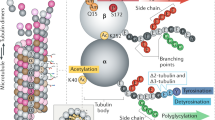
Abstract
Microtubules are polarized cytoskeletal filaments that serve as tracks for intracellular transport and form a scaffold that positions organelles and other cellular components and modulates cell shape and mechanics. In animal cells, the geometry, density and directionality of microtubule networks are major determinants of cellular architecture, polarity and proliferation. In dividing cells, microtubules form bipolar spindles that pull chromosomes apart, whereas in interphase cells, microtubules are organized in a cell type-specific fashion, which strongly correlates with cell physiology. In motile cells, such as fibroblasts and immune cells, microtubules are organized as radial asters, whereas in immotile epithelial and neuronal cells and in muscles, microtubules form parallel or antiparallel arrays and cortical meshworks. Here, we review recent work addressing how the formation of such microtubule networks is driven by the plethora of microtubule regulatory proteins. These include proteins that nucleate or anchor microtubule ends at different cellular structures and those that sever or move microtubules, as well as regulators of microtubule elongation, stability, bundling or modifications. The emerging picture, although still very incomplete, shows a remarkable diversity of cell-specific mechanisms that employ conserved building blocks to adjust microtubule organization in order to facilitate different cellular functions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kapitein, L. C. & Hoogenraad, C. C. Building the neuronal microtubule cytoskeleton. Neuron 87, 492–506 (2015).
Cuenca-Zamora, E. J., Ferrer-Marin, F., Rivera, J. & Teruel-Montoya, R. Tubulin in platelets: when the shape matters. Int. J. Mol. Sci. 20, 3484 (2019).
Caporizzo, M. A., Chen, C. Y. & Prosser, B. L. Cardiac microtubules in health and heart disease. Exp. Biol. Med. 244, 1255–1272 (2019).
Mitchison, T. J. & Field, C. M. Self-organization of cellular units. Annu. Rev. Cell Dev. Biol. 37, 23–42 (2021).
Roostalu, J. & Surrey, T. Microtubule nucleation: beyond the template. Nat. Rev. Mol. Cell Biol. 18, 702–710 (2017).
Tovey, C. A. & Conduit, P. T. Microtubule nucleation by γ-tubulin complexes and beyond. Essays Biochem. 62, 765–780 (2018).
Lin, T. C., Neuner, A. & Schiebel, E. Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends Cell Biol. 25, 296–307 (2015).
Hannak, E. et al. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is γ-tubulin dependent. J. Cell Biol. 157, 591–602 (2002).
Rogers, G. C., Rusan, N. M., Peifer, M. & Rogers, S. L. A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol. Biol. Cell 19, 3163–3178 (2008).
Tsuchiya, K. & Goshima, G. Microtubule-associated proteins promote microtubule generation in the absence of γ-tubulin in human colon cancer cells. J. Cell Biol. 220, e202104114 (2021).
Wang, S. et al. NOCA-1 functions with γ-tubulin and in parallel to Patronin to assemble non-centrosomal microtubule arrays in C. elegans. eLife 4, e08649 (2015). This study demonstrates how two pathways of microtubule minus-end organization can synergize or work in parallel to form non-centrosomal microtubule arrays in different cell types.
Sallee, M. D., Zonka, J. C., Skokan, T. D., Raftrey, B. C. & Feldman, J. L. Tissue-specific degradation of essential centrosome components reveals distinct microtubule populations at microtubule organizing centers. PLoS Biol. 16, e2005189 (2018).
Zheng, Y. et al. A perinuclear microtubule-organizing centre controls nuclear positioning and basement membrane secretion. Nat. Cell Biol. 22, 297–309 (2020). This paper describes an unconventional, γ-TuRC-independent pathway of microtubule organization acting at the nuclear envelope of fly fat body cells.
King, M. R. & Petry, S. Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. Nat. Commun. 11, 270 (2020).
Woodruff, J. B. et al. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169, 1066–1077 (2017).
Roostalu, J., Cade, N. I. & Surrey, T. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat. Cell Biol. 17, 1422–1434 (2015).
Manka, S. W. & Moores, C. A. Pseudo-repeats in doublecortin make distinct mechanistic contributions to microtubule regulation. EMBO Rep. 21, e51534 (2020).
Aher, A. et al. CLASP mediates microtubule repair by restricting lattice damage and regulating tubulin incorporation. Curr. Biol. 30, 2175–2183 (2020).
Abal, M. et al. Microtubule release from the centrosome in migrating cells. J. Cell Biol. 159, 731–737 (2002).
Goldspink, D. A. et al. Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres. Open. Biol. 7, 160274 (2017).
Delgehyr, N., Sillibourne, J. & Bornens, M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118, 1565–1575 (2005).
Lechler, T. & Fuchs, E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J. Cell Biol. 176, 147–154 (2007).
Lecland, N., Hsu, C. Y., Chemin, C., Merdes, A. & Bierkamp, C. Epidermal development requires ninein for spindle orientation and cortical microtubule organization. Life Sci. Alliance 2, e201900373 (2019).
Meng, W., Mushika, Y., Ichii, T. & Takeichi, M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell–cell contacts. Cell 135, 948–959 (2008). This landmark study presents the first functional description of a CAMSAP/Patronin family member and shows that it is involved in microtubule organization at cell–cell junctions.
Goodwin, S. S. & Vale, R. D. Patronin regulates the microtubule network by protecting microtubule minus ends. Cell 143, 263–274 (2010).
