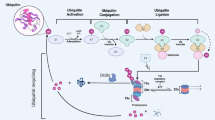
Abstract
Sumoylation is an essential post-translational modification that is catalysed by a small number of modifying enzymes but regulates thousands of target proteins in a dynamic manner. Small ubiquitin-like modifiers (SUMOs) can be attached to target proteins as one or more monomers or in the form of polymers of different types. Non-covalent readers recognize SUMO-modified proteins via SUMO interaction motifs. SUMO simultaneously modifies groups of functionally related proteins to regulate predominantly nuclear processes, including gene expression, the DNA damage response, RNA processing, cell cycle progression and proteostasis. Recent progress has increased our understanding of the cellular and pathophysiological roles of SUMO modifications, extending their functions to the regulation of immunity, pluripotency and nuclear body assembly in response to oxidative stress, which partly occurs through the recently characterized mechanism of liquid–liquid phase separation. Such progress in understanding the roles and regulation of sumoylation opens new avenues for the targeting of SUMO to treat disease, and indeed the first drug blocking sumoylation is currently under investigation in clinical trials as a possible anticancer agent.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mahajan, R., Delphin, C., Guan, T., Gerace, L. & Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88, 97–107 (1997).
Matunis, M. J., Coutavas, E. & Blobel, G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135, 1457–1470 (1996).
Hendriks, I. A. & Vertegaal, A. C. A comprehensive compilation of SUMO proteomics. Nat. Rev. Mol. Cell Biol. 17, 581–595 (2016).
Lamoliatte, F., McManus, F. P., Maarifi, G., Chelbi-Alix, M. K. & Thibault, P. Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat. Commun. 8, 14109 (2017).
Li, C. et al. Quantitative SUMO proteomics identifies PIAS1 substrates involved in cell migration and motility. Nat. Commun. 11, 834 (2020).
Kohler, J. B. et al. Targeting of SUMO substrates to a Cdc48-Ufd1-Npl4 segregase and STUbL pathway in fission yeast. Nat. Commun. 6, 8827 (2015).
Lumpkin, R. J. et al. Site-specific identification and quantitation of endogenous SUMO modifications under native conditions. Nat. Commun. 8, 1171 (2017).
Hendriks, I. A., D’Souza, R. C., Chang, J. G., Mann, M. & Vertegaal, A. C. System-wide identification of wild-type SUMO-2 conjugation sites. Nat. Commun. 6, 7289 (2015).
Hendriks, I. A. et al. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat. Struct. Mol. Biol. 21, 927–936 (2014).
Hendriks, I. A. et al. Site-specific characterization of endogenous SUMOylation across species and organs. Nat. Commun. 9, 2456 (2018).
Hendriks, I. A. et al. Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nat. Struct. Mol. Biol. 24, 325–336 (2017). Hendriks et al. uncover the complexity of the sumoylated proteome and frequent crosstalk between sumoylation and phosphorylation.
Tammsalu, T. et al. Proteome-wide identification of SUMO2 modification sites. Sci. Signal. 7, rs2 (2014).
Teramura, H. et al. Characterization of novel SUMO family genes in the rice genome. Genes Genet. Syst. 96, 25–32 (2021).
Kurepa, J. et al. The small ubiquitin-like modifier (SUMO) protein modification system in Arabidopsis. Accumulation of SUMO1 and -2 conjugates is increased by stress. J. Biol. Chem. 278, 6862–6872 (2003).
Liang, Y. C. et al. SUMO5, a novel poly-SUMO isoform, regulates PML nuclear bodies. Sci. Rep. 6, 26509 (2016).
Su, H. L. & Li, S. S. Molecular features of human ubiquitin-like SUMO genes and their encoded proteins. Gene 296, 65–73 (2002).
Wang, L. et al. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep. 15, 878–885 (2014).
Evdokimov, E., Sharma, P., Lockett, S. J., Lualdi, M. & Kuehn, M. R. Loss of SUMO1 in mice affects RanGAP1 localization and formation of PML nuclear bodies, but is not lethal as it can be compensated by SUMO2 or SUMO3. J. Cell Sci. 121, 4106–4113 (2008).
Zhang, F. P. et al. Sumo-1 function is dispensable in normal mouse development. Mol. Cell Biol. 28, 5381–5390 (2008).
Owerbach, D., McKay, E. M., Yeh, E. T., Gabbay, K. H. & Bohren, K. M. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem. Biophys. Res. Commun. 337, 517–520 (2005).
Nacerddine, K. et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell 9, 769–779 (2005).
Kunz, K., Piller, T. & Muller, S. SUMO-specific proteases and isopeptidases of the SENP family at a glance. J. Cell Sci. https://doi.org/10.1242/jcs.211904 (2018).
Odeh, H. M., Coyaud, E., Raught, B. & Matunis, M. J. The SUMO-specific isopeptidase SENP2 is targeted to intracellular membranes via a predicted N-terminal amphipathic alpha-helix. Mol. Biol. Cell 29, 1878–1890 (2018).
Karami, S. et al. Novel SUMO-protease SENP7S regulates beta-catenin signaling and mammary epithelial cell transformation. Sci. Rep. 7, 46477 (2017).
Eisenhardt, N. et al. A new vertebrate SUMO enzyme family reveals insights into SUMO-chain assembly. Nat. Struct. Mol. Biol. 22, 959–967 (2015).
