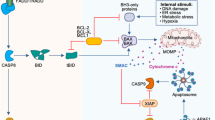
Abstract
The removal of functionally dispensable, infected or potentially neoplastic cells is driven by programmed cell death (PCD) pathways, highlighting their important roles in homeostasis, host defence against pathogens, cancer and a range of other pathologies. Several types of PCD pathways have been described, including apoptosis, necroptosis and pyroptosis; they employ distinct molecular and cellular processes and differ in their outcomes, such as the capacity to trigger inflammatory responses. Recent genetic and biochemical studies have revealed remarkable flexibility in the use of these PCD pathways and indicate a considerable degree of plasticity in their molecular regulation; for example, despite having a primary role in inducing pyroptosis, inflammatory caspases can also induce apoptosis, and conversely, apoptotic stimuli can trigger pyroptosis. Intriguingly, this flexibility is most pronounced in cellular responses to infection, while apoptosis is the dominant cell death process through which organisms prevent the development of cancer. In this Review, we summarize the mechanisms of the different types of PCD and describe the physiological and pathological processes that engage crosstalk between these pathways, focusing on infections and cancer. We discuss the intriguing notion that the different types of PCD could be seen as a single, coordinated cell death system, in which the individual pathways are highly interconnected and can flexibly compensate for one another.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Thompson, C. B. Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 (1995).
Ellis, R. E., Yuan, J. & Horvitz, H. R. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 7, 663–698 (1991).
Strasser, A., O’Connor, L. & Dixit, V. M. Apoptosis signaling. Ann. Rev. Biochem. 69, 217–245 (2000).
Green, D. R. The coming decade of cell death research: five riddles. Cell 177, 1094–1107 (2019).
Ke, F. F. S. et al. Embryogenesis and adult life in the absence of intrinsic apoptosis Effectors BAX, BAK, and BOK. Cell 173, 1217–1230 e1217 (2018).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000). This report describes the essential overlapping role of BAX and BAK for the execution of the intrinsic pathway of apoptosis during embryogenesis and tissue homeostasis.
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). Together with Murphy et al. (2013), this study identifies MLKL as the essential executioner of necroptosis.
Rock, K. L. & Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 3, 99–126 (2008).
Van Opdenbosch, N. & Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 50, 1352–1364 (2019).
Boatright, K. M. & Salvesen, G. S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15, 725–731 (2003).
Kerr, J. F. R., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Brit. J. Cancer 26, 239–257 (1972). This publication coins the term ‘apoptotis’ and describes the morphology observed during an apoptotic process.
Segawa, K. et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164–1168 (2014).
Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 (2014).
Singh, R., Letai, A. & Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 20, 175–193 (2019).
Cosentino, K. & Garcia-Saez, A. J. Bax and Bak pores: Are we closing the circle? Trends Cell Biol. 27, 266–275 (2017).
O’Neill, K. L., Huang, K., Zhang, J., Chen, Y. & Luo, X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 30, 973–988 (2016).
Salvesen, G. S. & Dixit, V. M. Caspase activation: the induced-proximity model. Proc. Natl Acad. Sci. USA 96, 10964–10967 (1999).
Galban, S. & Duckett, C. S. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 17, 54–60 (2010).
Ekert, P. G. & Vaux, D. L. The mitochondrial death squad: hardened killers or innocent bystanders? Curr. Opin. Cell Biol. 17, 626–630 (2005).
Ashkenazi, A. & Dixit, V. M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Kischkel, F. C. et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14, 5579–5588 (1995). Kischkel et al. provide a description of the death-inducing signalling complex that is critical for FAS to induce apoptosis.
Kataoka, T. The caspase-8 modulator c-FLIP. Crit. Rev. Immunol. 25, 31–58 (2005).
Billen, L. P., Shamas-Din, A. & Andrews, D. W. Bid: a Bax-like BH3 protein. Oncogene 27, S93–S104 (2008).
Brennan, M. A. & Cookson, B. T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 (2000).
Sansonetti, P. J. et al. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12, 581–590 (2000).
Lamkanfi, M. & Dixit, V. M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 227, 95–105 (2009).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Hornung, V. & Latz, E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur. J. Immunol. 40, 620–623 (2010).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Duncan, J. A. & Canna, S. W. The NLRC4 inflammasome. Immunol. Rev. 281, 115–123 (2018).
