauvant, C. Differential effect of COX1 and COX2 inhibitors on renal outcomes following ischemic acute kidney injury. Am. J. Nephrol. 40, 1–11 (2014).
Badr, K. F., Kelley, V. E., Rennke, H. G. & Brenner, B. M. Roles for thromboxane A2 and leukotrienes in endotoxin-induced acute renal failure. Kidney Int. 30, 74–480 (1986).
Awrty, E. A. & Loscalzo, J. in Platelets Second Edition (ed. Michelson, A.) 1099–1125 (Elsevier, 2006).
Mangano, D. T. et al. Aspirin and mortality from coronary bypass surgery. N Engl. J. Med. 347, 1309–1317 (2002).
Cao, L., Silvestry, S., Zhao, N., Diehl, J. & Sun, J. Effects of preoperative aspirin on cardiocerebral and renal complications in non-emergent cardiac surgery patients: a sub-group and cohort study. PLoS ONE 7, e30094 (2012).
Hur, M. et al. Preoperative aspirin use and acute kidney injury after cardiac surgery: a propensity-score matched observational study. PLoS ONE 12, e0177201 (2017).
Garg, A. X. et al. Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA 312, 2254–2264 (2014).
Devereaux, P. J. et al. Aspirin in patients undergoing noncardiac surgery. N. Engl. J. Med. 370, 1494–1503 (2014).
Karrowni, W. et al. Blood transfusion and the risk of acute kidney injury among patients with acute coronary syndrome undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 9, e003279 (2016).
Karkouti, K. Transfusion and risk of acute kidney injury in cardiac surgery. Br. J. Anaesth. 109 (Suppl. 1), i29–i38 (2012).
Stenberg, P. E., McEver, R. P., Shuman, M. A., Jacques, Y. V. & Bainton, D. F. A. Platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation J. Cell Biol. 101, 880–886 (1985).
Zizzi, H. C. et al. Quantification of P-selectin expression after renal ischemia and reperfusion. J. Pediatr. Surg. 32, 1010–1013 (1997).
Johnston, G. I., Cook, R. G. & McEver, R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell 56, 1033–1044 (1989).
Singbartl, K., Green, S. A. & Ley, K. Blocking P-selectin protects from ischemia reperfusion. Faseb J. 14, 48–54 (2000).
Singbartl, K., Forlow, S. B. & Ley, K. Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J. 15, 2337–2344 (2001).
Linden, M. D. & Furman, M. I. in Cardiovascular Biomarkers Pathophysiology and Disease Management (ed. Morrow, G. A.), 487–493 (Humana Press, 2005).
McEver, R. P. in Platelets Second Edition (ed. Michelson, A.) 231–249 (Elsevier, 2006).
Koo, D. D., Welsh, K. I., Roake, J. A., Morris, P. J. & Fuggle, S. V. Ischemia/reperfusion injury in human kidney transplantation: an immunhistochemical analysis of changes after reperfusion. Am. J. Pathol. 153, 557–566 (1998).
Patrono, C. Biosynthesis and pharmacological modulation of thromboxane in humans. Circulation 81, 12–15; discussion 122–123 (1990).
Lopez, L. R. et al. Platelet thromboxane (11-dehydro-Thromboxane B2) and aspirin response in patients with diabetes and coronary artery disease. World J. Diabetes 5, 115–127 (2014).
Klausner, J. M. et al. Vasodilating prostaglandins attenuate ischemic renal injury only if thromboxane is inhibited. Ann. Surg. 209, 219–224 (1989).
Ujike-Omori, H. et al. The urinary levels of prostanoid metabolites predict acute kidney injury in heterogeneous adult Japanese ICU patients: a prospective observational study. Clin. Exp. Nephrol. 19, 1024–1036 (2015).
Aukrust, P. et al. Elevated Circulating Levels of C-C chemokines in patients with congestive heart failure. Circulation 97, 1136–1143 (1998).
Yu, T. M. et al. RANTES mediates kidney ischemia reperfusion injury through a possible role of HIF-1alpha and LncRNA PRINS. Sci. Rep. 6, 18424 (2016).
Levine, S. P. & Wohl, H. Human platelet factor 4: purification and characterization by affinity chromatography. Purification of human platelet factor. J. Biol. Chem. 251, 324–328 (1976).
Kowalska, M. A., Rauova, L. & Poncz, M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb. Res. 4, 292–296 (2010).
Lapchak, P. H. et al. The role of platelet factor 4 in local and remote tissue damage in a mouse model of mesenteric ischemia/reperfusion injury. PLoS ONE 7, e39934 (2012).
Acknowledgements
J.J.T.H.R. is supported by The Netherlands Organisation for Health Research and Development (Clinical Fellowship grant #40-00703-97-12480) and by the Dutch Kidney Foundation (grant #KJP10.017).
Reviewer information
Nature Reviews Nephrology thanks B. Kerlin, A. Zarbock and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
M.P.B.J. researched data for the article and wrote the manuscript. M.P.B.J. and J.J.T.H.R. substantially contributed to the discussion of the content. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Thrombin
-
A serine protease that plays a key part in the coagulation cascade. Thrombin is generated from prothrombin by proteolytic cleavage that is mediated by blood coagulation factor X. This activation also releases prothrombin fragment 1.2, which can be used clinically as a coagulation marker.