Jiang, K. et al. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev. Cell 28, 295–309 (2014).
Hernandez-Vega, A. et al. Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Rep. 20, 2304–2312 (2017).
Imasaki, T. et al. CAMSAP2 organizes a γ-tubulin-independent microtubule nucleation centre. Preprint at bioRxiv https://doi.org/10.1101/2021.03.01.433304 (2021).
Wu, J. et al. Molecular pathway of microtubule organization at the Golgi apparatus. Dev. Cell 39, 44–60 (2016). This study provides a comprehensive analysis of the pathways of microtubule nucleation and anchoring at the Golgi apparatus.
Coquand, L. et al. CAMSAPs organize an acentrosomal microtubule network from basal varicosities in radial glial cells. J. Cell Biol. 220, e202003151 (2021).
Noordstra, I. et al. Control of apico-basal epithelial polarity by the microtubule minus-end-binding protein CAMSAP3 and spectraplakin ACF7. J. Cell Sci. 129, 4278–4288 (2016).
Khanal, I., Elbediwy, A., Diaz de la Loza Mdel, C., Fletcher, G. C. & Thompson, B. J. Shot and Patronin polarise microtubules to direct membrane traffic and biogenesis of microvilli in epithelia. J. Cell Sci. 129, 2651–2659 (2016).
Martin, M. & Akhmanova, A. Coming into focus: mechanisms of microtubule minus-end organization. Trends Cell Biol. 28, 574–588 (2018).
Dong, C. et al. CAMSAP3 accumulates in the pericentrosomal area and accompanies microtubule release from the centrosome via katanin. J. Cell Sci. 130, 1709–1715 (2017).
Gillard, G., Girdler, G. & Roper, K. A release-and-capture mechanism generates an essential non-centrosomal microtubule array during tube budding. Nat. Commun. 12, 4096 (2021). This paper shows how a microtubule array formed by severing and minus-end stabilization affects tissue morphogenesis.
Muroyama, A., Seldin, L. & Lechler, T. Divergent regulation of functionally distinct γ-tubulin complexes during differentiation. J. Cell Biol. 213, 679–692 (2016). This paper provides important insights into switching from a centrosomal to a non-centrosomal microtubule array during cell differentiation and into the role of γ-TuRC-associated proteins in this process.
Stiess, M. et al. Axon extension occurs independently of centrosomal microtubule nucleation. Science 327, 704–707 (2010).
Muroyama, A. & Lechler, T. Microtubule organization, dynamics and functions in differentiated cells. Development 144, 3012–3021 (2017).
Zhang, X. et al. Cell-type-specific alternative splicing governs cell fate in the developing cerebral cortex. Cell 166, 1147–1162 (2016).
Zhu, X. & Kaverina, I. Golgi as an MTOC: making microtubules for its own good. Histochem. Cell Biol. 140, 361–367 (2013).
Liang, X. et al. Growth cone-localized microtubule organizing center establishes microtubule orientation in dendrites. eLife 9, e56547 (2020).
Bernabe-Rubio, M. & Alonso, M. A. Routes and machinery of primary cilium biogenesis. Cell Mol. Life Sci. 74, 4077–4095 (2017).
Pitaval, A. et al. Microtubule stabilization drives 3D centrosome migration to initiate primary ciliogenesis. J. Cell Biol. 216, 3713–3728 (2017).
Garbrecht, J., Laos, T., Holzer, E., Dillinger, M. & Dammermann, A. An acentriolar centrosome at the C. elegans ciliary base. Curr. Biol. 31, 2418–2428.e8 (2021).
Magescas, J., Eskinazi, S., Tran, M. V. & Feldman, J. L. Centriole-less pericentriolar material serves as a microtubule organizing center at the base of C. elegans sensory cilia. Curr. Biol. 31, 2410–2417 (2021). Together with Garbrecht et al. (2021), this work provides molecular and functional insight into the formation of an acentriolar MTOC located at the ciliary base in worm neurons.
Spassky, N. & Meunier, A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423–436 (2017).
Clare, D. K. et al. Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat. Commun. 5, 4888 (2014).
Tateishi, K., Nishida, T., Inoue, K. & Tsukita, S. Three-dimensional organization of layered apical cytoskeletal networks associated with mouse airway tissue development. Sci. Rep. 7, 43783 (2017).
Mercey, O. et al. Massive centriole production can occur in the absence of deuterosomes in multiciliated cells. Nat. Cell Biol. 21, 1544–1552 (2019).
Usami, F. M. et al. Intercellular and intracellular cilia orientation is coordinated by CELSR1 and CAMSAP3 in oviduct multi-ciliated cells. J. Cell Sci. 134, jcs257006 (2021).
Robinson, A. M. et al. CAMSAP3 facilitates basal body polarity and the formation of the central pair of microtubules in motile cilia. Proc. Natl Acad. Sci. USA 117, 13571–13579 (2020).
Rios, R. M. The centrosome–Golgi apparatus nexus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130462 (2014).
Rivero, S., Cardenas, J., Bornens, M. & Rios, R. M. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 28, 1016–1028 (2009).
Gavilan, M. P. et al. The dual role of the centrosome in organizing the microtubule network in interphase. EMBO Rep. 19, e45942 (2018).
Yang, C. et al. EB1 and EB3 regulate microtubule minus end organization and Golgi morphology. J. Cell Biol. 216, 3179–3198 (2017).