Broz, P., Pelegrin, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). Together with Kayagaki et al. (2015), this study identifies GSDMD as the essential executioner of pyroptosis.
Man, S. M., Kanneganti, T. D. & Gasdermin, D. the long-awaited executioner of pyroptosis. Cell Res. 25, 1183–1184 (2015).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Kupz, A. et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8+ T cells. Nat. Immunol. 13, 162–169 (2012).
Ruhl, S. et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018). Ruhl et al. demonstrate that the ESCRT machinery can protect cells from gasdermin-driven lysis at the early stages of pyroptosis.
Salvamoser, R. et al. Characterisation of mice lacking the inflammatory caspases-1/11/12 reveals no contribution of caspase-12 to cell death and sepsis. Cell Death Differ. 26, 1124–1137 (2019).
Wang, S. et al. Identification and characterization of Ich-3, a member of the interleukin-1β converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J. Biol. Chem. 271, 20580–20587 (1996).
Kobori, M. et al. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 11, 123–130 (2004).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–122 (2011).
Rathinam, V. A. K., Zhao, Y. & Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 20, 527–533 (2019).
Lawlor, K. E. et al. XIAP loss triggers RIPK3- and caspase-8-driven IL-1β activation and cell death as a consequence of TLR-MyD88-induced cIAP1-TRAF2 degradation. Cell Rep. 20, 668–682 (2017).
Lawlor, K. E. et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 6, 6282 (2015).
Newton, K. & Manning, G. Necroptosis and inflammation. Annu. Rev. Biochem. 85, 743–763 (2016).
Newton, K. R. I. P. K. 1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 25, 347–353 (2015).
Khan, N., Lawlor, K. E., Murphy, J. M. & Vince, J. E. More to life than death: molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr. Opin. Immunol. 26, 76–89 (2014).
Gong, Y. N. et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300 e216 (2017).
Vaux, D. L., Haecker, G. & Strasser, A. An evolutionary perspective on apoptosis. Cell 76, 777–779 (1994).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Franchi, L., Eigenbrod, T., Munoz-Planillo, R. & Nunez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 (2009).
Sutterwala, F. S. et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204, 3235–3245 (2007).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
McAuley, J. L. et al. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog. 9, e1003392 (2013).
Ichinohe, T., Pang, I. K. & Iwasaki, A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 11, 404–410 (2010).
Lara-Tejero, M. et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203, 1407–1412 (2006).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Maltez, V. I. & Miao, E. A. Reassessing the evolutionary importance of inflammasomes. J. Immunol. 196, 956–962 (2016).
Tsuchiya, K. et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun. 10, 2091 (2019). This report demonstrates that caspase 1 can induce cell killing in the absence of gasdermin D.
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Brown, A. S. et al. Cooperation between monocyte-derived cells and lymphoid cells in the acute response to a bacterial lung pathogen. PLoS Pathog. 12, e1005691 (2016).
Mascarenhas, D. P. A. et al. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 13, e1006502 (2017).
Stewart, M. K. & Cookson, B. T. Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses. Nat. Rev. Microbiol. 14, 346–359 (2016).
Maltez, V. I. et al. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity 43, 987–997 (2015).
Wong Fok Lung, T., Pearson, J. S., Schuelein, R. & Hartland, E. L. The cell death response to enteropathogenic Escherichia coli infection. Cell. Microbiol. 16, 1736–1745 (2014).
Pearson, J. S. et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature 501, 247–251 (2013). This study identifies a process employed by bacteria to inhibit death receptor-induced apoptosis.
Philip, N. H. et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc. Natl Acad. Sci. USA 111, 7385–7390 (2014).
Weng, D. et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl Acad. Sci. USA 111, 7391–7396 (2014).
Wang, X. et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc. Natl Acad. Sci. USA 111, 15438–15443 (2014).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Zhang, T. et al. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell 180, 1115–1129.e1113 (2020).
Dondelinger, Y. et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 20, 1381–1392 (2013).
Alvarez-Diaz, S. et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45, 513–526 (2016).
