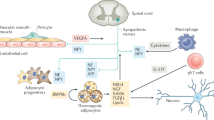
Abstract
Brown and beige adipocytes are mitochondria-enriched cells capable of dissipating energy in the form of heat. These thermogenic fat cells were originally considered to function solely in heat generation through the action of the mitochondrial protein uncoupling protein 1 (UCP1). In recent years, significant advances have been made in our understanding of the ontogeny, bioenergetics and physiological functions of thermogenic fat. Distinct subtypes of thermogenic adipocytes have been identified with unique developmental origins, which have been increasingly dissected in cellular and molecular detail. Moreover, several UCP1-independent thermogenic mechanisms have been described, expanding the role of these cells in energy homeostasis. Recent studies have also delineated roles for these cells beyond the regulation of thermogenesis, including as dynamic secretory cells and as a metabolic sink. This Review presents our current understanding of thermogenic adipocytes with an emphasis on their development, biological functions and roles in systemic physiology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cinti, S.Obesity, Type 2 Diabetes and the Adipose Organ: A Pictorial Atlas from Research to Clinical Applications 1st edn (Springer, 2017).
Wu, J., Cohen, P. & Spiegelman, B. M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250 (2013).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Lidell, M. E. et al. Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 (2013).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Shinoda, K. et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 21, 389–394 (2015).
Cypess, A. M. et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19, 635–639 (2013).
Ikeda, K., Maretich, P. & Kajimura, S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 29, 191–200 (2018).
Lepper, C. & Fan, C. M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436 (2010).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Atit, R. et al. β-Catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 296, 164–176 (2006).
Sanchez-Gurmaches, J. & Guertin, D. A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5, 4099 (2014).
Sebo, Z. L., Jeffery, E., Holtrup, B. & Rodeheffer, M. S. A mesodermal fate map for adipose tissue. Development 145, dev166801 (2018).
Wang, W. et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl Acad. Sci. USA 111, 14466–14471 (2014).
Zhang, L. et al. Generation of functional brown adipocytes from human pluripotent stem cells via progression through a paraxial mesoderm state. Cell Stem Cell 27, 784–797.e11 (2020). This study generates human brown adipocytes from pluripotent stem cells by a serum-free directed differentiation strategy.
Xue, B. et al. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J. Lipid Res. 48, 41–51 (2007).
Lee, Y. H., Petkova, A. P., Mottillo, E. P. & Granneman, J. G. In vivo identification of bipotential adipocyte progenitors recruited by β-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 (2012).
Berry, D. C., Jiang, Y. & Graff, J. M. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat. Commun. 7, 10184 (2016).
Liu, W. et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J. Cell Sci. 126, 3527–3532 (2013).
Oguri, Y. et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell 182, 563–577.e20 (2020).
Long, J. Z. et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 19, 810–820 (2014).
Vishvanath, L. et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23, 350–359 (2016).
Schulz, T. J. et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl Acad. Sci. USA 108, 143–148 (2011).
Rodeheffer, M. S., Birsoy, K. & Friedman, J. M. Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 (2008).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 (2013).
Cattaneo, P. et al. Parallel lineage-tracing studies establish fibroblasts as the prevailing in vivo adipocyte progenitor. Cell Rep. 30, 571–582.e2 (2020).
Finlin, B. S. et al. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Invest. 130, 2319–2331 (2020). This study reports that chronic activation of the β3-AR by mirabegron improves insulin sensitivity and activates beige fat in humans with obesity.
Finlin, B. S. et al. Human adipose beiging in response to cold and mirabegron. JCI Insight 3, e121510 (2018).
Min, S. Y. et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 22, 312–318 (2016).
Raajendiran, A. et al. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Rep. 27, 1528–1540.e7 (2019).
Singh, A. M. et al. Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nat. Commun. 11, 2758 (2020).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
Himms-Hagen, J. et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol. 279, C670–C681 (2000).
Barbatelli, G. et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. 298, E1244–E1253 (2010).
Shao, M. et al. Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885 (2019). This study reports the quantitative contribution of beige adipocyte biogenesis via de novo differentiation versus reinstallation of existing adipocytes in vivo.
Lee, Y. H., Petkova, A. P., Konkar, A. A. & Granneman, J. G. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 29, 286–299 (2015).
Tajima, K. et al. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat. Metab. 1, 886–898 (2019).
Berry, D. C. et al. Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metab. 25, 481 (2017).
