Abstract
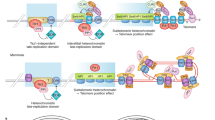
The regulation of telomere length in mammals is crucial for chromosome end-capping and thus for maintaining genome stability and cellular lifespan. This process requires coordination between telomeric protein complexes and the ribonucleoprotein telomerase, which extends the telomeric DNA. Telomeric proteins modulate telomere architecture, recruit telomerase to accessible telomeres and orchestrate the conversion of the newly synthesized telomeric single-stranded DNA tail into double-stranded DNA. Dysfunctional telomere maintenance leads to telomere shortening, which causes human diseases including bone marrow failure, premature ageing and cancer. Recent studies provide new insights into telomerase-related interactions (the ‘telomere replisome’) and reveal new challenges for future telomere structural biology endeavours owing to the dynamic nature of telomere architecture and the great number of structures that telomeres form. In this Review, we discuss recently determined structures of the shelterin and CTC1–STN1–TEN1 (CST) complexes, how they may participate in the regulation of telomere replication and chromosome end-capping, and how disease-causing mutations in their encoding genes may affect specific functions. Major outstanding questions in the field include how all of the telomere components assemble relative to each other and how the switching between different telomere structures is achieved.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41580-021-00353-x
References
Moyzis, R. K. et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl Acad. Sci. USA 85, 6622–6626 (1988).
Meyne, J., Ratliff, R. L. & Moyzis, R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl Acad. Sci. USA 86, 7049–7053 (1989).
Makarov, V. L., Hirose, Y. & Langmore, J. P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88, 657–666 (1997).
Lansdorp, P. M. et al. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 5, 685–691 (1996).
Vaziri, H. et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am. J. Hum. Genet. 52, 661–667 (1993).
Allsopp, R. C. et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl Acad. Sci. USA 89, 10114–10118 (1992).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14, 501–513 (2004).
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999).
Vulliamy, T. J., Knight, S. W., Mason, P. J. & Dokal, I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cell Mol. Dis. 27, 353–357 (2001).
Kim, N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Shay, J. W. & Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 33, 787–791 (1997).
Ye, J. Z. et al. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J. Biol. Chem. 279, 47264–47271 (2004).
Liu, D., O’Connor, M. S., Qin, J. & Songyang, Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J. Biol. Chem. 279, 51338–51342 (2004).
O’Connor, M. S., Safari, A., Xin, H., Liu, D. & Songyang, Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc. Natl Acad. Sci. USA 103, 11874–11879 (2006). Together with Liu et al. (J. Biol. Chem. 2004), this study identifies the full six-member shelterin complex, showing TIN2 binding both TRF1 and TRF2.
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 (2005).
Baumann, P. & Cech, T. R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
Lei, M., Podell, E. R. & Cech, T. R. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat. Struct. Mol. Biol. 11, 1223–1229 (2004).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999). This study reports that a substantial fraction of mammalian telomeres form a dsDNA loop to protect chromosome ends.
Stansel, R. M., de Lange, T. & Griffith, J. D. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 20, 5532–5540 (2001).
Nikitina, T. & Woodcock, C. L. Closed chromatin loops at the ends of chromosomes. J. Cell Biol. 166, 161–165 (2004).
Doksani, Y., Wu, J. Y., de Lange, T. & Zhuang, X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell 155, 345–356 (2013).
Marcand, S., Gilson, E. & Shore, D. A protein-counting mechanism for telomere length regulation in yeast. Science 275, 986–990 (1997).
Li, J. S. et al. TZAP: a telomere-associated protein involved in telomere length control. Science 355, 638–641 (2017). This study reports a telomeric DNA-binding protein that initiates trimming of long telomeres.
Greider, C. W. & Blackburn, E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337, 331–337 (1989).
Lingner, J. et al. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276, 561–567 (1997). This study and Greider and Blackburn (1989) report the first cloning of genes for the RNA and the catalytic (TERT) subunits of telomerase in ciliated protozoa, which led to the identification of the corresponding human factors.
Greider, C. W. Telomerase is processive. Mol. Cell Biol. 11, 4572–4580 (1991).
Roake, C. M. & Artandi, S. E. Regulation of human telomerase in homeostasis and disease. Nat. Rev. Mol. Cell Biol. 21, 384–397 (2020).
Schmidt, J. C. & Cech, T. R. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev. 29, 1095–1105 (2015).
Hockemeyer, D. & Collins, K. Control of telomerase action at human telomeres. Nat. Struct. Mol. Biol. 22, 848–852 (2015).
Wright, W. E., Piatyszek, M. A., Rainey, W. E., Byrd, W. & Shay, J. W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 18, 173–179 (1996).
Nandakumar, J. et al. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492, 285–289 (2012).