Gonzalez-Prieto, R. et al. Global non-covalent SUMO interaction networks reveal SUMO-dependent stabilization of the non-homologous end joining complex. Cell Rep. 34, 108691 (2021).
Hecker, C. M., Rabiller, M., Haglund, K., Bayer, P. & Dikic, I. Specification of SUMO1- and SUMO2-interacting motifs. J. Biol. Chem. 281, 16117–16127 (2006).
Nagaraj, N. et al. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 7, 548 (2011).
Cappadocia, L. & Lima, C. D. Ubiquitin-like protein conjugation: structures, chemistry, and mechanism. Chem. Rev. 118, 889–918 (2018).
Pichler, A., Fatouros, C., Lee, H. & Eisenhardt, N. SUMO conjugation - a mechanistic view. Biomol. Concepts 8, 13–36 (2017).
Wagner, K. et al. The SUMO isopeptidase SENP6 functions as a rheostat of chromatin residency in genome maintenance and chromosome dynamics. Cell Rep. 29, 480–494 e485 (2019).
Palvimo, J. J. PIAS proteins as regulators of small ubiquitin-related modifier (SUMO) modifications and transcription. Biochem. Soc. Trans. 35, 1405–1408 (2007).
Rodriguez, M. S., Dargemont, C. & Hay, R. T. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J. Biol. Chem. 276, 12654–12659 (2001).
Matic, I. et al. Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Mol. Cell 39, 641–652 (2010).
Bernier-Villamor, V., Sampson, D. A., Matunis, M. J. & Lima, C. D. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108, 345–356 (2002).
Streich, F. C. Jr. & Lima, C. D. Capturing a substrate in an activated RING E3/E2-SUMO complex. Nature 536, 304–308 (2016). In this study, the SUMO E3 ligase Siz1 is shown to force-feed K164 of PCNA in the E2 active site to mediate preferential sumoylation.
Hickey, C. M., Wilson, N. R. & Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 13, 755–766 (2012).
Cheng, J., Kang, X., Zhang, S. & Yeh, E. T. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 131, 584–595 (2007).
Kang, X. et al. SUMO-specific protease 2 is essential for suppression of polycomb group protein-mediated gene silencing during embryonic development. Mol. Cell 38, 191–201 (2010).
Li, J. et al. Desumoylase SENP6 maintains osteochondroprogenitor homeostasis by suppressing the p53 pathway. Nat. Commun. 9, 143 (2018).
Shin, E. J. et al. DeSUMOylating isopeptidase: a second class of SUMO protease. EMBO Rep. 13, 339–346 (2012).
Schulz, S. et al. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep. 13, 930–938 (2012).
Hutten, S., Chachami, G., Winter, U., Melchior, F. & Lamond, A. I. A role for the Cajal-body-associated SUMO isopeptidase USPL1 in snRNA transcription mediated by RNA polymerase II. J. Cell Sci. 127, 1065–1078 (2014).
Tatham, M. H. et al. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276, 35368–35374 (2001).
Swatek, K. N. et al. Insights into ubiquitin chain architecture using Ub-clipping. Nature 572, 533–537 (2019).
Kulathu, Y. & Komander, D. Atypical ubiquitylation- the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 13, 508–523 (2012).
Jentsch, S. & Psakhye, I. Control of nuclear activities by substrate-selective and protein-group SUMOylation. Annu. Rev. Genet. 47, 167–186 (2013).
Psakhye, I. & Jentsch, S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 151, 807–820 (2012).
Pfander, B., Moldovan, G. L., Sacher, M., Hoege, C. & Jentsch, S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436, 428–433 (2005).
Papouli, E. et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19, 123–133 (2005).
Garcia-Rodriguez, N., Wong, R. P. & Ulrich, H. D. Functions of Ubiquitin and SUMO in DNA replication and replication stress. Front. Genet. 7, 87 (2016).
Dhingra, N., Wei, L. & Zhao, X. Replication protein A (RPA) sumoylation positively influences the DNA damage checkpoint response in yeast. J. Biol. Chem. 294, 2690–2699 (2019).
Cappadocia, L., Kochanczyk, T. & Lima, C. D. DNA asymmetry promotes SUMO modification of the single-stranded DNA-binding protein RPA. EMBO J. 40, e103787, https://doi.org/10.15252/embj.2019103787 (2021).
Cappadocia, L., Pichler, A. & Lima, C. D. Structural basis for catalytic activation by the human ZNF451 SUMO E3 ligase. Nat. Struct. Mol. Biol. 22, 968–975 (2015).
Danielsen, J. R. et al. DNA damage-inducible SUMOylation of HERC2 promotes RNF8 binding via a novel SUMO-binding zinc finger. J. Cell Biol. 197, 179–187 (2012).
Husnjak, K. & Dikic, I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 81, 291–322 (2012).
Parker, J. L. & Ulrich, H. D. SIM-dependent enhancement of substrate-specific SUMOylation by a ubiquitin ligase in vitro. Biochem. J. 457, 435–440 (2014).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Lyon, A. S., Peeples, W. B. & Rosen, M. K. A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol. 22, 215–235 (2021).
Banani, S. F. et al. Compositional control of phase-separated cellular bodies. Cell 166, 651–663 (2016). This study reveals how SUMO polymers and SIM polymers form substructures via phase separation, providing a mechanistic explanation for the assembly of nuclear bodies.