Efimov, A. et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917–930 (2007). This study convincingly demonstrates that the Golgi apparatus serves as a major MTOC in mammalian cells.
Mukherjee, A., Brooks, P. S., Bernard, F., Guichet, A. & Conduit, P. T. D. Microtubules originate asymmetrically at the somatic Golgi and are guided via Kinesin2 to maintain polarity within neurons. eLife 9, e58943 (2020).
Valenzuela, A., Meservey, L., Nguyen, H. & Fu, M. M. Golgi outposts nucleate microtubules in cells with specialized shapes. Trends Cell Biol. 30, 792–804 (2020).
Oddoux, S. et al. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J. Cell Biol. 203, 205–213 (2013).
Gimpel, P. et al. Nesprin-1α-dependent microtubule nucleation from the nuclear envelope via Akap450 is necessary for nuclear positioning in muscle cells. Curr. Biol. 27, 2999–3009 (2017).
Yalgin, C. et al. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nat. Neurosci. 18, 1437–1445 (2015).
Ori-McKenney, K. M., Jan, L. Y. & Jan, Y. N. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921–930 (2012).
Yang, S. Z. & Wildonger, J. Golgi outposts locally regulate microtubule orientation in neurons but are not required for the overall polarity of the dendritic cytoskeleton. Genetics 215, 435–447 (2020).
Nguyen, M. M. et al. γ-Tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol. Biol. Cell 25, 2039–2050 (2014).
Fu, M. M. et al. The Golgi outpost protein TPPP nucleates microtubules and is critical for myelination. Cell 179, 132–146 (2019).
Weiner, A. T. et al. Endosomal Wnt signaling proteins control microtubule nucleation in dendrites. PLoS Biol. 18, e3000647 (2020). This paper shows that Wnt signalling pathway components associated with endosomes participate in organizing microtubules in fly neurons.
Hehnly, H. & Doxsey, S. Rab11 endosomes contribute to mitotic spindle organization and orientation. Dev. Cell 28, 497–507 (2014).
Krishnan, N. et al. Rab11 endosomes coordinate centrosome number and movement following mitotic exit. biorRxiv https://doi.org/10.1101/2021.08.11.455966 (2021).
Chen, J. V., Buchwalter, R. A., Kao, L. R. & Megraw, T. L. A splice variant of centrosomin converts mitochondria to microtubule-organizing centers. Curr. Biol. 27, 1928–1940 (2017).
Vergarajauregui, S. et al. AKAP6 orchestrates the nuclear envelope microtubule-organizing center by linking Golgi and nucleus via AKAP9. eLife 9, e61669 (2020). This study represents a comprehensive analysis of the MTOC associated with the nuclear envelope in cardiomyocytes.
Harris, T. J. & Peifer, M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev. Cell 12, 727–738 (2007).
Feldman, J. L. & Priess, J. R. A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization. Curr. Biol. 22, 575–582 (2012).
Sanchez, A. D. et al. Proximity labeling reveals non-centrosomal microtubule-organizing center components required for microtubule growth and localization. Curr. Biol. 31, 3586–3600 (2021).
Castiglioni, V. G. et al. Epidermal PAR-6 and PKC-3 are essential for larval development of C. elegans and organize non-centrosomal microtubules. eLife 9, e62067 (2020).
Toya, M. et al. CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc. Natl Acad. Sci. USA 113, 332–337 (2016).
Nashchekin, D., Fernandes, A. R. & St Johnston, D. Patronin/shot cortical foci assemble the noncentrosomal microtubule array that specifies the Drosophila anterior–posterior axis. Dev. Cell 38, 61–72 (2016).
Guerreiro, A. et al. WDR62 localizes katanin at spindle poles to ensure synchronous chromosome segregation. J. Cell Biol. 220, e202007171 (2021).
Huang, J., Liang, Z., Guan, C., Hua, S. & Jiang, K. WDR62 regulates spindle dynamics as an adaptor protein between TPX2/Aurora A and katanin. J. Cell Biol. 220, e202007167 (2021).
Petry, S., Groen, A. C., Ishihara, K., Mitchison, T. J. & Vale, R. D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777 (2013).
Sanchez-Huertas, C. et al. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat. Commun. 7, 12187 (2016).
Cunha-Ferreira, I. et al. The HAUS complex is a key regulator of non-centrosomal microtubule organization during neuronal development. Cell Rep. 24, 791–800 (2018). Together with ref. 80, this work demonstrates that branching microtubule nucleation has a role in the formation of microtubule arrays in different neuronal compartments.
Qu, X., Kumar, A., Blockus, H., Waites, C. & Bartolini, F. Activity-dependent nucleation of dynamic microtubules at presynaptic boutons controls neurotransmission. Curr. Biol. 29, 4231–4240 (2019).
Zenker, J. et al. A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science 357, 925–928 (2017). This paper shows how the cytokinetic bridge is transformed into an MTOC during early mammalian development.
Labat-de-Hoz, L. et al. A model for primary cilium biogenesis by polarized epithelial cells: role of the midbody remnant and associated specialized membranes. Front. Cell Dev. Biol. 8, 622918 (2020).
Desai, A. & Mitchison, T. J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117 (1997).
Brouhard, G. J. & Rice, L. M. Microtubule dynamics: an interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 19, 451–463 (2018).
Gudimchuk, N. B. & McIntosh, J. R. Regulation of microtubule dynamics, mechanics and function through the growing tip. Nat. Rev. Mol. Cell Biol. 22, 777–795 (2021).