Zhong, F. L. et al. TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends. Cell 150, 481–494 (2012).
Sexton, A. N., Youmans, D. T. & Collins, K. Specificity requirements for human telomere protein interaction with telomerase holoenzyme. J. Biol. Chem. 287, 34455–34464 (2012). Together with Nandakumar et. al. (2012) and Zhong et. al. (2012), this study identifies specific amino acids of the TPP1 OB fold that recruit telomerase and enhance its enzymatic processivity.
Chen, L. Y., Redon, S. & Lingner, J. The human CST complex is a terminator of telomerase activity. Nature 488, 540–544 (2012).
Gao, H., Cervantes, R. B., Mandell, E. K., Otero, J. H. & Lundblad, V. RPA-like proteins mediate yeast telomere function. Nat. Struct. Mol. Biol. 14, 208–214 (2007).
Miyake, Y. et al. RPA-like mammalian Ctc1–Stn1–Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell 36, 193–206 (2009).
Surovtseva, Y. V. et al. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol. Cell 36, 207–218 (2009). Together with Miyake et al. (2009), this study shows that the RPA-like CST complex, previously characterized in yeast telomeres, has mammalian and plant counterparts with telomeric functions.
Casteel, D. E. et al. A DNA polymerase-α primase cofactor with homology to replication protein A-32 regulates DNA replication in mammalian cells. J. Biol. Chem. 284, 5807–5818 (2009).
Azzalin, C. M., Reichenbach, P., Khoriauli, L., Giulotto, E. & Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318, 798–801 (2007).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998). This study shows that interfering with TRF2 accumulation at telomeres causes dramatic chromosome end fusions.
Smogorzewska, A. et al. Control of human telomere length by TRF1 and TRF2. Mol. Cell Biol. 20, 1659–1668 (2000).
Kim, S. H., Kaminker, P. & Campisi, J. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 23, 405–412 (1999).
Li, B. & de Lange, T. Rap1 affects the length and heterogeneity of human telomeres. Mol. Biol. Cell 14, 5060–5068 (2003).
Ye, J. Z. et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 (2004).
Liu, D. et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 6, 673–680 (2004).
Colgin, L. M., Baran, K., Baumann, P., Cech, T. R. & Reddel, R. R. Human POT1 facilitates telomere elongation by telomerase. Curr. Biol. 13, 942–946 (2003).
Bianchi, A., Smith, S., Chong, L., Elias, P. & de Lange, T. TRF1 is a dimer and bends telomeric DNA. EMBO J. 16, 1785–1794 (1997).
Broccoli, D., Smogorzewska, A., Chong, L. & de Lange, T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17, 231–235 (1997).
van Steensel, B. & de Lange, T. Control of telomere length by the human telomeric protein TRF1. Nature 385, 740–743 (1997).
Bilaud, T. et al. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 17, 236–239 (1997).
Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018 (2003).
Lim, C., Zaug, A. J., Kim, H. J. & Cech, T. R. Reconstitution of human shelterin complexes reveals unexpected stoichiometry and dual pathways to enhance telomerase processivity. Nat. Commun. 8, 1075 (2017).
Rai, R., Chen, Y., Lei, M. & Chang, S. TRF2–RAP1 is required to protect telomeres from engaging in homologous recombination-mediated deletions and fusions. Nat. Commun. 7, 10881 (2016).
Sfeir, A., Kabir, S., van Overbeek, M., Celli, G. B. & de Lange, T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science 327, 1657–1661 (2010).
Lototska, L. et al. Human RAP1 specifically protects telomeres of senescent cells from DNA damage. EMBO Rep. 21, e49076 (2020).
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007).
Xin, H. et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 (2007).
Lei, M., Zaug, A. J., Podell, E. R. & Cech, T. R. Switching human telomerase on and off with hPOT1 protein in vitro. J. Biol. Chem. 280, 20449–20456 (2005).
Rice, C. et al. Structural and functional analysis of the human POT1–TPP1 telomeric complex. Nat. Commun. 8, 14928 (2017).
Chen, C. et al. Structural insights into POT1–TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat. Commun. 8, 14929 (2017).
Kar, A., Willcox, S. & Griffith, J. D. Transcription of telomeric DNA leads to high levels of homologous recombination and T-loops. Nucleic Acids Res. 44, 9369–9380 (2016).
Hu, C. et al. Structural and functional analyses of the mammalian TIN2–TPP1–TRF2 telomeric complex. Cell Res. 27, 1485–1502 (2017). This study reports the crystal structure of the key ‘bridge’ region of mammalian shelterin, which connects its dsDNA-binding and ssDNA-binding subunits.
Chen, L. Y. et al. Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol. Cell 47, 839–850 (2012).