Cuijpers, S. A. G. & Vertegaal, A. C. O. Guiding mitotic progression by crosstalk between post-translational modifications. Trends Biochem. Sci. 43, 251–268 (2018).
Vaughan, R. M., Kupai, A. & Rothbart, S. B. Chromatin regulation through ubiquitin and ubiquitin-like histone modifications. Trends Biochem. Sci. 46, 258–269 (2021).
Perry, J. J., Tainer, J. A. & Boddy, M. N. A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem. Sci. 33, 201–208 (2008).
Hay, R. T. Decoding the SUMO signal. Biochem. Soc. Trans. 41, 463–473 (2013).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10, 547–555 (2008).
Tatham, M. H. et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat. Cell Biol. 10, 538–546 (2008).
Akimov, V. et al. UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat. Struct. Mol. Biol. 25, 631–640 (2018).
Sriramachandran, A. M. et al. Arkadia/RNF111 is a SUMO-targeted ubiquitin ligase with preference for substrates marked with SUMO1-capped SUMO2/3 chain. Nat. Commun. 10, 3678 (2019).
Kumar, R., Gonzalez-Prieto, R., Xiao, Z., Verlaan-de Vries, M. & Vertegaal, A. C. O. The STUbL RNF4 regulates protein group SUMOylation by targeting the SUMO conjugation machinery. Nat. Commun. 8, 1809 (2017).
Prudden, J. et al. SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 26, 4089–4101 (2007).
Yin, Y. et al. SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes Dev. 26, 1196–1208 (2012).
Galanty, Y., Belotserkovskaya, R., Coates, J. & Jackson, S. P. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 26, 1179–1195 (2012).
Horigome, C. et al. PolySUMOylation by Siz2 and Mms21 triggers relocation of DNA breaks to nuclear pores through the Slx5/Slx8 STUbL. Genes Dev. 30, 931–945 (2016).
Churikov, D. et al. SUMO-dependent relocalization of eroded telomeres to nuclear pore complexes controls telomere recombination. Cell Rep. 15, 1242–1253 (2016).
Lecona, E. et al. USP7 is a SUMO deubiquitinase essential for DNA replication. Nat. Struct. Mol. Biol. 23, 270–277 (2016). Lecona et al. identify a STUbP which maintains a SUMO-rich, ubiquitin-poor chromatin state required for replication.
Hendriks, I. A., Schimmel, J., Eifler, K., Olsen, J. V. & Vertegaal, A. C. O. Ubiquitin-specific protease 11 (USP11) deubiquitinates hybrid small ubiquitin-like modifier (SUMO)-ubiquitin chains to counteract RING finger protein 4 (RNF4). J. Biol. Chem. 290, 15526–15537 (2015).
Pfeiffer, A. et al. Ataxin-3 consolidates the MDC1-dependent DNA double-strand break response by counteracting the SUMO-targeted ubiquitin ligase RNF4. EMBO J. 36, 1066–1083 (2017).
Lin, C. H., Liu, S. Y. & Lee, E. H. SUMO modification of Akt regulates global SUMOylation and substrate SUMOylation specificity through Akt phosphorylation of Ubc9 and SUMO1. Oncogene 35, 595–607 (2016).
Hietakangas, V. et al. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol. Cell Biol. 23, 2953–2968 (2003).
Hietakangas, V. et al. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl Acad. Sci. USA 103, 45–50 (2006).
Mohideen, F. et al. A molecular basis for phosphorylation-dependent SUMO conjugation by the E2 UBC9. Nat. Struct. Mol. Biol. 16, 945–952 (2009).
Stehmeier, P. & Muller, S. Phospho-regulated SUMO interaction modules connect the SUMO system to CK2 signaling. Mol. Cell 33, 400–409 (2009).
Aichem, A. et al. The ubiquitin-like modifier FAT10 interferes with SUMO activation. Nat. Commun. 10, 4452 (2019).
Wei, B. et al. Mitotic phosphorylation of SENP3 regulates DeSUMOylation of chromosome-associated proteins and chromosome stability. Cancer Res. 78, 2171–2178 (2018).
Liebelt, F. & Vertegaal, A. C. Ubiquitin-dependent and independent roles of SUMO in proteostasis. Am. J. Physiol. Cell Physiol. 311, C284–C296 (2016).
Golebiowski, F. et al. System-wide changes to SUMO modifications in response to heat shock. Sci. Signal. 2, ra24 (2009).
Liebelt, F. et al. SUMOylation and the HSF1-regulated chaperone network converge to promote proteostasis in response to heat shock. Cell Rep. 26, 236–249 e234 (2019).
Keiten-Schmitz, J. et al. The nuclear SUMO-targeted ubiquitin quality control network regulates the dynamics of cytoplasmic stress granules. Mol. Cell 79, 54–67 e57 (2020).
Marmor-Kollet, H. et al. Spatiotemporal proteomic analysis of stress granule disassembly using APEX reveals regulation by SUMOylation and links to ALS pathogenesis. Mol. Cell 80, 876–891 e876 (2020).
Kim, Y. S., Keyser, S. G. & Schneekloth, J. S. Jr Synthesis of 2′,3′,4′-trihydroxyflavone (2-D08), an inhibitor of protein sumoylation. Bioorg. Med. Chem. Lett. 24, 1094–1097 (2014).