Rosenwald, M., Perdikari, A., Rulicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Altshuler-Keylin, S. et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 24, 402–419 (2016).
Lu, X. et al. Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal. 11, eaap8526 (2018).
Roh, H. C. et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab. 27, 1121–1137.e5 (2018).
Gnad, T. et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014).
Bordicchia, M. et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 122, 1022–1036 (2012).
Fisher, F. M. et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Ohno, H., Shinoda, K., Spiegelman, B. M. & Kajimura, S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404 (2012).
Inagaki, T., Sakai, J. & Kajimura, S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 17, 480–495 (2016).
Sidossis, L. & Kajimura, S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest. 125, 478–486 (2015).
Sun, W. et al. Cold-induced epigenetic programming of the sperm enhances brown adipose tissue activity in the offspring. Nat. Med. 24, 1372–1383 (2018).
Jiang, Y., Berry, D. C. & Graff, J. M. Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329 (2017).
Bronnikov, G., Houstek, J. & Nedergaard, J. β-Adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via β1 but not via β3 adrenoceptors. J. Biol. Chem. 267, 2006–2013 (1992).
McQueen, A. E. et al. The C-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure. J. Biol. Chem. 292, 16122–16134 (2017).
Goh, Y. Y. et al. Angiopoietin-like 4 interacts with integrins β1 and β5 to modulate keratinocyte migration. Am. J. Pathol. 177, 2791–2803 (2010).
Zhu, Y. et al. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab. 24, 420–433 (2016). This study identifies the role of the gap junction in beige fat biogenesis via propagation of the sympathetically derived cAMP signal to neighbouring adipocytes.
Chen, Y. et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185 (2019).
Jun, H. et al. Adrenergic-independent signaling via CHRNA2 regulates beige fat activation. Dev. Cell 54, 106–116.e5 (2020).
Wu, Y., Kinnebrew, M. A., Kutyavin, V. I. & Chawla, A. Distinct signaling and transcriptional pathways regulate peri-weaning development and cold-induced recruitment of beige adipocytes. Proc. Natl Acad. Sci. USA 117, 6883–6889 (2020).
Song, A. et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Invest. 130, 247–257 (2019).
Lee, K. Y. et al. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 38, e99291 (2019).
Min, S. Y. et al. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc. Natl Acad. Sci. USA 116, 17970–17979 (2019). This study reports diverse adipocyte progenitors in human adipose tissue that give rise to beige adipocytes.
Xue, R. et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat. Med. 21, 760–768 (2015).
Sun, W. et al. Single-nucleus RNA-seq reveals a new type of brown adipocyte regulating thermogenesis. Nature 587, 98–102 (2020). This study employs single-nucleus RNA-sequencing to characterize adipocyte heterogeneity in mice and humans, and identifies a subpopulation of adipocytes that uses acetate to regulate the thermogenic capacity of neighbouring adipocytes.
Schwalie, P. C. et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 (2018). This study, by single-cell RNA-sequencing analysis, identifies distinct subpopulations of adipose precursor cells, including adipogenesis-regulatory cells, in mouse adipose tissue.
Hepler, C. et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7, e39636 (2018). This study reveals the functional heterogeneity of visceral WAT perivascular cells and identifies fibro-inflammatory progenitors.
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501 (2019). This study employs single-cell RNA sequencing to identify mesenchymal progenitor cells that give rise to adipocytes in mice and humans.
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Kajimura, S. et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 22, 1397–1409 (2008).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011).
Cohen, P. et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156, 304–316 (2014).
Ohno, H., Shinoda, K., Ohyama, K., Sharp, L. Z. & Kajimura, S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504, 163–167 (2013).
Berg, F., Gustafson, U. & Andersson, L. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet. 2, e129 (2006).
Gaudry, M. J. et al. Inactivation of thermogenic UCP1 as a historical contingency in multiple placental mammal clades. Sci. Adv. 3, e1602878 (2017).
Ricquier, D. & Kader, J. C. Mitochondrial protein alteration in active brown fat: a sodium dodecyl sulfate-polyacrylamide gel electrophoretic study. Biochem. Biophys. Res. Commun. 73, 577–583 (1976).
Nicholls, D. G. Hamster brown-adipose-tissue mitochondria. Purine nucleotide control of the ion conductance of the inner membrane, the nature of the nucleotide binding site. Eur. J. Biochem. 62, 223–228 (1976).