Chen, Y. et al. A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins. Science 319, 1092–1096 (2008).
Fairall, L., Chapman, L., Moss, H., de Lange, T. & Rhodes, D. Structure of the TRFH dimerization domain of the human telomeric proteins TRF1 and TRF2. Mol. Cell 8, 351–361 (2001).
Bianchi, A. et al. TRF1 binds a bipartite telomeric site with extreme spatial flexibility. EMBO J. 18, 5735–5744 (1999).
Nishikawa, T., Nagadoi, A., Yoshimura, S., Aimoto, S. & Nishimura, Y. Solution structure of the DNA-binding domain of human telomeric protein, hTRF1. Structure 6, 1057–1065 (1998).
Court, R., Chapman, L., Fairall, L. & Rhodes, D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. EMBO Rep. 6, 39–45 (2005).
Smith, S. & de Lange, T. TRF1, a mammalian telomeric protein. Trends Genet. 13, 21–26 (1997).
Griffith, J., Bianchi, A. & de Lange, T. TRF1 promotes parallel pairing of telomeric tracts in vitro. J. Mol. Biol. 278, 79–88 (1998).
Seimiya, H., Muramatsu, Y., Smith, S. & Tsuruo, T. Functional subdomain in the ankyrin domain of tankyrase 1 required for poly(ADP-ribosyl)ation of TRF1 and telomere elongation. Mol. Cell Biol. 24, 1944–1955 (2004).
Poulet, A. et al. The N-terminal domains of TRF1 and TRF2 regulate their ability to condense telomeric DNA. Nucleic Acids Res. 40, 2566–2576 (2012).
Saint-Leger, A. et al. The basic N-terminal domain of TRF2 limits recombination endonuclease action at human telomeres. Cell Cycle 13, 2469–2474 (2014).
Necasova, I., Janouskova, E., Klumpler, T. & Hofr, C. Basic domain of telomere guardian TRF2 reduces D-loop unwinding whereas Rap1 restores it. Nucleic Acids Res. 45, 12599 (2017).
Schmutz, I., Timashev, L., Xie, W., Patel, D. J. & de Lange, T. TRF2 binds branched DNA to safeguard telomere integrity. Nat. Struct. Mol. Biol. 24, 734–742 (2017).
Konishi, A., Izumi, T. & Shimizu, S. TRF2 protein interacts with core histones to stabilize chromosome ends. J. Biol. Chem. 291, 20798–20810 (2016).
Wang, R. C., Smogorzewska, A. & de Lange, T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119, 355–368 (2004).
Fouche, N. et al. The basic domain of TRF2 directs binding to DNA junctions irrespective of the presence of TTAGGG repeats. J. Biol. Chem. 281, 37486–37495 (2006).
Williamson, J. R., Raghuraman, M. K. & Cech, T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell 59, 871–880 (1989).
Sundquist, W. I. & Klug, A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature 342, 825–829 (1989).
Pedroso, I. M., Hayward, W. & Fletcher, T. M. The effect of the TRF2 N-terminal and TRFH regions on telomeric G-quadruplex structures. Nucleic Acids Res. 37, 1541–1554 (2009).
Janouskova, E. et al. Human Rap1 modulates TRF2 attraction to telomeric DNA. Nucleic Acids Res. 43, 2691–2700 (2015).
Choi, K. H., Farrell, A. S., Lakamp, A. S. & Ouellette, M. M. Characterization of the DNA binding specificity of Shelterin complexes. Nucleic Acids Res. 39, 9206–9223 (2011).
Erdel, F. et al. Telomere recognition and assembly mechanism of mammalian shelterin. Cell Rep. 18, 41–53 (2017).
Hanaoka, S., Nagadoi, A. & Nishimura, Y. Comparison between TRF2 and TRF1 of their telomeric DNA-bound structures and DNA-binding activities. Protein Sci. 14, 119–130 (2005).
Nishikawa, T. et al. Structure of the DNA-binding domain of human telomeric protein, TRF1 and its interaction with telomeric DNA. Nucleic Acids Res. Suppl. 1, 273–274 (2001).
Takai, K. K., Hooper, S., Blackwood, S., Gandhi, R. & de Lange, T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 285, 1457–1467 (2010).
Pike, A. M., Strong, M. A., Ouyang, J. P. T. & Greider, C. W. TIN2 functions with TPP1/POT1 to stimulate telomerase processivity. Mol. Cell Biol. 39, e00593-18 (2019).
Nelson, N. D. et al. The C-terminal extension unique to the long isoform of the shelterin component TIN2 enhances its interaction with TRF2 in a phosphorylation- and dyskeratosis congenita cluster-dependent fashion. Mol. Cell Biol. 38, e00025-18 (2018).