Fujino, N., Kubo, H. & Maciewicz, R. A. Phenotypic screening identifies Axl kinase as a negative regulator of an alveolar epithelial cell phenotype. Lab. Invest. 97, 1047–1062 (2017).
Hay, R. T. Role of ubiquitin-like proteins in transcriptional regulation. Ernst Scher. Res. Found. Workshop https://doi.org/10.1007/3-540-37633-x_10 (2006).
Rodriguez, M. S. et al. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18, 6455–6461 (1999).
Schimmel, J. et al. Uncovering SUMOylation dynamics during cell-cycle progression reveals FoxM1 as a key mitotic SUMO target protein. Mol. Cell 53, 1053–1066 (2014).
Ninova, M. et al. The SUMO ligase Su(var)2-10 controls hetero- and euchromatic gene expression via establishing H3K9 trimethylation and negative feedback regulation. Mol. Cell 77, 571–585 e574 (2020).
Ivanov, A. V. et al. PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell 28, 823–837 (2007).
Paakinaho, V. et al. SUMOylation regulates the protein network and chromatin accessibility at glucocorticoid receptor-binding sites. Nucleic Acids Res. 49, 1951–1971 (2021).
Lin, X. et al. Opposed regulation of corepressor CtBP by SUMOylation and PDZ binding. Mol. Cell 11, 1389–1396 (2003).
Chakrabarti, S. R. & Nucifora, G. The leukemia-associated gene TEL encodes a transcription repressor which associates with SMRT and mSin3A. Biochem. Biophys. Res. Commun. 264, 871–877 (1999).
Lin, D. Y. et al. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell 24, 341–354 (2006).
Ryu, H. Y., Zhao, D., Li, J., Su, D. & Hochstrasser, M. Histone sumoylation promotes Set3 histone-deacetylase complex-mediated transcriptional regulation. Nucleic Acids Res. 48, 12151–12168 (2020).
Rawat, P. et al. Stress-induced nuclear condensation of NELF drives transcriptional downregulation. Mol. Cell 81, 1013–1026 e1011 (2021).
Ninova, M. et al. Su(var)2-10 and the SUMO pathway link piRNA-guided target recognition to chromatin silencing. Mol. Cell 77, 556–570 e556 (2020).
Kim, H. et al. PIE-1 SUMOylation promotes germline fates and piRNA-dependent silencing in C. elegans. Elife https://doi.org/10.7554/eLife.63300 (2021).
Kim, H. et al. HDAC1 SUMOylation promotes Argonaute-directed transcriptional silencing in C. elegans. Elife https://doi.org/10.7554/eLife.63299 (2021).
Chen, P. et al. piRNA-mediated gene regulation and adaptation to sex-specific transposon expression in D. melanogaster male germline. Genes Dev. 35, 914–935 (2021).
Neyret-Kahn, H. et al. Sumoylation at chromatin governs coordinated repression of a transcriptional program essential for cell growth and proliferation. Genome Res. 23, 1563–1579 (2013).
Schwertman, P., Bekker-Jensen, S. & Mailand, N. Regulation of DNA double-strand break repair by ubiquitin and ubiquitin-like modifiers. Nat. Rev. Mol. Cell Biol. 17, 379–394 (2016).
Dantuma, N. P. & van Attikum, H. Spatiotemporal regulation of posttranslational modifications in the DNA damage response. EMBO J. 35, 6–23 (2016).
Dingler, F. A. et al. Two aldehyde clearance systems are essential to prevent lethal formaldehyde accumulation in mice and humans. Mol. Cell 80, 996–1012 e1019 (2020).
Stingele, J., Schwarz, M. S., Bloemeke, N., Wolf, P. G. & Jentsch, S. A DNA-dependent protease involved in DNA-protein crosslink repair. Cell 158, 327–338 (2014).
Borgermann, N. et al. SUMOylation promotes protective responses to DNA-protein crosslinks. EMBO J. https://doi.org/10.15252/embj.2019101496 (2019).
Schellenberg, M. J. et al. ZATT (ZNF451)-mediated resolution of topoisomerase 2 DNA-protein cross-links. Science 357, 1412–1416 (2017). Schellenberg et al. report that sumoylation plays an important role in the repair of DPCs.
Tian, T. et al. The ZATT-TOP2A-PICH axis drives extensive replication fork reversal to promote genome stability. Mol. Cell 81, 198–211 e196 (2021).
Fielden, J. et al. TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nat. Commun. 11, 1274 (2020).
Sun, Y. et al. A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Sci. Adv. https://doi.org/10.1126/sciadv.aba6290 (2020).
Liu, J. C. Y. et al. Mechanism and function of DNA replication-independent DNA-protein crosslink repair via the SUMO-RNF4 pathway. EMBO J. 40, e107413 (2021).
Pozzi, B. et al. SUMO conjugation to spliceosomal proteins is required for efficient pre-mRNA splicing. Nucleic Acids Res. 45, 6729–6745 (2017).
Vethantham, V., Rao, N. & Manley, J. L. Sumoylation regulates multiple aspects of mammalian poly(A) polymerase function. Genes Dev. 22, 499–511 (2008).
Du, Y. et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 46, 5195–5208 (2018).
Raman, N., Weir, E. & Muller, S. The AAA ATPase MDN1 acts as a SUMO-targeted regulator in mammalian pre-ribosome remodeling. Mol. Cell 64, 607–615 (2016).