Aquila, H., Link, T. A. & Klingenberg, M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. 4, 2369–2376 (1985).
Bouillaud, F., Ricquier, D., Thibault, J. & Weissenbach, J. Molecular approach to thermogenesis in brown adipose tissue: cDNA cloning of the mitochondrial uncoupling protein. Proc. Natl Acad. Sci. USA 82, 445–448 (1985).
Enerback, S. et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94 (1997).
Arsenijevic, D. et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 26, 435–439 (2000).
Gong, D. W. et al. Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J. Biol. Chem. 275, 16251–16257 (2000).
Klingenberg, M. UCP1 — a sophisticated energy valve. Biochimie 134, 19–27 (2017).
Ricquier, D. UCP1, the mitochondrial uncoupling protein of brown adipocyte: a personal contribution and a historical perspective. Biochimie 134, 3–8 (2017).
Winkler, E. & Klingenberg, M. Effect of fatty acids on H+ transport activity of the reconstituted uncoupling protein. J. Biol. Chem. 269, 2508–2515 (1994).
Jezek, P., Orosz, D. E., Modriansky, M. & Garlid, K. D. Transport of anions and protons by the mitochondrial uncoupling protein and its regulation by nucleotides and fatty acids. A new look at old hypotheses. J. Biol. Chem. 269, 26184–26190 (1994).
Urbankova, E., Voltchenko, A., Pohl, P., Jezek, P. & Pohl, E. E. Transport kinetics of uncoupling proteins. Analysis of UCP1 reconstituted in planar lipid bilayers. J. Biol. Chem. 278, 32497–32500 (2003).
Schreiber, R. et al. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab. 26, 753–763.e7 (2017).
Shin, H. et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab. 26, 764–777.e5 (2017).
Anderson, C. M. et al. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep. 10, 505–515 (2015).
Putri, M. et al. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem. Biophys. Commun. 457, 520–525 (2015).
Simcox, J. et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 26, 509–522.e6 (2017). This study identifies a mechanism whereby FFAs from adipose tissue promote acylcarnitine production in the liver, which provides fuel for cold-induced thermogenesis.
Chouchani, E. T. et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016).
Wang, G. et al. Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol. Cell 74, 844–857.e7 (2019).
Ukropec, J., Anunciado, R. P., Ravussin, Y., Hulver, M. W. & Kozak, L. P. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1–/– mice. J. Biol. Chem. 281, 31894–31908 (2006).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017). This paper provides direct evidence of a UCP1-independent mechanism in beige fat that controls thermogenesis and glucose homeostasis.
de Meis, L. Uncoupled ATPase activity and heat production by the sarcoplasmic reticulum Ca2+-ATPase. Regulation by ADP. J. Biol. Chem. 276, 25078–25087 (2001).
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579 (2012).
Tajima, K. et al. Wireless optogenetics protects against obesity via stimulation of non-canonical fat thermogenesis. Nat. Commun. 11, 1730 (2020).
Aquilano, K. et al. Low-protein/high-carbohydrate diet induces AMPK-dependent canonical and non-canonical thermogenesis in subcutaneous adipose tissue. Redox Biol. 36, 101633 (2020).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015). This study identifies a UCP1-independent thermogenic mechanism that involves creatine futile cycling.
Kazak, L. et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 26, 660–671.e3 (2017).
Kazak, L. et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab. 1, 360–370 (2019).
Guan, H. P. et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med. 8, 1122–1128 (2002).
Flachs, P. et al. Induction of lipogenesis in white fat during cold exposure in mice: link to lean phenotype. Int. J. Obes. 41, 372–380 (2017).
Reidy, S. P. & Weber, J. M. Accelerated substrate cycling: a new energy-wasting role for leptin in vivo. Am. J. Physiol. 282, E312–E317 (2002).
Silva, J. E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 86, 435–464 (2006).
DosSantos, R. A., Alfadda, A., Eto, K., Kadowaki, T. & Silva, J. E. Evidence for a compensated thermogenic defect in transgenic mice lacking the mitochondrial glycerol-3-phosphate dehydrogenase gene. Endocrinology 144, 5469–5479 (2003).
Anunciado-Koza, R., Ukropec, J., Koza, R. A. & Kozak, L. P. Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J. Biol. Chem. 283, 27688–27697 (2008).