Grill, S. et al. Two separation-of-function isoforms of human TPP1 dictate telomerase regulation in somatic and germ cells. Cell Rep. 27, 3511–3521.e7 (2019).
Zemp, I. & Lingner, J. The shelterin component TPP1 is a binding partner and substrate for the deubiquitinating enzyme USP7. J. Biol. Chem. 289, 28595–28606 (2014).
Bhanot, M. & Smith, S. TIN2 stability is regulated by the E3 ligase Siah2. Mol. Cell Biol. 32, 376–384 (2012).
Janovic, T., Stojaspal, M., Veverka, P., Horakova, D. & Hofr, C. Human telomere repeat binding factor TRF1 replaces TRF2 bound to shelterin core hub TIN2 when TPP1 is absent. J. Mol. Biol. 431, 3289–3301 (2019).
Kim, J. K. et al. Structural basis for shelterin bridge assembly. Mol. Cell 68, 698–714.e5 (2017).
Zhang, Y. et al. Phosphorylation of TPP1 regulates cell cycle-dependent telomerase recruitment. Proc. Natl Acad. Sci. USA 110, 5457–5462 (2013).
Gray, J. T., Celander, D. W., Price, C. M. & Cech, T. R. Cloning and expression of genes for the Oxytricha telomere-binding protein: specific subunit interactions in the telomeric complex. Cell 67, 807–814 (1991).
Yang, Q., Zheng, Y. L. & Harris, C. C. POT1 and TRF2 cooperate to maintain telomeric integrity. Mol. Cell Biol. 25, 1070–1080 (2005).
Timashev, L. A. & de Lange, T. Characterization of T-loop formation by TRF2. Nucleus 11, 164–177 (2020).
Amiard, S. et al. A topological mechanism for TRF2-enhanced strand invasion. Nat. Struct. Mol. Biol. 14, 147–154 (2007).
Subramaniam, S. The cryo-EM revolution: fueling the next phase. IUCrJ 6, 1–2 (2019).
Cheng, Y. Single-particle cryo-EM — how did it get here and where will it go. Science 361, 876–880 (2018).
Jiang, J. et al. Structure of Tetrahymena telomerase reveals previously unknown subunits, functions, and interactions. Science 350, aab4070 (2015).
Nguyen, T. H. D. et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature 557, 190–195 (2018).
Schmidt, J. C., Zaug, A. J. & Cech, T. R. Live cell imaging reveals the dynamics of telomerase recruitment to telomeres. Cell 166, 1188–1197.e9 (2016).
Schmidt, J. C., Zaug, A. J., Kufer, R. & Cech, T. R. Dynamics of human telomerase recruitment depend on template-telomere base pairing. Mol. Biol. Cell 29, 869–880 (2018).
Laprade, H. et al. Single-molecule imaging of telomerase RNA reveals a recruitment-retention model for telomere elongation. Mol. Cell 79, 115–126.e6 (2020).
Lin, J. et al. TRF1 and TRF2 use different mechanisms to find telomeric DNA but share a novel mechanism to search for protein partners at telomeres. Nucleic Acids Res. 42, 2493–2504 (2014).
Hockemeyer, D., Sfeir, A. J., Shay, J. W., Wright, W. E. & de Lange, T. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 24, 2667–2678 (2005).
Denchi, E. L. & de Lange, T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071 (2007). This study establishes roles of shelterin components in subduing specific signalling pathways that detect DNA damage but need to be repressed at natural chromosome ends.
Flynn, R. L. et al. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature 471, 532–536 (2011).
Wang, H., Nora, G. J., Ghodke, H. & Opresko, P. L. Single molecule studies of physiologically relevant telomeric tails reveal POT1 mechanism for promoting G-quadruplex unfolding. J. Biol. Chem. 286, 7479–7489 (2011).
Zaug, A. J., Podell, E. R. & Cech, T. R. Human POT1 disrupts telomeric G-quadruplexes allowing telomerase extension in vitro. Proc. Natl Acad. Sci. USA 102, 10864–10869 (2005).
Zahler, A. M., Williamson, J. R., Cech, T. R. & Prescott, D. M. Inhibition of telomerase by G-quartet DNA structures. Nature 350, 718–720 (1991).
Hwang, H., Buncher, N., Opresko, P. L. & Myong, S. POT1–TPP1 regulates telomeric overhang structural dynamics. Structure 20, 1872–1880 (2012).
Jansson, L. I. et al. Telomere DNA G-quadruplex folding within actively extending human telomerase. Proc. Natl Acad. Sci. USA 116, 9350–9359 (2019).