Estevez-Gallego, J. et al. Structural model for differential cap maturation at growing microtubule ends. eLife 9, e50155 (2020).
Manka, S. W. & Moores, C. A. The role of tubulin–tubulin lattice contacts in the mechanism of microtubule dynamic instability. Nat. Struct. Mol. Biol. 25, 607–615 (2018).
LaFrance, B. J. et al. Structural transitions in the GTP cap visualized by cryo-electron microscopy of catalytically inactive microtubules. Proc. Natl Acad. Sci. USA 119 (2022).
Brouhard, G. J. et al. XMAP215 is a processive microtubule polymerase. Cell 132, 79–88 (2008).
Feng, C. et al. Patronin-mediated minus end growth is required for dendritic microtubule polarity. J. Cell Biol. 218, 2309–2328 (2019).
Akhmanova, A. & Steinmetz, M. O. Control of microtubule organization and dynamics: two ends in the limelight. Nat. Rev. Mol. Cell Biol. 16, 711–726 (2015).
Bouchet, B. P. et al. Mesenchymal cell invasion requires cooperative regulation of persistent microtubule growth by SLAIN2 and CLASP1. Dev. Cell 39, 708–723 (2016).
Grigoriev, I. et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr. Biol. 18, 177–182 (2008).
van der Vaart, B. et al. CFEOM1-associated kinesin KIF21A is a cortical microtubule growth inhibitor. Dev. Cell 27, 145–160 (2013).
Homma, N. et al. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 114, 229–239 (2003).
Maor-Nof, M. et al. Axonal pruning is actively regulated by the microtubule-destabilizing protein kinesin superfamily protein 2A. Cell Rep. 3, 971–977 (2013).
Jaworski, J. et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61, 85–100 (2009).
Straube, A. & Merdes, A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr. Biol. 17, 1318–1325 (2007).
Hooikaas, P. J. et al. Kinesin-4 KIF21B limits microtubule growth to allow rapid centrosome polarization in T cells. eLife 9, e62876 (2020). This paper shows that keeping microtubule arrays sparse can be important for rapid microtubule reorganization in immune cells.
Muhia, M. et al. The Kinesin KIF21B regulates microtubule dynamics and is essential for neuronal morphology, synapse function, and learning and memory. Cell Rep. 15, 968–977 (2016).
Bearce, E. A., Erdogan, B. & Lowery, L. A. TIPsy tour guides: how microtubule plus-end tracking proteins (+TIPs) facilitate axon guidance. Front. Cell Neurosci. 9, 241 (2015).
Baas, P. W., Rao, A. N., Matamoros, A. J. & Leo, L. Stability properties of neuronal microtubules. Cytoskeleton 73, 442–460 (2016).
Cuveillier, C. et al. MAP6 is an intraluminal protein that induces neuronal microtubules to coil. Sci. Adv. 6, eaaz4344 (2020). This study demonstrates that the protein responsible for strong stabilization of neuronal microtubules localizes to microtubule lumen and induces their deformation.
Bodakuntla, S., Jijumon, A. S., Villablanca, C., Gonzalez-Billault, C. & Janke, C. Microtubule-associated proteins: structuring the cytoskeleton. Trends Cell Biol. 29, 804–819 (2019).
Freal, A. et al. Feedback-driven assembly of the axon initial segment. Neuron 104, 305–321 (2019).
Feng, C. et al. Trim9 and Klp61F promote polymerization of new dendritic microtubules along parallel microtubules. J. Cell Sci. 134, jcs258437 (2021).
Janson, M. E., de Dood, M. E. & Dogterom, M. Dynamic instability of microtubules is regulated by force. J. Cell Biol. 161, 1029–1034 (2003).
Xu, Z. et al. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356, 328–332 (2017).
Thery, M. & Blanchoin, L. Microtubule self-repair. Curr. Opin. Cell Biol. 68, 144–154 (2021).
Schaedel, L. et al. Microtubules self-repair in response to mechanical stress. Nat. Mater. 14, 1156–1163 (2015).
Vemu, A. et al. Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 361, eaau1504 (2018).
Triclin, S. et al. Self-repair protects microtubules from destruction by molecular motors. Nat. Mater. 20, 883–891 (2021).
Andreu-Carbo, M., Fernandes, S., Velluz, M. C., Kruse, K. & Aumeier, C. Motor usage imprints microtubule stability along the shaft. Dev. Cell 57, 5–18 (2022).
Dimitrov, A. et al. Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues. Science 322, 1353–1356 (2008).
de Forges, H. et al. Localized mechanical stress promotes microtubule rescue. Curr. Biol. 26, 3399–3406 (2016).
Shima, T. et al. Kinesin-binding-triggered conformation switching of microtubules contributes to polarized transport. J. Cell Biol. 217, 4164–4183 (2018).
Peet, D. R., Burroughs, N. J. & Cross, R. A. Kinesin expands and stabilizes the GDP-microtubule lattice. Nat. Nanotechnol. 13, 386–391 (2018). Together with Shima et al. (2018), this work convincingly demonstrates that kinesin 1 can cause expansion of the microtubule lattice.
He, L. et al. Cortical anchoring of the microtubule cytoskeleton is essential for neuron polarity. eLife 9, e55111 (2020).
Dogterom, M. & Koenderink, G. H. Actin–microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 20, 38–54 (2019).