Long, J. Z. et al. The secreted enzyme pm20d1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166, 424–435 (2016).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Cooney, G. J., Caterson, I. D. & Newsholme, E. A. The effect of insulin and noradrenaline on the uptake of 2-[1–14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett. 188, 257–261 (1985).
Guerra, C. et al. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J. Clin. Invest. 108, 1205–1213 (2001).
Dallner, O. S., Chernogubova, E., Brolinson, K. A. & Bengtsson, T. β3-Adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology 147, 5730–5739 (2006).
Olsen, J. M. et al. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J. Cell Biol. 207, 365–374 (2014).
Lowell, B. B. et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 (1993).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
de Souza, C. J., Hirshman, M. F. & Horton, E. S. CL-316,243, a β3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes 46, 1257–1263 (1997).
Roberts-Toler, C., O’Neill, B. T. & Cypess, A. M. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity 23, 1765–1770 (2015).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Berbee, J. F. et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 6, 6356 (2015).
Bartelt, A. et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun. 8, 15010 (2017). This study reports a possible atheroprotective role of thermogenic fat via increased cholesterol flux through HDL.
Balaz, M. et al. Inhibition of mevalonate pathway prevents adipocyte browning in mice and men by affecting protein prenylation. Cell Metab. 29, 901–916.e8 (2019).
Worthmann, A. et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 23, 839–849 (2017).
Sponton, C. H. et al. The regulation of glucose and lipid homeostasis via PLTP as a mediator of BAT–liver communication. EMBO Rep. 21, e49828 (2020).
Neinast, M. D. et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 29, 417–429.e4 (2019).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019). This study reports the role of thermogenic fat in BCAA metabolism and identified the first mitochondrial BCAA transporter.
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Huffman, K. M. et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32, 1678–1683 (2009).
Pietilainen, K. H. et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 5, e51 (2008).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Newgard, C. B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 (2012).
Liu, J. et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics 13, 104 (2017).
Guasch-Ferre, M. et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 (2016).
Felig, P., Marliss, E. & Cahill, G. F. Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 281, 811–816 (1969).
Crown, S. B., Marze, N. & Antoniewicz, M. R. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PloS ONE 10, e0145850 (2015).
Green, C. R. et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 12, 15–21 (2016).
Wallace, M. et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 14, 1021–1031 (2018).
Su, X. et al. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: effects of obesity and weight loss. Obesity 23, 329–334 (2015).
Gunawardana, S. C. & Piston, D. W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61, 674–682 (2012).
Ali Khan, A. et al. Comparative secretome analyses of primary murine white and brown adipocytes reveal novel adipokines. Mol. Cell Proteom. 17, 2358–2370 (2018).
Villarroya, J., Cereijo, R., Giralt, M. & Villarroya, F. Secretory proteome of brown adipocytes in response to camp-mediated thermogenic activation. Front. Physiol. 10, 67 (2019).
Deshmukh, A. S. et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab. 30, 963–975.e7 (2019).
Villarroya, J. et al. New insights into the secretory functions of brown adipose tissue. J. Endocrinol. 243, R19–R27 (2019).
Whittle, A. J. et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012).
Svensson, K. J. et al. A secreted slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell Metab. 23, 454–466 (2016).
Kristof, E. et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp. Cell Res. 377, 47–55 (2019).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012).
Mahdaviani, K., Chess, D., Wu, Y., Shirihai, O. & Aprahamian, T. R. Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism 65, 26–35 (2016).
Cereijo, R. et al. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab. 28, 750–763.e6 (2018).
Campderros, L. et al. Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity 27, 1606–1616 (2019).
Nisoli, E., Tonello, C., Benarese, M., Liberini, P. & Carruba, M. O. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology 137, 495–503 (1996).
Zeng, X. et al. Innervation of thermogenic adipose tissue via a calsyntenin 3β-S100b axis. Nature 569, 229–235 (2019).
Wang, G. X. et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20, 1436–1443 (2014).
Kong, X. et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 28, 631–643.e3 (2018).
Ruan, C. C. et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab. 28, 476–489.e5 (2018).
Lynes, M. D. et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 23, 631–637 (2017). This study reports a cold-inducible batokine, 12,13-diHOME, that stimulates fatty acid uptake in brown fat.
Stanford, K. I. et al. 12,13-DiHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 27, 1111–1120.e3 (2018).