Patrick, E. M., Slivka, J. D., Payne, B., Comstock, M. J. & Schmidt, J. C. Observation of processive telomerase catalysis using high-resolution optical tweezers. Nat. Chem. Biol. 16, 801–809 (2020).
Latrick, C. M. & Cech, T. R. POT1–TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 29, 924–933 (2010).
Paudel, B. P. et al. A mechanism for the extension and unfolding of parallel telomeric G-quadruplexes by human telomerase at single-molecule resolution. eLife 9, e56428 (2020).
Wang, F. et al. Human CST has independent functions during telomere duplex replication and C-strand fill-in. Cell Rep. 2, 1096–1103 (2012).
Huang, C., Dai, X. & Chai, W. Human Stn1 protects telomere integrity by promoting efficient lagging-strand synthesis at telomeres and mediating C-strand fill-in. Cell Res. 22, 1681–1695 (2012).
Wang, Y., Brady, K. S., Caiello, B. P., Ackerson, S. M. & Stewart, J. A. Human CST suppresses origin licensing and promotes AND-1/Ctf4 chromatin association. Life Sci. Alliance 2, e201800270 (2019).
Stewart, J. A. et al. Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 31, 3537–3549 (2012).
Zhang, M. et al. Mammalian CST averts replication failure by preventing G-quadruplex accumulation. Nucleic Acids Res. 47, 5243–5259 (2019).
Wang, F., Stewart, J. & Price, C. M. Human CST abundance determines recovery from diverse forms of DNA damage and replication stress. Cell Cycle 13, 3488–3498 (2014).
Barazas, M. et al. The CST complex mediates end protection at double-strand breaks and promotes PARP inhibitor sensitivity in BRCA1-deficient cells. Cell Rep. 23, 2107–2118 (2018).
Mirman, Z. et al. 53BP1-RIF1-shieldin counteracts DSB resection through CST- and Polα-dependent fill-in. Nature 560, 112–116 (2018).
Bochkareva, E., Korolev, S., Lees-Miller, S. P. & Bochkarev, A. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. EMBO J. 21, 1855–1863 (2002).
Bochkareva, E., Belegu, V., Korolev, S. & Bochkarev, A. Structure of the major single-stranded DNA-binding domain of replication protein A suggests a dynamic mechanism for DNA binding. EMBO J. 20, 612–618 (2001).
Shastrula, P. K., Rice, C. T., Wang, Z., Lieberman, P. M. & Skordalakes, E. Structural and functional analysis of an OB-fold in human Ctc1 implicated in telomere maintenance and bone marrow syndromes. Nucleic Acids Res. 46, 972–984 (2018).
Bryan, C., Rice, C., Harkisheimer, M., Schultz, D. C. & Skordalakes, E. Structure of the human telomeric Stn1–Ten1 capping complex. PLoS ONE 8, e66756 (2013).
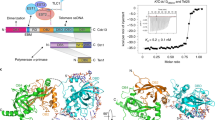
Lim, C. et al. The structure of human CST reveals a decameric assembly bound to telomeric DNA. Science 368, 1081–1085 (2020). This study uses cryo-EM to solve the structure of the CST complex and reveals that ssDNA binding can trigger its assembly into a 2-MDa decameric supercomplex.
Bhattacharjee, A., Wang, Y., Diao, J. & Price, C. M. Dynamic DNA binding, junction recognition and G4 melting activity underlie the telomeric and genome-wide roles of human CST. Nucleic Acids Res. 45, 12311–12324 (2017).
Hom, R. A. & Wuttke, D. S. Human CST prefers G-rich but not necessarily telomeric sequences. Biochemistry 56, 4210–4218 (2017).
Chen, L. Y., Majerska, J. & Lingner, J. Molecular basis of telomere syndrome caused by CTC1 mutations. Genes Dev. 27, 2099–2108 (2013).
Gu, P. et al. CTC1–STN1 coordinates G- and C-strand synthesis to regulate telomere length. Aging Cell 17, e12783 (2018).
Feng, X. et al. CTC1–STN1 terminates telomerase while STN1–TEN1 enables C-strand synthesis during telomere replication in colon cancer cells. Nat. Commun. 9, 2827 (2018).
Fan, J. & Pavletich, N. P. Structure and conformational change of a replication protein A heterotrimer bound to ssDNA. Genes Dev. 26, 2337–2347 (2012).
Gao, Y. et al. Structures and operating principles of the replisome. Science 363, eaav7003 (2019).
Ge, Y. et al. Structural insights into telomere protection and homeostasis regulation by yeast CST complex. Nat. Struct. Mol. Biol. 27, 752–762 (2020).
Wan, B. et al. The Tetrahymena telomerase p75–p45–p19 subcomplex is a unique CST complex. Nat. Struct. Mol. Biol. 22, 1023–1026 (2015).