Noordstra, I. & Akhmanova, A. Linking cortical microtubule attachment and exocytosis. F1000Res 6, 469 (2017).
Basu, S. et al. CLASP2-dependent microtubule capture at the neuromuscular junction membrane requires LL5β and actin for focal delivery of acetylcholine receptor vesicles. Mol. Biol. Cell 26, 938–951 (2015).
Rahimov, F. & Kunkel, L. M. The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J. Cell Biol. 201, 499–510 (2013).
Nelson, D. M. et al. Variable rescue of microtubule and physiological phenotypes in mdx muscle expressing different miniaturized dystrophins. Hum. Mol. Genet. 27, 2090–2100 (2018).
Gawor, M. & Proszynski, T. J. The molecular cross talk of the dystrophin–glycoprotein complex. Ann. N. Y. Acad. Sci. 1412, 62–72 (2018).
Ayalon, G., Davis, J. Q., Scotland, P. B. & Bennett, V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135, 1189–1200 (2008).
Leterrier, C. et al. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc. Natl Acad. Sci. USA 108, 8826–8831 (2011).
Shaw, R. M. et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128, 547–560 (2007).
Roll-Mecak, A. The tubulin code in microtubule dynamics and information encoding. Dev. Cell 54, 7–20 (2020).
Janke, C. & Magiera, M. M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 21, 307–326 (2020).
Katrukha, E. A., Jurriens, D., Salas Pastene, D. M. & Kapitein, L. C. Quantitative mapping of dense microtubule arrays in mammalian neurons. eLife 10, e67925 (2021). This paper uses stimulated emission depletion and expansion microscopy to measure the distribution and relative abundance of different microtubule subsets in dendrites.
Peris, L. et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell Biol. 185, 1159–1166 (2009).
Valenstein, M. L. & Roll-Mecak, A. Graded control of microtubule severing by tubulin glutamylation. Cell 164, 911–921 (2016).
Cai, D., McEwen, D. P., Martens, J. R., Meyhofer, E. & Verhey, K. J. Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 7, e1000216 (2009).
Guardia, C. M., Farias, G. G., Jia, R., Pu, J. & Bonifacino, J. S. BORC functions upstream of Kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 17, 1950–1961 (2016).
Tas, R. P. et al. Differentiation between oppositely oriented microtubules controls polarized neuronal transport. Neuron 96, 1264–1271 (2017). This paper introduces Motor-PAINT and demonstrates that different microtubule subsets have different orientations within dendrites.
Sirajuddin, M., Rice, L. M. & Vale, R. D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335–344 (2014).
Monroy, B. Y. et al. A combinatorial MAP code dictates polarized microtubule transport. Dev. Cell 53, 60–72 (2020). This paper dissects the effects of an array of neuronal MAPs on the motility of several types of transporting kinesins.
Magiera, M. M., Singh, P., Gadadhar, S. & Janke, C. Tubulin posttranslational modifications and emerging links to human disease. Cell 173, 1323–1327 (2018).
Magiera, M. M. et al. Excessive tubulin polyglutamylation causes neurodegeneration and perturbs neuronal transport. EMBO J. 37, e100440 (2018).
Shashi, V. et al. Loss of tubulin deglutamylase CCP1 causes infantile-onset neurodegeneration. EMBO J. 37, e100540 (2018). Together with Magiera et al. (EMBO J, 2018), this work provides one of the best examples of how misregulation of microtubule post-translational modifications can lead to human disease.
Bodakuntla, S. et al. Tubulin polyglutamylation is a general traffic-control mechanism in hippocampal neurons. J. Cell Sci. 133, jcs241802 (2020).
Bodakuntla, S. et al. Distinct roles of α- and β-tubulin polyglutamylation in controlling axonal transport and in neurodegeneration. EMBO J. 40, e108498 (2021).
Gadadhar, S. et al. Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science 371, eabd4914 (2021).
Lechler, T. & Mapelli, M. Spindle positioning and its impact on vertebrate tissue architecture and cell fate. Nat. Rev. Mol. Cell Biol. 22, 691–708 (2021).
Jimenez, A. J. et al. Acto-myosin network geometry defines centrosome position. Curr. Biol. 31, 1206–1220 (2021).
Yi, J. et al. Centrosome repositioning in T cells is biphasic and driven by microtubule end-on capture-shrinkage. J. Cell Biol. 202, 779–792 (2013).
Zheng, Y. et al. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10, 1172–1180 (2008).
del Castillo, U., Winding, M., Lu, W. & Gelfand, V. I. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. eLife 4, e10140 (2015).
Del Castillo, U., Muller, H. J. & Gelfand, V. I. Kinetochore protein Spindly controls microtubule polarity in Drosophila axons. Proc. Natl Acad. Sci. USA 117, 12155–12163 (2020).
Lu, W. & Gelfand, V. I. Moonlighting motors: kinesin, dynein, and cell polarity. Trends Cell Biol. 27, 505–514 (2017).
Palacios, I. M. & St Johnston, D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development 129, 5473–5485 (2002).
Lu, W., Fox, P., Lakonishok, M., Davidson, M. W. & Gelfand, V. I. Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr. Biol. 23, 1018–1023 (2013).
Mattie, F. J. et al. Directed microtubule growth, +TIPs, and kinesin-2 are required for uniform microtubule polarity in dendrites. Curr. Biol. 20, 2169–2177 (2010).