Chen, Y. et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat. Commun. 7, 11420 (2016).
Thomou, T. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455 (2017).
Sun, K., Tordjman, J., Clement, K. & Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 (2013).
Lackey, D. E. et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am. J. Physiol. 306, E233–E246 (2014).
Muir, L. A. et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity 24, 597–605 (2016).
Divoux, A. et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010).
Reggio, S. et al. Increased basement membrane components in adipose tissue during obesity: links with TGFβ and metabolic phenotypes. J. Clin. Endocrinol. Metab. 101, 2578–2587 (2016).
Henegar, C. et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 9, R14 (2008).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
Hasegawa, Y. et al. Repression of adipose tissue fibrosis through a PRDM16–GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. 27, 180–194.e6 (2018).
Wang, W. et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 30, 174–189.e5 (2019).
Heaton, J. M. The distribution of brown adipose tissue in the human. J. Anat. 112, 35–39 (1972).
Hany, T. F. et al. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur. J. Nucl. Med. Mol. Imaging 29, 1393–1398 (2002).
Cohade, C., Osman, M., Pannu, H. K. & Wahl, R. L. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J. Nucl. Med. 44, 170–176 (2003).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Leitner, B. P. et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl Acad. Sci.USA 114, 8649–8654 (2017). This study maps brown fat in six distinct anatomical depots in young men, comparing lean individuals and individuals with obesity.
Chen, K. Y. et al. Brown adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 24, 210–222 (2016).
Sharp, L. Z. et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS ONE 7, e49452 (2012).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Hanssen, M. J. et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21, 863–865 (2015).
Chondronikola, M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014).
Lee, P. et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63, 3686–3698 (2014).
Hanssen, M. J. et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 65, 1179–1189 (2016). This study shows that short-term cold exposure can lead to the recruitment of brown fat in humans with obesity.
Vijgen, G. H. et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 97, E1229–E1233 (2012).
Raiko, J., Orava, J., Savisto, N. & Virtanen, K. A. High brown fat activity correlates with cardiovascular risk factor levels cross-sectionally and subclinical atherosclerosis at 5-year follow-up. Arterioscler. Thromb. Vasc. Biol. 40, 1289–1295 (2020). This study finds that the presence of cold-induced brown fat activity correlates with lower cardiovascular risk factors and decreased carotid intima-media thickness and higher carotid elasticity on 5-year follow-up.
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021). This study finds that brown fat in humans is associated with protection from cardio-metabolic diseases, particularly in individuals that are overweight and obese.
Ma, S. et al. Caloric restriction reprograms the single-cell transcriptional landscape of rattus norvegicus aging. Cell 180, 984–1001.e22 (2020).
Yoneshiro, T. et al. Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int. J. Obes. 37, 993–998 (2013).
Bakker, L. E. et al. Brown adipose tissue volume in healthy lean South Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol. 2, 210–217 (2014).
Vosselman, M. J., Vijgen, G. H., Kingma, B. R., Brans, B. & van Marken Lichtenbelt, W. D. Frequent extreme cold exposure and brown fat and cold-induced thermogenesis: a study in a monozygotic twin. PloS ONE 9, e101653 (2014).
Riveros-McKay, F. et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 15, e1007603 (2019).
Zhang, F. et al. An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab. 27, 252–262.e3 (2018).
Fitzgibbons, T. P. et al. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am. J. Physiol. Heart Circ. Physiol. 301, H1425–H1437 (2011).
Sacks, H. S. et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 94, 3611–3615 (2009).
Lynch, C. J. & Adams, S. H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736 (2014).
Villarroya, F., Cereijo, R., Villarroya, J., Gavalda-Navarro, A. & Giralt, M. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab. 27, 954–961 (2018).
Sakamoto, T. et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol. 310, E676–E687 (2016).
Goto, T. et al. Proinflammatory cytokine interleukin-1β suppresses cold-induced thermogenesis in adipocytes. Cytokine 77, 107–114 (2016).
Valladares, A., Roncero, C., Benito, M. & Porras, A. TNF-α inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Lett. 493, 6–11 (2001).
Chiang, S. H. et al. The protein kinase IKKε regulates energy balance in obese mice. Cell 138, 961–975 (2009).
Mowers, J. et al. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKε and TBK1. eLife 2, e01119 (2013).
Kumari, M. et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J. Clin. Invest. 126, 2839–2854 (2016).