Lue, N. F. et al. The telomere capping complex CST has an unusual stoichiometry, makes multipartite interaction with G-tails, and unfolds higher-order G-tail structures. PLoS Genet. 9, e1003145 (2013).
Gelinas, A. D. et al. Telomere capping proteins are structurally related to RPA with an additional telomere-specific domain. Proc. Natl Acad. Sci. USA 106, 19298–19303 (2009).
Sun, J. et al. Structural bases of dimerization of yeast telomere protein Cdc13 and its interaction with the catalytic subunit of DNA polymerase α. Cell Res. 21, 258–274 (2011).
Rice, C. & Skordalakes, E. Structure and function of the telomeric CST complex. Comput. Struct. Biotechnol. J. 14, 161–167 (2016).
Zeng, Z. et al. Structural basis for Tetrahymena telomerase processivity factor Teb1 binding to single-stranded telomeric-repeat DNA. Proc. Natl Acad. Sci. USA 108, 20357–20361 (2011).
Goulian, M., Heard, C. J. & Grimm, S. L. Purification and properties of an accessory protein for DNA polymerase α/primase. J. Biol. Chem. 265, 13221–13230 (1990).
Zhao, Y. et al. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 138, 463–475 (2009).
Kratz, K. & de Lange, T. Protection of telomeres 1 proteins POT1a and POT1b can repress ATR signaling by RPA exclusion, but binding to CST limits ATR repression by POT1b. J. Biol. Chem. 293, 14384–14392 (2018).
Wan, M., Qin, J., Songyang, Z. & Liu, D. OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J. Biol. Chem. 284, 26725–26731 (2009).
Tesmer, V. M., Smith, E. M., Danciu, O., Padmanaban, S. & Nandakumar, J. Combining conservation and species-specific differences to determine how human telomerase binds telomeres. Proc. Natl Acad. Sci. USA 116, 26505–26515 (2019).
Wu, P., Takai, H. & de Lange, T. Telomeric 3′ overhangs derive from resection by Exo1 and Apollo and fill-in by POT1b-associated CST. Cell 150, 39–52 (2012).
Feng, X., Hsu, S. J., Kasbek, C., Chaiken, M. & Price, C. M. CTC1-mediated C-strand fill-in is an essential step in telomere length maintenance. Nucleic Acids Res. 45, 4281–4293 (2017).
Diotti, R., Kalan, S., Matveyenko, A. & Loayza, D. DNA-directed polymerase subunits play a vital role in human telomeric overhang processing. Mol. Cancer Res. 13, 402–410 (2015).
Porcella, S. Y. et al. Separable, Ctf4-mediated recruitment of DNA polymerase α for initiation of DNA synthesis at replication origins and lagging-strand priming during replication elongation. PLoS Genet. 16, e1008755 (2020).
Kilkenny, M. L. et al. The human CTF4-orthologue AND-1 interacts with DNA polymerase α/primase via its unique C-terminal HMG box. Open. Biol. 7, 170217 (2017).
Nakaoka, H., Nishiyama, A., Saito, M. & Ishikawa, F. Xenopus laevis Ctc1–Stn1–Ten1 (xCST) protein complex is involved in priming DNA synthesis on single-stranded DNA template in Xenopus egg extract. J. Biol. Chem. 287, 619–627 (2012).
Ganduri, S. & Lue, N. F. STN1–POLA2 interaction provides a basis for primase-pol α stimulation by human STN1. Nucleic Acids Res. 45, 9455–9466 (2017).
Nunez-Ramirez, R. et al. Flexible tethering of primase and DNA pol α in the eukaryotic primosome. Nucleic Acids Res. 39, 8187–8199 (2011).
Leteurtre, F. et al. Accelerated telomere shortening and telomerase activation in Fanconi’s anaemia. Br. J. Haematol. 105, 883–893 (1999).
Ball, S. E. et al. Progressive telomere shortening in aplastic anemia. Blood 91, 3582–3592 (1998).
Shay, J. W. & Wright, W. E. Telomeres and telomerase: three decades of progress. Nat. Rev. Genet. 20, 299–309 (2019).
Savage, S. A. Beginning at the ends: telomeres and human disease. F1000Res https://doi.org/10.12688/f1000research.14068.1 (2018).
Podlevsky, J. D., Bley, C. J., Omana, R. V., Qi, X. & Chen, J. J. The telomerase database. Nucleic Acids Res. 36, D339–D343 (2008).
Guo, Y. et al. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood 124, 2767–2774 (2014).
Bisht, K., Smith, E. M., Tesmer, V. M. & Nandakumar, J. Structural and functional consequences of a disease mutation in the telomere protein TPP1. Proc. Natl Acad. Sci. USA 113, 13021–13026 (2016).