Haindl, M., Harasim, T., Eick, D. & Muller, S. The nucleolar SUMO-specific protease SENP3 reverses SUMO modification of nucleophosmin and is required for rRNA processing. EMBO Rep. 9, 273–279 (2008).
Finkbeiner, E., Haindl, M. & Muller, S. The SUMO system controls nucleolar partitioning of a novel mammalian ribosome biogenesis complex. EMBO J. 30, 1067–1078 (2011).
Rao, H. B. et al. A SUMO-ubiquitin relay recruits proteasomes to chromosome axes to regulate meiotic recombination. Science 355, 403–407 (2017). This article describes crosstalk between sumoylation and the ubiquitin–proteasome system as being required for meiosis.
He, W. et al. SUMO fosters assembly and functionality of the MutSgamma complex to facilitate meiotic crossing over. Dev. Cell 56, 2073–2088 e2073 (2021).
Qiao, H. et al. Antagonistic roles of ubiquitin ligase HEI10 and SUMO ligase RNF212 regulate meiotic recombination. Nat. Genet. 46, 194–199 (2014).
Divekar, N. S., Davis-Roca, A. C., Zhang, L., Dernburg, A. F. & Wignall, S. M. A degron-based strategy reveals new insights into Aurora B function in C. elegans. PLoS Genet. 17, e1009567 (2021).
Pelisch, F. et al. A SUMO-dependent protein network regulates chromosome congression during oocyte meiosis. Mol. Cell 65, 66–77 (2017).
Lallemand-Breitenbach, V. & de The, H. PML nuclear bodies: from architecture to function. Curr. Opin. Cell Biol. 52, 154–161 (2018).
Weis, K. et al. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell 76, 345–356 (1994).
de The, H. et al. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66, 675–684 (1991).
Feric, M. et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 (2016).
Lallemand-Breitenbach, V. et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J. Exp. Med. 193, 1361–1371 (2001).
Lang, M. et al. Three-dimensional organization of promyelocytic leukemia nuclear bodies. J. Cell Sci. 123, 392–400 (2010).
Chung, I., Osterwald, S., Deeg, K. I. & Rippe, K. PML body meets telomere: the beginning of an ALTernate ending? Nucleus 3, 263–275 (2012).
Zhang, J. M., Genois, M. M., Ouyang, J., Lan, L. & Zou, L. Alternative lengthening of telomeres is a self-perpetuating process in ALT-associated PML bodies. Mol. Cell 81, 1027–1042 e1024 (2021).
Min, J., Wright, W. E. & Shay, J. W. Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Genes Dev. 33, 814–827 (2019).
Li, S. J. & Hochstrasser, M. A new protease required for cell-cycle progression in yeast. Nature 398, 246–251 (1999).
Seufert, W., Futcher, B. & Jentsch, S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature 373, 78–81 (1995).
Hayashi, T. et al. Ubc9 is essential for viability of higher eukaryotic cells. Exp. Cell Res. 280, 212–221 (2002).
Cuijpers, S. A. G. et al. Chromokinesin KIF4A teams up with stathmin 1 to regulate abscission in a SUMO-dependent manner. J. Cell Sci. https://doi.org/10.1242/jcs.248591 (2020).
Zhang, X. D. et al. SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Mol. Cell 29, 729–741 (2008).
Gonzalez-Prieto, R., Cuijpers, S. A., Kumar, R., Hendriks, I. A. & Vertegaal, A. C. c-Myc is targeted to the proteasome for degradation in a SUMOylation-dependent manner, regulated by PIAS1, SENP7 and RNF4. Cell Cycle 14, 1859–1872 (2015).
Sabo, A., Doni, M. & Amati, B. SUMOylation of Myc-family proteins. PLoS ONE 9, e91072 (2014).
Sun, X. X. et al. SUMO protease SENP1 deSUMOylates and stabilizes c-Myc. Proc. Natl Acad. Sci. USA 115, 10983–10988 (2018).
Wen, D., Wu, J., Wang, L. & Fu, Z. SUMOylation promotes nuclear import and stabilization of polo-like Kinase 1 to support its mitotic function. Cell Rep. 21, 2147–2159 (2017).
Perez de Castro, I. et al. A SUMOylation motif in aurora-A: implications for spindle dynamics and oncogenesis. Front. Oncol. 1, 50 (2011).
Fernandez-Miranda, G. et al. SUMOylation modulates the function of aurora-B kinase. J. Cell Sci. 123, 2823–2833 (2010).
Ban, R., Nishida, T. & Urano, T. Mitotic kinase aurora-B is regulated by SUMO-2/3 conjugation/deconjugation during mitosis. Genes Cell 16, 652–669 (2011).
Pelisch, F. et al. Dynamic SUMO modification regulates mitotic chromosome assembly and cell cycle progression in Caenorhabditis elegans. Nat. Commun. 5, 5485 (2014).
Lee, C. C., Li, B., Yu, H. & Matunis, M. J. Sumoylation promotes optimal APC/C activation and timely anaphase. Elife https://doi.org/10.7554/eLife.29539 (2018).
Eifler, K. et al. SUMO targets the APC/C to regulate transition from metaphase to anaphase. Nat. Commun. 9, 1119 (2018).