- α-Granules
-
The most abundant storage platelet granules (50–80 granules per platelet) containing membrane-bound proteins such as P-selectin and soluble proteins such as platelet factor 4 (PF4) that are either expressed on the platelet surface or released into the extracellular space following platelet activation.
- Glycocalyx
-
A layer of proteoglycans that line the luminal endothelial surface, providing a physical barrier that prevents the adhesion and subsequent activation of platelets by endothelial components.
- Prostacyclin
-
(PGI2). A prostaglandin member of the eicosanoid family of lipid molecules that is released from the endothelium. PGI2 release inhibits platelet activation and has a role in the maintenance of a nonthrombotic barrier between the vessel wall and the blood. PGI2 is also an effective vasodilator.
- Ectonucleoside triphosphate diphosphohydrolase 1
-
(NTPDase 1). A membrane-anchored glycoprotein with ecto-apyrase activity that rapidly hydrolyses ATP and ADP into AMP.
- Weibel–Palade bodies
-
Endothelial-specific secretory granules that contain a variety of bioactive molecules that play a part in inflammation and haemostasis and are released by exocytosis upon endothelial activation.
- Extrinsic coagulation pathway
-
A branch of the coagulation pathway that begins with initiation of the coagulation cascade in response to tissue factor exposure (for example, as a result of tissue injury), leading to thrombin generation.
- Danger-associated molecular patterns
-
(DAMPs). Molecules released by stressed cells undergoing necrosis that act as endogenous danger signals to promote and exacerbate the inflammatory response.
- Opsonization
-
A process in which particles (such as bacteria) are marked for immune cell destruction through phagocytosis.
- Haemoconcentration
-
A decrease in plasma volume that causes an increase in the concentration of circulating blood cells. Haemoconcentration facilitates the inflammatory process by allowing increased endothelial cell–leukocyte contact.
- Caecal ligation and puncture
-
A method used in animal models to induce polymicrobial sepsis for studying the progression and characteristics of human sepsis.
- Chimeric mice
-
Mice composed of cells with distinct genotypes.
- Intravital imaging
-
A form of microscopy that allows the capture of images of biological processes in vivo at a high resolution.
- Leukotrienes
-
A family of eicosanoid inflammatory mediators (such as leukotriene B4) that are produced in leukocytes by oxidation of arachidonic acid and the essential fatty acid eicosapentaenoic acid by the enzyme arachidonate 5-lipoxygenase.
- Systemic inflammatory response syndrome
-
(SIRS). An excessive immune response triggered by a non-infectious agent as a result of trauma, burn or acute pancreatitis.
- Reactive oxygen species
-
(ROS). Highly reactive chemical species containing oxygen, which have important roles in processes such as cell signalling, homeostasis and defence against pathogens.
- Histone citrullination
-
An epigenetic post-translational modification in which arginine is converted to citrulline on histones, thereby affecting chromatin structure.
- Phosphodiesterase inhibitors
-
A class of platelet activation inhibitors that interfere with the breakdown of intracellular cyclic nucleotides (cGMP and cAMP) by reversible binding to phosphodiesterases, thereby increasing their concentration.
- Thienopyridines
-
A class of small-molecule inhibitors that irreversibly bind to the ADP-binding pocket of P2Y purinoreceptor 12 (P2Y12) on platelets.
- Thrombotic microangiopathy
-
A pathology that results in thrombosis in capillaries and arterioles owing to an endothelial injury.
- Prostanoid
-
A type of biologically active lipid that forms a subclass of the eicosanoid family. Prostanoids are formed from the metabolism of arachidonic acid by the action of cyclooxygenase (COX) enzymes and include prostaglandin E2 (PGE2), thromboxanes (such as thromboxane A2 (TxA2)), prostacyclin (PGI2), prostaglandin F2A (PGF2A) and prostaglandin D2 (PGD2), each of which is involved in some aspect of the inflammatory response.
- Propensity score
-
A statistical matching technique that attempts to estimate the effect of an intervention by accounting for the covariates that predict receiving the treatment.
Rights and permissions
About this article
Cite this article
Jansen, M.P.B., Florquin, S. & Roelofs, J.J.T.H. The role of platelets in acute kidney injury. Nat Rev Nephrol 14, 457–471 (2018). https://doi.org/10.1038/s41581-018-0015-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0015-5
This article is cited by
-
Pioneering predictions of AKI and AKIN severity in burn patients: a comprehensive CBC approach
Scientific Reports (2024)
-
Construction and validation of an early warning model for predicting the acute kidney injury in elderly patients with sepsis
Aging Clinical and Experimental Research (2022)
-
Immunopathophysiology of trauma-related acute kidney injury
Nature Reviews Nephrology (2021)
-
Preliminary algorithm for a personalized diagnosis of cardiovascular disease and dependent renal complications using decision tree
Proceedings of the Indian National Science Academy (2021)
-
Intraoperative blood transfusion volume is an independent risk factor for postoperative acute kidney injury in type A acute aortic dissection
BMC Cardiovascular Disorders (2020)