Keller, R. B. et al. CTC1 mutations in a patient with dyskeratosis congenita. Pediatr. Blood Cancer 59, 311–314 (2012).
Anderson, B. H. et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat. Genet. 44, 338–342 (2012).
Simon, A. J. et al. Mutations in STN1 cause Coats plus syndrome and are associated with genomic and telomere defects. J. Exp. Med. 213, 1429–1440 (2016).
Gu, P. & Chang, S. Functional characterization of human CTC1 mutations reveals novel mechanisms responsible for the pathogenesis of the telomere disease Coats plus. Aging Cell 12, 1100–1109 (2013).
Wang, Y. & Chai, W. Pathogenic CTC1 mutations cause global genome instabilities under replication stress. Nucleic Acids Res. 46, 3981–3992 (2018).
Maciejowski, J. & de Lange, T. Telomeres in cancer: tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 18, 175–186 (2017).
Makarov, V. L., Lejnine, S., Bedoyan, J. & Langmore, J. P. Nucleosomal organization of telomere-specific chromatin in rat. Cell 73, 775–787 (1993).
Tommerup, H., Dousmanis, A. & de Lange, T. Unusual chromatin in human telomeres. Mol. Cell. Biol. 14, 5777–5785 (1994).
Wu, P. & de Lange, T. No overt nucleosome eviction at deprotected telomeres. Mol. Cell. Biol. 28, 5724–5735 (2008).
Galati, A. et al. The human telomeric protein TRF1 specifically recognizes nucleosomal binding sites and alters nucleosome structure. J. Mol. Biol. 360, 377–385 (2006).
Galati, A. et al. TRF2 controls telomeric nucleosome organization in a cell cycle phase-dependent manner. PLoS ONE 7, e34386 (2012).
Soman, A. et al. The human telomeric nucleosome displays distinct structural and dynamic properties. Nucleic Acids Res. 48, 5383–5396 (2020).
Ichikawa, Y., Morohashi, N., Nishimura, Y., Kurumizaka, H. & Shimizu, M. Telomeric repeats act as nucleosome-disfavouring sequences in vivo. Nucleic Acids Res. 42, 1541–1552 (2014).
James, T. C. et al. Distribution patterns of Hp1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur. J. Cell Biol. 50, 170–180 (1989).
Gottschling, D. E., Aparicio, O. M., Billington, B. L. & Zakian, V. A. Position effect at S. cerevisiae telomeres—reversible repression of Pol II transcription. Cell 63, 751–762 (1990).
Garcia-Cao, M., O’Sullivan, R., Peters, A. H., Jenuwein, T. & Blasco, M. A. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat. Genet. 36, 94–99 (2004).
Arnoult, N., Van Beneden, A. & Decottignies, A. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1α. Nat. Struct. Mol. Biol. 19, 948–956 (2012).
Blasco, M. A. The epigenetic regulation of mammalian telomeres. Nat. Rev. Genet. 8, 299–309 (2007).
Dejardin, J. & Kingston, R. E. Purification of proteins associated with specific genomic loci. Cell 136, 175–186 (2009).
Grolimund, L. et al. A quantitative telomeric chromatin isolation protocol identifies different telomeric states. Nat. Commun. 4, 2848 (2013).
Pfeiffer, V., Crittin, J., Grolimund, L. & Lingner, J. The THO complex component Thp2 counteracts telomeric R-loops and telomere shortening. EMBO J. 32, 2861–2871 (2013).
Ribes-Zamora, A., Indiviglio, S. M., Mihalek, I., Williams, C. L. & Bertuch, A. A. TRF2 interaction with Ku heterotetramerization interface gives insight into c-NHEJ prevention at human telomeres. Cell Rep. 5, 194–206 (2013).
Fisher, T. S. & Zakian, V. A. Ku: a multifunctional protein involved in telomere maintenance. DNA Repair 4, 1215–1226 (2005).
Celli, G. B., Denchi, E. L. & de Lange, T. Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat. Cell Biol. 8, 885–U162 (2006).
McKay, S. J. & Cooke, H. hnRNP A2/B1 binds specifically to single stranded vertebrate telomeric repeat TTAGGGn. Nucleic Acids Res. 20, 6461–6464 (1992).
Ishikawa, F., Matunis, M. J., Dreyfuss, G. & Cech, T. R. Nuclear proteins that bind the pre-mRNA 3′ splice site sequence r(UUAG/G) and the human telomeric DNA sequence d(TTAGGG)n. Mol. Cell Biol. 13, 4301–4310 (1993).
LaBranche, H. et al. Telomere elongation by hnRNP A1 and a derivative that interacts with telomeric repeats and telomerase. Nat. Genet. 19, 199–202 (1998).