Schatzle, P. et al. Activity-dependent actin remodeling at the base of dendritic spines promotes microtubule entry. Curr. Biol. 28, 2081–2093 (2018).
Merriam, E. B. et al. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J. Neurosci. 33, 16471–16482 (2013).
Slater, P. G. et al. XMAP215 promotes microtubule-F-actin interactions to regulate growth cone microtubules during axon guidance in Xenopus laevis. J. Cell Sci. 132, jcs224311 (2019).
Sanchez-Huertas, C. et al. The +TIP Navigator-1 is an actin-microtubule crosslinker that regulates axonal growth cone motility. J. Cell Biol. 219, e201905199 (2020).
Kundu, T., Dutta, P., Nagar, D., Maiti, S. & Ghose, A. Coupling of dynamic microtubules to F-actin by Fmn2 regulates chemotaxis of neuronal growth cones. J. Cell Sci. 134, jcs252916 (2021).
Burute, M. & Kapitein, L. C. Cellular logistics: unraveling the interplay between microtubule organization and intracellular transport. Annu. Rev. Cell Dev. Biol. 35, 29–54 (2019).
Douanne, T. & Griffiths, G. M. Cytoskeletal control of the secretory immune synapse. Curr. Opin. Cell Biol. 71, 87–94 (2021).
Kopf, A. et al. Microtubules control cellular shape and coherence in amoeboid migrating cells. J. Cell Biol. 219, e201907154 (2020). This study provides convincing support for the concept that one of the functions of the microtubule network is to preserve the integrity of highly branched cells during cell migration in complex environments.
Vertii, A. et al. The centrosome undergoes Plk1-independent interphase maturation during inflammation and mediates cytokine release. Dev. Cell 37, 377–386 (2016).
Etienne-Manneville, S. Microtubules in cell migration. Annu. Rev. Cell Dev. Biol. 29, 471–499 (2013).
Martin, M., Veloso, A., Wu, J., Katrukha, E. A. & Akhmanova, A. Control of endothelial cell polarity and sprouting angiogenesis by non-centrosomal microtubules. eLife 7, e 33864 (2018).
Pasquier, E., André, N. & Braguer, D. Targeting microtubules to inhibit angiogenesis and disrupt tumour vasculature: implications for cancer treatment. Curr. Cancer Drug Targets 7, 566–581 (2007).
Blasky, A. J., Mangan, A. & Prekeris, R. Polarized protein transport and lumen formation during epithelial tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 31, 575–591 (2015).
Booth, A. J. R., Blanchard, G. B., Adams, R. J. & Roper, K. A dynamic microtubule cytoskeleton directs medial actomyosin function during tube formation. Dev. Cell 29, 562–576 (2014).
Henderson, D. J., Long, D. A. & Dean, C. H. Planar cell polarity in organ formation. Curr. Opin. Cell Biol. 55, 96–103 (2018).
Matis, M., Russler-Germain, D. A., Hu, Q., Tomlin, C. J. & Axelrod, J. D. Microtubules provide directional information for core PCP function. eLife 3, e02893 (2014). This paper combines experiments with modelling to demonstrate the interplay between planar cell polarity signalling and the apical microtubule cytoskeleton.
Shimada, Y., Yonemura, S., Ohkura, H., Strutt, D. & Uemura, T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell 10, 209–222 (2006).
Kimura, T., Saito, H., Kawasaki, M. & Takeichi, M. CAMSAP3 is required for mTORC1-dependent ependymal cell growth and lateral ventricle shaping in mouse brains. Development 148, dev195073 (2021).
Herawati, E. et al. Multiciliated cell basal bodies align in stereotypical patterns coordinated by the apical cytoskeleton. J. Cell Biol. 214, 571–586 (2016).
Oddoux, S. et al. Misplaced Golgi elements produce randomly oriented microtubules and aberrant cortical arrays of microtubules in dystrophic skeletal muscle fibers. Front. Cell Dev. Biol. 7, 176 (2019).
Robison, P. et al. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 352, aaf0659 (2016). This paper demonstrates that specific interactions between microtubules and intermediate filaments contribute to the mechanics of heart cell contraction.
Salomon, A. K. et al. Desmin intermediate filaments and tubulin detyrosination stabilize growing microtubules in the cardiomyocyte. Preprint at bioRxiv https://doi.org/10.1101/2021.05.26.445641 (2021).
Yu, X. et al. MARK4 controls ischaemic heart failure through microtubule detyrosination. Nature 594, 560–565 (2021).
Zile, M. R. et al. Cardiocyte cytoskeleton in patients with left ventricular pressure overload hypertrophy. J. Am. Coll. Cardiol. 37, 1080–1084 (2001).
Chen, C. Y. et al. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat. Med. 24, 1225–1233 (2018).
Aiken, J. & Holzbaur, E. L. F. Cytoskeletal regulation guides neuronal trafficking to effectively supply the synapse. Curr. Biol. 31, R633–R650 (2021).
Koppers, M. & Farias, G. G. Organelle distribution in neurons: logistics behind polarized transport. Curr. Opin. Cell Biol. 71, 46–54 (2021).
Baas, P. W. Microtubules and neuronal polarity: lessons from mitosis. Neuron 22, 23–31 (1999).
Hertzler, J. I. et al. Kinetochore proteins suppress neuronal microtubule dynamics and promote dendrite regeneration. Mol. Biol. Cell 31, 2125–2138 (2020).