Yadav, H. et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 14, 67–79 (2011).
Koncarevic, A. et al. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology 153, 3133–3146 (2012).
Guo, T. et al. Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. eLife 3, e03245 (2014).
Rajbhandari, P. et al. Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife 8, e49501 (2019).
Rajbhandari, P. et al. IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell 172, 218–233 e217 (2018). This study characterizes adipocytes and stromal cells identifying crosstalk between immune cells and thermogenic adipocytes.
Wolf, Y. et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18, 665–674 (2017).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Chung, K. J. et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat. Immunol. 18, 654–664 (2017).
Hu, B. et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Lee, M. W. et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015).
Zhang, X. et al. Functional inactivation of mast cells enhances subcutaneous adipose tissue browning in mice. Cell Rep. 28, 792–803.e4 (2019).
Finlin, B. S. et al. Mast cells promote seasonal white adipose beiging in humans. Diabetes 66, 1237–1246 (2017).
Lynch, L. et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab. 24, 510–519 (2016).
Cypess, A. M. et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl Acad. Sci. USA 109, 10001–10005 (2012).
Cypess, A. M. et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
O’Mara, A. E. et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Invest. 130, 2209–2219 (2020). This study shows that chronic treatment with mirabegron increases human brown fat activity, which is associated with increased HDL and improved insulin sensitivity.
Blondin, D. P. et al. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab. 32, 287–300.e7 (2020).
Broeders, E. P. et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 22, 418–426 (2015).
Ramage, L. E. et al. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab. 24, 130–141 (2016).
Yoneshiro, T., Aita, S., Kawai, Y., Iwanaga, T. & Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 95, 845–850 (2012).
Ohyama, K. et al. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 65, 1410–1423 (2016).
Wang, S. et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 466, 247–253 (2015).
Jiang, J. et al. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metabolism 77, 58–64 (2017).
Acknowledgements
The authors apologize for being unable to cite large numbers of important contributions to the field due to space limitations. This work was supported by the American Diabetes Association Pathway Program (1-17-ACE-17) to P.C., and by the National Institutes of Health (NIH) (DK097441, DK125281, DK126160, DK127575, DK125283) and the Edward Mallinckrodt, Jr. Foundation to S.K.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks M. Saito, F. Villarroya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Cristae
-
Folds in the inner membrane of a mitochondrion where active electron transport takes place.
- Catecholamines
-
Monoamine neurotransmitters that mediate cold-induced thermogenesis.
- Thiazolidinediones
-
(Also known as glitazones). Synthetic ligands of PPARγ typically used in the treatment of type 2 diabetes. They also increase thermogenesis and have been shown to promote recruitment of beige thermogenic adipocytes.
- Dermomyotome
-
Epithelial tissue present during development that combines a dermatome (giving rise to the epidermis) and a myotome (giving rise to skeletal muscle) before they separate in embryogenesis.
- Retroperitoneal WAT
-
White adipose tissue (WAT) in the area between the posterior portion of the parietal peritoneum and the posterior abdominal wall muscles.
- β3-Adrenergic signalling
-
Signalling potently stimulated by cold that is mediated by catecholamines that bind to β3-adrenergic receptors (β3-ARs), G protein-coupled receptors that activate adenylate cyclase to produce a second messenger cAMP.
- Inguinal WAT
-
Subcutaneous adipose tissue located at the juncture of the lower portion of the anterior abdominal wall and legs. Inguinal white adipose tissue (WAT) contains high levels of beige adipocytes.
- Insulin resistance
-
Insulin acts on the insulin receptor on the plasma membrane of target organs and triggers insulin signalling to stimulate anabolic reactions. Insulin action is impaired under insulin resistance conditions, which can eventually lead to type 2 diabetes.
- Mitophagy
-
A selective mechanism to degrade defective mitochondria by autophagy.
- Natriuretic peptides
-
Peptide hormones that induce sodium excretion by the kidney, including atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP) and dendroaspis natriuretic peptide (DNP).
- Myokine
-
A secretory molecule released from skeletal muscle. Examples include IL-6 and irisin, both of which are known to activate thermogenic fat biogenesis.
- Adiponectin
-
A fat-selective secretory protein hormone (adipokine) that is involved in regulating glucose and lipid homeostasis. In general, adiponectin levels are positively correlated with metabolic health.
- Ancillary/niche cells
-
Supporting cells releasing paracrine factors.