Nogusa, S. et al. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza a virus. Cell Host Microbe 20, 13–24 (2016). RIPK3-mediated initiation of two distinct cell death pathways can be used for protection against viral infection.
Rodrigue-Gervais, I. G. et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe 15, 23–35 (2014).
Kuriakose, T. et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 1, aag2045 (2016).
Shan, B., Pan, H., Najafov, A. & Yuan, J. Necroptosis in development and diseases. Genes Dev. 32, 327–340 (2018).
Tsujimoto, Y., Gorham, J., Cossman, J., Jaffe, E. & Croce, C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 229, 1390–1393 (1985).
Vaux, D. L., Cory, S. & Adams, J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988). This report identifies the function of BCL-2.
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010). This study demonstrates somatic copy number amplifications of the genomic regions that encode MCL-1 or BCL-XL in a substantial fraction of human cancers.
Strasser, A., Harris, A. W., Bath, M. L. & Cory, S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348, 331–333 (1990). Strasser et al. provide the first demonstration that blocking apoptosis promotes the development of cancer.
Egle, A., Harris, A. W., Bouillet, P. & Cory, S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc. Natl Acad. Sci. USA 101, 6164–6169 (2004).
Michalak, E. M. et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ. 16, 684–696 (2009).
Strasser, A., Harris, A. W. & Cory, S. Em-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene 8, 1–9 (1993).
Strasser, A., Harris, A. W., Jacks, T. & Cory, S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 79, 329–339 (1994).
Newton, K. & Strasser, A. Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of fas or FADD/MORT1 signaling: implications for cancer therapy. J. Exp. Med. 191, 195–200 (2000).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003).
Jeffers, J. R. et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4, 321–328 (2003). Together with Villunger et al. (2003), this study demonstrates that PUMA directly transcriptionally activated by p53 is critical for the killing of cells by DNA damage-inducing anticancer agents.
Bouillet, P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999). Bouillet et al. provide the first demonstration that BH3-only proteins are essential for the initiation of apoptosis.
Cragg, M. S., Harris, C., Strasser, A. & Scott, C. L. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat. Rev. Cancer 9, 321–326 (2009).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005). This study describes the first BH3-mimetic drug for cancer therapy.
Merino, D. et al. BH3-mimetic drugs: blazing the trail for new cancer medicines. Cancer Cell 34, 879–891 (2018).
Montero, J. & Letai, A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 25, 56–64 (2018).
Roberts, A. W. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2016). Roberts et al. present clinical trial data from using the BCL-2 inhibitor venetoclax in CLL, which led to FDA approval of this drug.
Kelly, G. L. et al. Targeting of MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev. 28, 58–70 (2014).
Kotschy, A. et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538, 477–482 (2016).
Brennan, M. S. et al. Humanized Mcl-1 mice enable accurate preclinical evaluation of MCL-1 inhibitors destined for clinical use. Blood 132, 1573–1583 (2018).
Seifert, L. et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 532, 245–249 (2016).
Wang, W. et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell 34, 757–774 e757 (2018).
Patel, S. et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. 27, 161–175 (2020).
Baker, K. J., Houston, A. & Brint, E. IL-1 family members in cancer; two sides to every story. Front. Immunol. 10, 1197 (2019).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, cancer. Cell 140, 883–899 (2010).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020). This study demonstrates the potential utility of activators of pyroptosis in cancer therapy.
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017). This article demonstrates that caspase-3-mediated cleavage of a gasdermin causes pyroptotic death in cells treated with chemotherapeutic drugs.
Liu, Y. et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 5, eaax7969 (2020).
Yuan, J., Amin, P. & Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19–33 (2019).
Caccamo, A. et al. Necroptosis activation in Alzheimer’s disease. Nat. Neurosci. 20, 1236–1246 (2017).
Ito, Y. et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 353, 603–608 (2016).
Heneka, M. T., McManus, R. M. & Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 19, 610–621 (2018).
Ising, C. et al. NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673 (2019).
Rohn, T. T. The role of caspases in Alzheimer’s disease; potential novel therapeutic opportunities. Apoptosis 15, 1403–1409 (2010).