Cheerambathur, D. K. et al. The kinetochore–microtubule coupling machinery is repurposed in sensory nervous system morphogenesis. Dev. Cell 48, 864–872 (2019).
Zhao, G., Oztan, A., Ye, Y. & Schwarz, T. L. Kinetochore proteins have a post-mitotic function in neurodevelopment. Dev. Cell 48, 873–882 (2019).
Guedes-Dias, P. & Holzbaur, E. L. F. Axonal transport: driving synaptic function. Science 366, eaaw9997 (2019).
Yogev, S., Cooper, R., Fetter, R., Horowitz, M. & Shen, K. Microtubule organization determines axonal transport dynamics. Neuron 92, 449–460 (2016). This paper carefully analyses the number and length of microtubules in worm axons and shows that cargoes frequently pause at microtubule ends.
Guedes-Dias, P. et al. Kinesin-3 responds to local microtubule dynamics to target synaptic cargo delivery to the presynapse. Curr. Biol. 29, 268–282 (2019).
Nirschl, J. J., Magiera, M. M., Lazarus, J. E., Janke, C. & Holzbaur, E. L. α-Tubulin tyrosination and CLIP-170 phosphorylation regulate the initiation of dynein-driven transport in neurons. Cell Rep. 14, 2637–2652 (2016).
Moughamian, A. J., Osborn, G. E., Lazarus, J. E., Maday, S. & Holzbaur, E. L. Ordered recruitment of dynactin to the microtubule plus-end is required for efficient initiation of retrograde axonal transport. J. Neurosci. 33, 13190–13203 (2013).
Stone, M. C., Roegiers, F. & Rolls, M. M. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell 19, 4122–4129 (2008).
Goodwin, P. R., Sasaki, J. M. & Juo, P. Cyclin-dependent kinase 5 regulates the polarized trafficking of neuropeptide-containing dense-core vesicles in Caenorhabditis elegans motor neurons. J. Neurosci. 32, 8158–8172 (2012).
Harterink, M. et al. Light-controlled intracellular transport in Caenorhabditis elegans. Curr. Biol. 26, R153–R154 (2016).
Baas, P. W., Deitch, J. S., Black, M. M. & Banker, G. A. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl Acad. Sci. USA 85, 8335–8339 (1988). This paper demonstrates that the microtubule array in dendrites from cultured neurons has mixed polarity.
Yau, K. W. et al. Dendrites in vitro and in vivo contain microtubules of opposite polarity and axon formation correlates with uniform plus-end-out microtubule orientation. J. Neurosci. 36, 1071–1085 (2016). This paper demonstrates mixed polarity of the microtubule arrays in dendrites in brain tissue and uses laser-based microsurgery to quantify them.
Ayloo, S., Guedes-Dias, P., Ghiretti, A. E. & Holzbaur, E. L. F. Dynein efficiently navigates the dendritic cytoskeleton to drive the retrograde trafficking of BDNF/TrkB signaling endosomes. Mol. Biol. Cell 28, 2543–2554 (2017).
Kapitein, L. C. et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 20, 290–299 (2010).
van Beuningen, S. F. B. et al. TRIM46 controls neuronal polarity and axon specification by driving the formation of parallel microtubule arrays. Neuron 88, 1208–1226 (2015).
Rao, A. N. et al. Cytoplasmic dynein transports axonal microtubules in a polarity-sorting manner. Cell Rep. 19, 2210–2219 (2017).
Muralidharan, H. & Baas, P. W. Mitotic motor KIFC1 is an organizer of microtubules in the axon. J. Neurosci. 39, 3792–3811 (2019).
Yau, K. W. et al. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron 82, 1058–1073 (2014).
Cao, Y. et al. Microtubule minus-end binding protein CAMSAP2 and Kinesin-14 motor KIFC3 control dendritic microtubule organization. Curr. Biol. 30, 899–908 (2020).
Boiarska, Z. & Passarella, D. Microtubule-targeting agents and neurodegeneration. Drug Discov. Today 26, 604–615 (2021).
Consolati, T. et al. Microtubule nucleation properties of single human γTuRCs explained by their cryo-EM structure. Dev. Cell 53, 603–617 (2020).
Liu, P. et al. Insights into the assembly and activation of the microtubule nucleator γ-TuRC. Nature 578, 467–471 (2020).
Wieczorek, M. et al. Asymmetric molecular architecture of the human γ-tubulin ring complex. Cell 180, 165–175 (2020).
Kuo, Y. W., Trottier, O., Mahamdeh, M. & Howard, J. Spastin is a dual-function enzyme that severs microtubules and promotes their regrowth to increase the number and mass of microtubules. Proc. Natl Acad. Sci. USA 116, 5533–5541 (2019).
Kuo, Y. W. & Howard, J. Cutting, amplifying, and aligning microtubules with severing enzymes. Trends Cell Biol. 31, 50–61 (2021).
Jiang, K. et al. Microtubule minus-end regulation at spindle poles by an ASPM–katanin complex. Nat. Cell Biol. 19, 480–492 (2017).
Chakraborty, S., Mahamid, J. & Baumeister, W. Cryoelectron tomography reveals nanoscale organization of the cytoskeleton and its relation to microtubule curvature inside cells. Structure 28, 991–1003 (2020).
Muller, A. et al. 3D FIB-SEM reconstruction of microtubule–organelle interaction in whole primary mouse β cells. J. Cell Biol. 220, e202010039 (2021). This study uses electron microscopy to visualize the complete microtubule cytoskeleton and membrane organelles in entire β cells.