- Epididymal WAT
-
Visceral adipose tissue attached to the epididymis. Epididymal white adipose tissue (WAT) is known to have lower beiging propensity relative to inguinal WAT of mice.
- γδ T cells
-
A subset of T cells that express a distinctive set (γ and δ-chains) of T cell receptor (TCR) on their surface, distinct from that of conventional T cells (αβ T cells).
- Group 2 innate lymphoid cells
-
(ILC2s). A subset of innate lymphoid cells that produce type 2 cytokines, such as IL-5 and IL-13.
- Methionine-enkephalin
-
(MetEnk). An endogenous opioid peptide that acts on δ-opioid receptor and μ-opioid receptor to a lesser extent.
- Mast cells
-
Immune cells that release histamine and other substances during inflammatory and allergic reactions.
- Invariant natural killer T cells
-
(iNKT cells). Specialized T cells that recognize lipid antigens.
- α-Galactosylceramide
-
(α-GalCer). A synthetic glycolipid that stimulates invariant natural killer T cells.
- Liraglutide
-
A glucagon-like peptide 1 receptor (GLP1R) agonist that acts as an incretin mimetic and increases insulin secretion.
- Respiratory chain
-
(Also known as electron transport chain). Multiple protein complexes that transfer electrons from electron donors, such as NADH, to electron acceptors, thereby generating a proton (H+) gradient across the mitochondrial inner membrane.
- Oxidative phosphorylation
-
A metabolic process in which cells use series of enzymes to oxidize glucose, fatty acids and other metabolites to produce ATP.
- Acylcarnitine
-
A metabolite derived from carnitine and acyl-coenzyme A (acyl-CoA). Generation of acylcarnitine allows the transport of fatty acids into the mitochondrial matrix for oxidation.
- Sulfenylation
-
A post-translational protein modification involving the addition of a sulfenyl group to cysteine residues.
- Succinylation
-
A post-translational modification in which a succinyl group is added to proteins at lysine residues.
- Sirtuin
-
A protein with NAD-dependent deacetylase activity that plays key roles in cellular homeostasis, including ageing, transcription, stress response, inflammation and apoptosis.
- 5′ AMP-activated protein kinase
-
(AMPK). A heterotrimeric enzyme complex that is activated in response to low cellular ATP, including low glucose and hypoxia, and stimulates glucose and fatty acid catabolism and autophagy.
- Leptin
-
An adipocyte-derived hormone that regulates food intake and energy expenditure.
- N-Acyl amino acids
-
Lipids that contain a fatty-acid tail covalently conjugated to an amino acid head group.
- Triglyceride-rich lipoproteins
-
Lipoproteins that transport triglycerides and cholesterol; these include very low-density lipoprotein (VLDL) and chylomicrons.
- Reverse cholesterol transport
-
A process in which cholesterol from peripheral organs is returned to the liver via the circulation.
- Mevalonate pathway
-
A metabolic pathway for the synthesis of sterols and isoprenoids.
- Bariatric surgery
-
A surgical procedure that promotes weight loss. These procedures include the Roux-en-Y gastric bypass, sleeve gastrectomy, adjustable gastric band and biliopancreatic diversion with duodenal switch.
- Oxylipin
-
An oxygenated lipid derived from polyunsaturated fatty acid.
- β-Hydroxybutyrate
-
(BHB). A major form of ketone bodies that is generated through fatty acid oxidation or leucine oxidation.
- 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography
-
(FDG-PET/CT). An imaging-based technique that measures the uptake of a radioactive glucose analogue.
- Chenodeoxycholic acid
-
A primary bile acid synthesized in the liver.
Rights and permissions
About this article
Cite this article
Cohen, P., Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol 22, 393–409 (2021). https://doi.org/10.1038/s41580-021-00350-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-021-00350-0
This article is cited by
-
WRN loss accelerates abnormal adipocyte metabolism in Werner syndrome
Cell & Bioscience (2024)
-
Deep learning enables the quantification of browning capacity of human adipose samples
Journal of Big Data (2024)
-
Futile lipid cycling: from biochemistry to physiology
Nature Metabolism (2024)
-
EPAC1 boosts thermogenic adipocyte formation
Nature Cell Biology (2024)
-
Ubiquitin ligase RNF20 coordinates sequential adipose thermogenesis with brown and beige fat-specific substrates
Nature Communications (2024)