Paradis, E., Douillard, H., Koutroumanis, M., Goodyer, C. & LeBlanc, A. Amyloid β peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates bax expression in human neurons. J. Neurosci. 16, 7533–7539 (1996).
Kitamura, Y. et al. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 780, 260–269 (1998).
Graham, S. H., Chen, J. & Clark, R. S. Bcl-2 family gene products in cerebral ischemia and traumatic brain injury. J. Neurotrauma 17, 831–841 (2000).
Del, Re,D. P., Amgalan, D., Linkermann, A., Liu, Q. & Kitsis, R. N. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 99, 1765–1817 (2019).
Newton, K. et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 23, 1565–1576 (2016).
Fang, X. et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl Acad. Sci. USA 116, 2672–2680 (2019).
Voet, S., Srinivasan, S., Lamkanfi, M. & van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 11, e10248 (2019).
Varfolomeev, E. E. et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998).
Newton, K., Harris, A. W., Bath, M. L., Smith, K. G. C. & Strasser, A. A dominant interfering mutant of FADD/Mort1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 17, 706–718 (1998).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Guasparri, I., Keller, S. A. & Cesarman, E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199, 993–1003 (2004).
Turner, S., Kenshole, B. & Ruby, J. Viral modulation of the host response via crmA/SPI-2 expression. Immunol. Cell Biol. 77, 236–241 (1999).
He, S. & Han, J. Manipulation of host cell death pathways by herpes simplex virus. Curr. Top. Microbiol. Immunol. (2020).
Pauleau, A. L. et al. Structure-function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA. Oncogene 26, 7067–7080 (2007).
Wang, T. et al. Necroptosis is dispensable for motor neuron degeneration in a mouse model of ALS. Cell Death Differ. 27, 1728–1739 (2020).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
Newton, K. et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature 575, 679–682 (2019). Together with Fritsch et al. (2019), this article demonstrates the ability of caspase-8 to coordinate the activation of several distinct cell death pathways.
Schwarzer, R., Jiao, H., Wachsmuth, L., Tresch, A. & Pasparakis, M. FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity 52, 978–993.e6 (2020).
Tummers, B. et al. Caspase-8-dependent inflammatory responses are controlled by its adaptor, FADD, and necroptosis. Immunity 52, 994–1006.e8 (2020).
Chen, K. W. et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 38, e101638 (2019).
Vince, J. E. et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep. 25, 2339–2353 (2018).
Lampson, B. L. & Davids, M. S. The development and current use of BCL-2 inhibitors for the treatment of chronic lymphocytic leukemia. Curr. Hematol. Malig. Rep. 12, 11–19 (2017).
Marsden, V. et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419, 634–637 (2002).
Bock, F. J. & Tait, S. W. G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 21, 85–100 (2020).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009).
Iwai, K. & Tokunaga, F. Linear polyubiquitination: a new regulator of NF-κB activation. EMBO Rep. 10, 706–713 (2009).
Shim, J. H. et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 (2005).
Chen, G. & Goeddel, D. V. TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635 (2002).
Grossmann, M. et al. The anti-apoptotic activities of rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 19, 6351–6360 (2000).
Baldwin, A. S. Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol. Rev. 246, 327–345 (2012).
Meinzer, U. et al. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe 11, 337–351 (2012).
Mukherjee, S. et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312, 1211–1214 (2006).
Peltzer, N. & Walczak, H. Cell Death and inflammation — a vital but dangerous Liaison. Trends Immunol. 40, 387–402 (2019).
Lei, X. et al. Enterovirus 71 3C inhibits cytokine expression through cleavage of the TAK1/TAB1/TAB2/TAB3 complex. J. Virol. 88, 9830–9841 (2014).
Zhou, X. et al. A Vibrio parahaemolyticus T3SS effector mediates pathogenesis by independently enabling intestinal colonization and inhibiting TAK1 activation. Cell Rep. 3, 1690–1702 (2013).
He, C. et al. Bacterial nucleotidyl cyclase inhibits the host innate immune response by suppressing TAK1 activation. Infect. Immun. 85, e00239-17 (2017).
Nilsson, J. A. & Cleveland, J. L. Myc pathways provoking cell suicide and cancer. Oncogene 22, 9007–9021 (2003).