Liu, S., Hoess, P. & Ries, J. Super-resolution microscopy for structural cell biology. Annu. Rev. Biophys. 51, https://doi.org/10.1146/annurev-biophys-102521-112912 (2022).
Chen, F., Tillberg, P. W. & Boyden, E. S. Optical imaging. Expansion microscopy. Science 347, 543–548 (2015).
Gros, O. J., Damstra, H. G. J., Kapitein, L. C., Akhmanova, A. & Berger, F. Dynein self-organizes while translocating the centrosome in T-cells. Mol. Biol. Cell 32, 855–868 (2021).
Damstra, H. G. J. et al. Visualizing cellular and tissue ultrastructure using ten-fold robust expansion microscopy (TREx). eLife 11, e73775 (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Torsten Wittmann, who co-reviewed with Alessandro Dema, Jawdat Al-Bassam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Centrosome
-
A non-membranous organelle which consists of two centrioles surrounded by a proteinaceous matrix that nucleates and anchors microtubules.
- Axon
-
A neuronal compartment that transmits signals.
- Dendrites
-
Neuronal compartments that receive signals.
- Centrioles
-
Cylindrical structures with a core of nine microtubule triplets. A centrosome contains two orthogonally arranged centrioles, the mother and the daughter centriole. A mother centriole is assembled one cell cycle earlier than the daughter centriole.
- Condensates
-
Membraneless structures which can form by the physical process of liquid–liquid phase separation, whereby a well-mixed solution of macromolecules such as proteins or nucleic acids spontaneously separates into two phases, a dense phase and a dilute phase.
- Axoneme
-
A microtubule-based cytoskeletal structure that forms the core of a cilium or a flagellum; it contains nine microtubule doublets, and in motile cilia also a central pair of microtubules
- End binding proteins
-
(EBs). Conserved proteins that specifically bind to growing microtubule ends because they preferentially associate with the microtubule lattice in which β-tubulin is bound to GTP.
- Dendritic arborization neurons
-
Neurons of a larval sensory type in Drosophila with specific dendritic morphologies.
- Radial glial cells
-
Progenitor cells responsible for producing neurons of the cerebral cortex.
- Oligodendrocytes
-
Myelinating glia cells of the central nervous system.
- Growth cone
-
An actin-supported extension of a developing or regenerating axon or dendrite.
- Fat body
-
An insect organ distributed throughout the body that has an essential role in energy storage and utilization.
- Adherens junctions
-
Protein assemblies at cell–cell junctions in epithelial and endothelial cells; they contain transmembrane proteins called cadherins and are linked to the actin cytoskeleton.
- Desmosomes
-
Adhesive protein complexes localized to intercellular junctions, responsible for maintaining the mechanical integrity of tissues.
- Presynaptic boutons
-
Neurotransmitter-producing knoblike enlargements at the end of an axon involved in forming a synapse with another neuron.
- Immunological synapse
-
The interface between an antigen-presenting cell or a target cell and a lymphocyte, such as a B cell or T cell, or a natural killer cell.
- Catastrophes
-
Abrupt transitions from microtubule growth to shortening associated with loss of a GTP cap; they can occur spontaneously or be triggered by obstacles to microtubule growth or different cellular factors.
- Rescues
-
Abrupt transitions from microtubule shortening to growth associated with regaining a GTP cap; these events are thought to be induced by cellular factors.
- Axon initial segment
-
A plasma membrane-associated compartment at the base of an axon, which generates and shapes the action potential.
- Focal adhesions
-
Integrin-containing multi-protein assemblies that establish mechanical links between intracellular actin bundles and the extracellular matrix.
- Z-discs
-
Fine dense lines forming sarcomere boundaries, which stabilize actin filaments and allow force transmission between sarcomeres.
- Sarcomeres
-
Basic contractile units of a muscle fibre; they contain actin and myosin filaments.
- Gap junction
-
A channel that physically connects adjacent cells, mediating rapid exchange of small molecules.
- Cytoplasmic streaming
-
Intracellular movement of the fluid substance (cytoplasm), transporting nutrients, macromolecules and organelles.
- Dendritic spines
-
Membranous protrusions from a neuronal dendrite that receive input from an axon at the synapse.
- Dendritic cells
-
Antigen-presenting cells that form an important role in the adaptive immune system.
- mTORC1
-
(Mammalian (or mechanistic) target of rapamycin complex 1). A protein complex that functions as a nutrient, energy and redox sensor and controls protein synthesis.
Rights and permissions
About this article
Cite this article
Akhmanova, A., Kapitein, L.C. Mechanisms of microtubule organization in differentiated animal cells. Nat Rev Mol Cell Biol 23, 541–558 (2022). https://doi.org/10.1038/s41580-022-00473-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-022-00473-y
This article is cited by
-
Map-1a regulates Sertoli cell BTB dynamics through the cytoskeletal organization of microtubule and F-actin
Reproductive Biology and Endocrinology (2024)
-
CAMSAPs and nucleation-promoting factors control microtubule release from γ-TuRC
Nature Cell Biology (2024)
-
Principles of organelle positioning in motile and non-motile cells
EMBO Reports (2024)
-
Minimal Mechanisms of Microtubule Length Regulation in Living Cells
Bulletin of Mathematical Biology (2024)
-
Compressive forces stabilize microtubules in living cells
Nature Materials (2023)