Potjer, T. P. et al. Multigene panel sequencing of established and candidate melanoma susceptibility genes in a large cohort of Dutch non-CDKN2A/CDK4 melanoma families. Int. J. Cancer 144, 2453–2464 (2019).
Pelusi, S. et al. Rare pathogenic variants predispose to hepatocellular carcinoma in nonalcoholic fatty liver disease. Sci. Rep. 9, 3682 (2019).
Guacci, A. et al. Identification of a novel truncating mutation in PALB2 gene by a multigene sequencing panel for mutational screening of breast cancer risk-associated and related genes. J. Clin. Laboratory Anal. 32, e22418 (2018).
Aoude, L. G. et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J. Natl Cancer Inst. 107, dju408 (2015).
Vulliamy, T. et al. Telomere length measurement can distinguish pathogenic from non-pathogenic variants in the shelterin component, TIN2. Clin. Genet. 81, 76–81 (2012).
Sasa, G. S., Ribes-Zamora, A., Nelson, N. D. & Bertuch, A. A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin. Genet. 81, 470–478 (2012).
Vulliamy, T. J. et al. Differences in disease severity but similar telomere lengths in genetic subgroups of patients with telomerase and shelterin mutations. PLoS ONE 6, e24383 (2011).
Robles-Espinoza, C. D. et al. POT1 loss-of-function variants predispose to familial melanoma. Nat. Genet. 46, 478–481 (2014).
Polvi, A. et al. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am. J. Hum. Genet. 90, 540–549 (2012).
Netravathi, M. et al. Whole exome sequencing in an Indian family links Coats plus syndrome and dextrocardia with a homozygous novel CTC1 and a rare HES7 variation. BMC Med. Genet. 16, 5 (2015).
Walne, A. J. et al. Mutations in the telomere capping complex in bone marrow failure and related syndromes. Haematologica 98, 334–338 (2013).
Acknowledgements
The authors thank T. de Lange for helpful comments and suggestions. C.L. thanks the members of the Cech laboratory for their support and advice during his postdoctoral years at the University of Colorado Boulder. This work was funded in part by the National Institutes of Health (NIH; R00GM131023) and the Steenbock Career Award from the University of Wisconsin-Madison to C.L. T.R.C. is an investigator at the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
T.R.C. is on the board of directors of Merck and a consultant for STORM Therapeutics and Eikon Therapeutics.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks Ming Lei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Human Gene Mutation Database: http://www.hgmd.cf.ac.uk/
Telomerase Database: http://telomerase.asu.edu
Glossary
- G-overhang
-
A telomeric single-stranded DNA 3′ tail consisting of TTAGGG repeats, which is the substrate of telomerase.
- Telomerase
-
A ribonucleoprotein that uses its RNA as a template to synthesize TTAGGG repeats, thereby extending telomeres.
- Processive
-
The ability of an enzyme to perform multiple catalytic reactions without releasing its substrate.
- Telomeric repeat-containing RNA
-
(TERRA). A long non-coding RNA involved in regulating telomerase activity at telomeres.
- Holliday junction
-
A branched DNA structure comprising four double-stranded arms.
- TEL patch
-
(TPP1 glutamate (E) and leucine (L)-rich patch). A small group of amino acids on the surface of the shelterin protein TPP1. The TEL patch directly recruits telomerase to telomeres and then stimulates its activity.
- G-quadruplexes
-
(G4s). Tertiary structures in which groups of four guanines in single-stranded DNA form tetrads through hydrogen bonds.
- Replication protein A
-
(RPA). A eukaryotic single-stranded DNA-binding protein complex involved in DNA replication and repair.
- Telomeric C-strand
-
The telomeric DNA strand consisting of CCCTAA repeats, which pair with the telomerase-synthesized TTAGGG repeats.
- Replisome
-
A multisubunit protein complex that carries out replication of DNA.
Rights and permissions
About this article
Cite this article
Lim, C.J., Cech, T.R. Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization. Nat Rev Mol Cell Biol 22, 283–298 (2021). https://doi.org/10.1038/s41580-021-00328-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-021-00328-y
This article is cited by
-
CST–polymerase α-primase solves a second telomere end-replication problem
Nature (2024)
-
The Role of Glia Telomere Dysfunction in the Pathogenesis of Central Nervous System Diseases
Molecular Neurobiology (2024)
-
Kinesin Family Member-18A (KIF18A) Promotes Cell Proliferation and Metastasis in Hepatocellular Carcinoma
Digestive Diseases and Sciences (2024)
-
Methods that shaped telomerase research
Biogerontology (2024)
-
Effects of hTERT transfection on the telomere and telomerase of Periplaneta americana cells in vitro
AMB Express (2023)