Fanidi, A., Harrington, E. A. & Evan, G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature 359, 554–556 (1992).
Bissonnette, R. P., Echeverri, F., Mahboubi, A. & Green, D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature 359, 552–554 (1992).
Gong, J. N. et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: pivotal role of MCL1. Blood 128, 1834–1844 (2016).
Nassour, J. et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 565, 659–663 (2019). Nassour et al. demonstrate the role of autophagic cell death in the removal of cells with chromosomal instability.
Poillet-Perez, L. & White, E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 33, 610–619 (2019).
Yamamoto, K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020).
Ofengeim, D. et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 114, E8788–E8797 (2017).
Taabazuing, C. Y., Okondo, M. C. & Bachovchin, D. A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 24, 507–514.e504 (2017).
Man, S. M. et al. Salmonella infection induces recruitment of caspase-8 to the inflammasome to modulate IL-1β production. J. Immunol. 191, 5239–5246 (2013).
Van Opdenbosch, N. et al. Caspase-1 engagement and TLR-induced c-FLIP expression suppress ASC/caspase-8-dependent apoptosis by inflammasome sensors NLRP1β and NLRC4. Cell Rep. 21, 3427–3444 (2017).
Chung, H. et al. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Differ. 23, 1331–1346 (2016).
Doerflinger, M. et al. Flexible usage and interconnectivity of diverse cell death pathways protects against intracellular infection. Immunity https://doi.org/10.1016/j.immuni.2020.07.004 (2020).
Heilig, R. et al. Caspase-1 cleaves Bid to release mitochondrial SMAC and drive secondary necrosis in the absence of GSDMD. Life Sci. Alliance 3, e202000735 (2020).
Sarhan, J. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA 115, E10888–E10897 (2018).
Muendlein, H. I. et al. cFLIPL protects macrophages from LPS-induced pyroptosis via inhibition of complex II formation. Science 367, 1379–1384 (2020).
Kesavardhana, S., Subbarao Malireddi, R. K. & Kanneganti, T. D. Caspases in cell death, inflammation, and gasdermin-induced pyroptosis. Annu. Rev. Immunol. 38, 567–595 (2020).
Rauch, I. et al. NAIP–NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46, 649–659 (2017).
Hausmann, A. et al. Intestinal epithelial NAIP/NLRC4 restricts systemic dissemination of the adapted pathogen Salmonella Typhimurium due to site-specific bacterial PAMP expression. Mucosal Immunol. 13, 530–544 (2020).
Strasser, A. & Vaux, D. L. Cell death in the origin and treatment of cancer. Mol. Cell 78, 1045–1054 (2020).
Ascierto, P. A. et al. Future perspectives in melanoma research ‘Melanoma Bridge’, Napoli, November 30th–3rd December 2016. J. Transl Med. 15, 236 (2017).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Miller, J. F. & Sadelain, M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell 27, 439–449 (2015).
Speir, M. et al. Eliminating Legionella by inhibiting BCL-XL to induce macrophage apoptosis. Nat. Microbiol. 1, 15034 (2016).
Kostic, V., Jackson-Lewis, V., de Bilbao, F., Dubois-Dauphin, M. & Przedborski, S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science 277, 559–562 (1997).
Kroemer, G. & Levine, B. Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 9, 1004–1010 (2008).
Holze, C. et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 19, 130–140 (2018).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012). This study is the first demonstration of the mechanism of programmed cell death by ferroptosis.
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Martinez-Lostao, L., Anel, A. & Pardo, J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. 21, 5047–5056 (2015).
Lowin, B., Peitsch, M. C. & Tschopp, J. Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr. Top. Microbiol. Immunol. 198, 1–24 (1995).
Waterhouse, N. J. et al. A central role for Bid in granzyme B-induced apoptosis. J. Biol. Chem. 280, 4476–4482 (2005).
Lopez, J. A. et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 121, 2659–2668 (2013).
Li, X., McKinstry, K. K., Swain, S. L. & Dalton, D. K. IFN-γ acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J. Immunol. 179, 939–949 (2007).
Barthson, J. et al. Cytokines tumor necrosis factor-α and interferon-γ induce pancreatic β-cell apoptosis through STAT1-mediated Bim protein activation. J. Biol. Chem. 286, 39632–39643 (2011).