Yatskevich, S. et al. Molecular mechanisms of APC/C release from spindle assembly checkpoint inhibition by APC/C SUMOylation. Cell Rep. 34, 108929 (2021).
Su, X. B. et al. SUMOylation stabilizes sister kinetochore biorientation to allow timely anaphase. J. Cell Biol. https://doi.org/10.1083/jcb.202005130 (2021).
Hahn, W. C. et al. An expanded universe of cancer targets. Cell 184, 1142–1155 (2021).
Jansen, N. S. & Vertegaal, A. C. O. A chain of events: regulating target proteins by SUMO polymers. Trends Biochem. Sci. 46, 113–123 (2021).
Liebelt, F. et al. The poly-SUMO2/3 protease SENP6 enables assembly of the constitutive centromere-associated network by group deSUMOylation. Nat. Commun. 10, 3987 (2019).
Fu, H. et al. SENP6-mediated M18BP1 deSUMOylation regulates CENP-A centromeric localization. Cell Res. 29, 254–257 (2019).
Mitra, S. et al. Genetic screening identifies a SUMO protease dynamically maintaining centromeric chromatin. Nat. Commun. 11, 501 (2020).
Decque, A. et al. Sumoylation coordinates the repression of inflammatory and anti-viral gene-expression programs during innate sensing. Nat. Immunol. 17, 140–149 (2016). Decque et al. reveal that sumoylation acts as a repressor of antiviral immunity and inflammation by blocking gene expression of type I interferon.
Hannoun, Z., Maarifi, G. & Chelbi-Alix, M. K. The implication of SUMO in intrinsic and innate immunity. Cytokine Growth Factor. Rev. 29, 3–16 (2016).
Hu, Z. et al. SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function. Mol. Cell 81, 940–952 e945 (2021).
Cui, Y. et al. SENP7 potentiates cGAS activation by relieving SUMO-mediated inhibition of cytosolic DNA sensing. PLoS Pathog. 13, e1006156 (2017).
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Ding, X. et al. Protein SUMOylation is required for regulatory T cell expansion and function. Cell Rep. 16, 1055–1066 (2016).
Hu, M. M., Liao, C. Y., Yang, Q., Xie, X. Q. & Shu, H. B. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J. Exp. Med. 214, 973–989 (2017).
Hua, G., Ganti, K. P. & Chambon, P. Glucocorticoid-induced tethered transrepression requires SUMOylation of GR and formation of a SUMO-SMRT/NCoR1-HDAC3 repressing complex. Proc. Natl Acad. Sci. USA 113, E635–E643 (2016).
Hua, G., Paulen, L. & Chambon, P. GR SUMOylation and formation of an SUMO-SMRT/NCoR1-HDAC3 repressing complex is mandatory for GC-induced IR nGRE-mediated transrepression. Proc. Natl Acad. Sci. USA 113, E626–E634 (2016).
Tian, S., Poukka, H., Palvimo, J. J. & Janne, O. A. Small ubiquitin-related modifier-1 (SUMO-1) modification of the glucocorticoid receptor. Biochem. J. 367, 907–911 (2002).
Barry, R. et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat. Commun. 9, 3001 (2018).
Kroonen, J. S. & Vertegaal, A. C. O. Targeting SUMO signaling to wrestle cancer. Trends Cancer 7, 496–510 (2021).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 6, 38 (2020).
Theurillat, I. et al. Extensive SUMO modification of repressive chromatin factors distinguishes pluripotent from somatic cells. Cell Rep. 32, 108146 (2020).
Cossec, J. C. et al. SUMO safeguards somatic and pluripotent cell identities by enforcing distinct chromatin states. Cell Stem Cell 23, 742–757 e748 (2018).
Yan, Y. L. et al. DPPA2/4 and SUMO E3 ligase PIAS4 opposingly regulate zygotic transcriptional program. PLoS Biol. 17, e3000324 (2019).
Borkent, M. et al. A serial shRNA screen for roadblocks to reprogramming identifies the protein modifier SUMO2. Stem Cell Rep. 6, 704–716 (2016).
Seeler, J. S. & Dejean, A. SUMO and the robustness of cancer. Nat. Rev. Cancer 17, 184–197 (2017).
Du, L. et al. Role of SUMO activating enzyme in cancer stem cell maintenance and self-renewal. Nat. Commun. 7, 12326 (2016).
Eifler, K. & Vertegaal, A. C. O. SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem. Sci. 40, 779–793 (2015).
Kessler, J. D. et al. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science 335, 348–353 (2012).
Yu, B. et al. Oncogenesis driven by the Ras/Raf pathway requires the SUMO E2 ligase Ubc9. Proc. Natl Acad. Sci. USA 112, E1724–E1733 (2015).
Hoellein, A. et al. Myc-induced SUMOylation is a therapeutic vulnerability for B-cell lymphoma. Blood 124, 2081–2090 (2014).
Lightcap, E. S. et al. A small-molecule SUMOylation inhibitor activates antitumor immune responses and potentiates immune therapies in preclinical models. Sci. Transl. Med. 13, eaba7791 (2021). Lightcap et al. demonstrate the potential of sumoylation inhibition to reduce tumour growth in mice by activating interferon signalling.
Kumar, S. et al. Targeting pancreatic cancer by TAK-981: a SUMOylation inhibitor that activates the immune system and blocks cancer cell cycle progression in a preclinical model. Gut https://doi.org/10.1136/gutjnl-2021-324834 (2022). Kumar et al. demonstrate the potential of sumoylation inhibition to reduce tumour growth in mice by activating interferon signalling.
Filippopoulou, C., Simos, G. & Chachami, G. The role of sumoylation in the response to hypoxia: an overview. Cells https://doi.org/10.3390/cells9112359 (2020).
van Hagen, M., Overmeer, R. M., Abolvardi, S. S. & Vertegaal, A. C. RNF4 and VHL regulate the proteasomal degradation of SUMO-conjugated hypoxia-inducible factor-2alpha. Nucleic Acids Res. 38, 1922–1931 (2010).
Cui, C. P. et al. SENP1 promotes hypoxia-induced cancer stemness by HIF-1alpha deSUMOylation and SENP1/HIF-1alpha positive feedback loop. Gut 66, 2149–2159 (2017).
Shangguan, X. et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat. Commun. 12, 1812 (2021).
Langston, S. P. et al. Discovery of TAK-981, a first-in-class inhibitor of SUMO-activating enzyme for the treatment of cancer. J. Med. Chem. 64, 2501–2520 (2021). Langston et al. present a highly potent and specific SUMO E1 inhibitor that is currently under investigation in phase I clinical trials.
Correa-Vazquez, J. F. et al. The Sumo proteome of proliferating and neuronal-differentiating cells reveals Utf1 among key Sumo targets involved in neurogenesis. Cell Death Dis. 12, 305 (2021).
Tirard, M. et al. In vivo localization and identification of SUMOylated proteins in the brain of His6-HA-SUMO1 knock-in mice. Proc. Natl Acad. Sci. USA 109, 21122–21127 (2012).
Ochaba, J. et al. PIAS1 regulates mutant huntingtin accumulation and Huntington’s disease-associated phenotypes in vivo. Neuron 90, 507–520 (2016).
Steffan, J. S. et al. SUMO modification of Huntingtin and Huntington’s disease pathology. Science 304, 100–104 (2004).
Guo, L. et al. A cellular system that degrades misfolded proteins and protects against neurodegeneration. Mol. Cell 55, 15–30 (2014).
Marinello, M. et al. SUMOylation by SUMO2 is implicated in the degradation of misfolded ataxin-7 via RNF4 in SCA7 models. Dis. Model Mech. https://doi.org/10.1242/dmm.036145 (2019).
Bernstock, J. D. et al. SUMOylation in brain ischemia: patterns, targets, and translational implications. J. Cereb. Blood Flow. Metab. 38, 5–16 (2018).
Yang, W., Sheng, H., Warner, D. S. & Paschen, W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J. Cereb. Blood Flow. Metab. 28, 269–279 (2008).
Tai, D. J. et al. MeCP2 SUMOylation rescues Mecp2-mutant-induced behavioural deficits in a mouse model of Rett syndrome. Nat. Commun. 7, 10552 (2016).
Gupta, M. K. et al. UBC9-mediated sumoylation favorably impacts cardiac function in compromised hearts. Circ. Res. 118, 1894–1905 (2016).
Gupta, M. K. et al. Sumo E2 enzyme UBC9 is required for efficient protein quality control in cardiomyocytes. Circ. Res. 115, 721–729 (2014).
Dehnavi, S. et al. The role of protein SUMOylation in the pathogenesis of atherosclerosis. J. Clin. Med. https://doi.org/10.3390/jcm8111856 (2019).
Cai, Z. et al. Ablation of adenosine monophosphate-activated protein kinase alpha1 in vascular smooth muscle cells promotes diet-induced atherosclerotic calcification in vivo. Circ. Res. 119, 422–433 (2016).
Bondalapati, S., Eid, E., Mali, S. M., Wolberger, C. & Brik, A. Total chemical synthesis of SUMO-2-Lys63-linked diubiquitin hybrid chains assisted by removable solubilizing tags. Chem. Sci. 8, 4027–4034 (2017).
Mulder, M. P. C. et al. Total chemical synthesis of SUMO and SUMO-based probes for profiling the activity of SUMO-specific proteases. Angew. Chem. Int. Ed. 57, 8958–8962 (2018).
Weller, C. E. et al. Aromatic thiol-mediated cleavage of N-O bonds enables chemical ubiquitylation of folded proteins. Nat. Commun. 7, 12979 (2016).
Vertegaal, A. C. Uncovering ubiquitin and ubiquitin-like signaling networks. Chem. Rev. 111, 7923–7940 (2011).
Fukuda, I. et al. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem. Biol. 16, 133–140 (2009).
He, X. et al. Probing the roles of SUMOylation in cancer cell biology by using a selective SAE inhibitor. Nat. Chem. Biol. 13, 1164–1171 (2017).
Biederstadt, A. et al. SUMO pathway inhibition targets an aggressive pancreatic cancer subtype. Gut 69, 1472–1482 (2020).
Lv, Z. et al. Molecular mechanism of a covalent allosteric inhibitor of SUMO E1 activating enzyme. Nat. Commun. 9, 5145 (2018).
Benoit, Y. D. et al. Targeting SUMOylation dependency in human cancer stem cells through a unique SAE2 motif revealed by chemical genomics. Cell Chem. Biol. https://doi.org/10.1016/j.chembiol.2021.04.014 (2021).
Tahk, S. et al. Control of specificity and magnitude of NF-kappa B and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc. Natl Acad. Sci. USA 104, 11643–11648 (2007).
Lopez, I. et al. An unanticipated tumor-suppressive role of the SUMO pathway in the intestine unveiled by Ubc9 haploinsufficiency. Oncogene 39, 6692–6703 (2020).
Orosa-Puente, B. et al. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 362, 1407–1410 (2018). This article describes how sumoylation enables plants to regulate branching of roots towards water by regulating the auxin response.
Rytz, T. C. et al. SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO ligase SIZ1 during heat stress. Plant Cell 30, 1077–1099 (2018).
Augustine, R. C., York, S. L., Rytz, T. C. & Vierstra, R. D. Defining the SUMO system in maize: SUMOylation is up-regulated during endosperm development and rapidly induced by stress. Plant. Physiol. 171, 2191–2210 (2016).
Crozet, P. et al. SUMOylation represses SnRK1 signaling in Arabidopsis. Plant J. 85, 120–133 (2016).
Lin, X. L. et al. An Arabidopsis SUMO E3 ligase, SIZ1, negatively regulates photomorphogenesis by promoting COP1 activity. PLoS Genet. 12, e1006016 (2016).
Cheng, X. et al. Sumoylation of Turnip mosaic virus RNA polymerase promotes viral infection by counteracting the host NPR1-mediated immune response. Plant Cell 29, 508–525 (2017).
Acknowledgements
The author apologizes to researchers whose contributions were not cited here owing to space constraints. The author gratefully acknowledges the reviewers for their constructive criticism and F. Trulsson for assisting with Fig. 1b,c. Work in the author’s laboratory is supported by the European Research Council (ERC), the Netherlands Organization for Scientific Research (NWO) and the Dutch Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author receives funding from Millennium-Takeda for research on the SUMO E1 inhibitor TAK981.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Niels Mailand and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- RAN
-
A GTPase involved in nucleocytoplasmic transport of proteins and RNAs.
- Pluripotency
-
Ability of stem cells to differentiate into any cell type required to form an organism.
- Liquid–liquid phase separation
-
(LLPS). Biophysical process of tightly interacting proteins enabling the formation of small cellular domains that are separated from their surrounding environment to enhance biochemical reactions.
- Pseudogenes
-
Segments of DNA that resemble a gene but lack critical elements and are therefore not expressed.
- ZNF451
-
E4 enzyme that generates small ubiquitin-like modifier (SUMO) polymers.
- E4 elongase
-
Enzyme that generates small ubiquitin-like modifier (SUMO) polymers.
- Nuclear bodies
-
Membraneless nuclear substructure, assembled by liquid–liquid phase separation.
- SP-RING
-
Siz/PIAS-RING domain present in Siz/PIAS SUMO E3 ligases catalyzing sumoylation of target proteins. This domain is a hybrid between RING and U-box domains as it contains a single zinc coordination site.
- Helicase
-
Motor protein that separates two hybridized nucleic acid strands.
- PML nuclear bodies
-
Dynamic nuclear bodies that contain promyelocytic leukaemia protein (PML) as a marker. These small nuclear substructures are assembled by phase separation.
- Top-down mass spectrometry
-
Mass spectrometry method for analysis of intact proteins.
- DNA–protein crosslinks
-
(DPCs). Crosslinks between DNA and a protein that constitute a replication-blocking lesion.
- Topoisomerase 2
-
(TOP2). Decatenating enzyme that adjusts the topology of DNA by producing a double-strand break in DNA to manage tangles and supercoils, and subsequently religating the broken DNA molecule.
- N 6-Adenosine methyltransferase complex
-
An enzyme that methylates adenosine at the N6 position in eukaryotic mRNAs, forming N6-methyladenosine.
- Chromosome congression
-
Alignment of chromosomes at the spindle equator, at equal distances from both spindle poles.
- Retinoic acid
-
A derivative of vitamin A that regulates cell growth, cell differentiation and organogenesis.
- Alternative lengthening of telomeres
-
(ALT). A process that enables cells to maintain telomere length independently of the enzyme telomerase.
- Innate immunity
-
The first line of defence against invading pathogens, which contrasts with the adaptive immune system, which is the second line of defence.
- Dendritic cells
-
Cells that present antigens to the adaptive immune system to stimulate acquired immunity.
- Cancer stem cells
-
A subpopulation of cancer cells which are capable of self-renewal.
Rights and permissions
About this article
Cite this article
Vertegaal, A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat Rev Mol Cell Biol 23, 715–731 (2022). https://doi.org/10.1038/s41580-022-00500-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-022-00500-y
This article is cited by
-
Sumoylation of SAP130 regulates its interaction with FAF1 as well as its protein stability and transcriptional repressor function
BMC Molecular and Cell Biology (2024)
-
Mechanisms and functions of SUMOylation in health and disease: a review focusing on immune cells
Journal of Biomedical Science (2024)
-
UBR5 promotes antiviral immunity by disengaging the transcriptional brake on RIG-I like receptors
Nature Communications (2024)
-
SENP3 mediates the activation of the Wnt/β-catenin signaling pathway to accelerate the growth and metastasis of oesophagal squamous cell carcinoma in mice
Functional & Integrative Genomics (2024)
-
Post translational modifications of connexin 43 in ventricular arrhythmias after myocardial infarction
Molecular Biology Reports (2024)