Acknowledgements
We are grateful to the present and past members of our laboratories, our collaborators and the mentors that we have had the pleasure of working with. Our work is supported by grants and fellowships from the Australian National Health and Medical Research Council (Project Grants 1186575 and 1145728 to M.J.H., 1143105 to M.J.H. and A.S., 1159658 to M.J.H. and S.B., Program Grant 1016701 to A.S., and Fellowships 1020363, to A.S., and 1156095, to M.J.H.), by the Leukemia and Lymphoma Society of America (grant LLS SCOR 7001-13 to A.S. and M.J.H.), by the Cancer Council of Victoria (project grants 1147328, to M.J.H., and 1052309, to A.S.) and by a Venture Grant to M.J.H. and A.S.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks J. Yuan, A. Oberst and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- BCL-2 (B cell leukaemia/lymphoma-2) family
-
A family of proteins — named after its original member, BCL-2 — that regulate the intrinsic apoptotic pathway.
- Complement system
-
Evolutionarily ancient system of protein cascades capable of lysing bacteria by perforating their outer membrane, opsonizing the pathogens and activating the cells of the immune system.
- Damage-associated molecular patterns
-
(DAMPs, also known as alarmins). Intracellular molecules, such as HMGB1 or S100A8, whose release from cells undergoing lytic cell death triggers distinct receptors in innate immune cells and causes inflammation.
- Pathogen-associated molecular patterns
-
(PAMPs). Evolutionarily conserved molecular components of pathogens, such as LPS expressed by Gram-negative bacteria, that cause inflammatory responses by innate immune cells.
- Pattern recognition receptors
-
Membrane-associated or cytosolic receptors capable of recognizing and responding to PAMPs through the induction of pro-inflammatory responses.
- TAM receptors
-
Family of receptor tyrosine kinases (TYRO3, AXL, MERTK) that promote apoptotic cell clearance by binding to phosphatidylserine exposed on apoptotic cells using GAS6 and protein S as bridging ligands.
- BH3-only proteins
-
Pro-apoptotic members of the BCL-2 protein family that share only one of the four BCL-2 homology (BH) domains, namely the BH3 domain, with the remainder of the family. BH3-only proteins are induced transcriptionally and/or activated post-translationally in response to developmental cues or cytotoxic stimuli that initiate the intrinsic apoptotic cell death pathway.
- Mitochondrial outer membrane permeabilization
-
(MOMP). Perforation of the outer mitochondrial membrane, causing leakage of content from the mitochondrial intermembrane space, including the apoptosis inducers cytochrome c and SMAC. MOMP can result in the translocation of mitochondrial DNA into the cytosol, leading to the production of type I interferons and thereby driving inflammatory responses.
- Inhibitor of apoptosis proteins
-
(IAPs). Family of proteins with structural homology (that is, baculovirus IAP repeats). Some of the IAP proteins have an E3 ubiquitin ligase function, allowing them to ubiquitylate their target proteins. XIAP inhibits apoptosis by binding to and promoting the degradation of caspases 3 and 7, whereas cIAP1 and cIAP2 promote pro-survival signalling from TNFR1 by enhancing NF-κB activation.
- Death receptors
-
Subsets of the TNFR superfamily that contain an intracellular death domain, which upon ligation can induce killing of the cells on which they are expressed through FADD adaptor protein-mediated activation of caspase 8.
- cFLIP
-
(cellular CASP8 and FADD-like apoptosis regulator). Protein with structural similarity to caspase 8 but that lacks enzymatic activity. There are two forms of FLIP: FLIP short and FLIP long. FLIP short inhibits apoptosis by preventing the activation of caspase 8; FLIP long can form heterodimers with caspase 8, and this heterodimer inhibits necroptosis by cleaving RIPK1. High levels of FLIP long can also inhibit caspase 8 activation and apoptosis.
- Inflammasomes
-
Multimeric protein complexes, activated by various events, including ion flux, reactive oxygen species and mitochondrial dysfunction. They comprise sensors, such as NLR molecules, and their formation often depends on the adaptor protein ASC and pro-caspase 1, which together cause the autocatalytic activation of caspase 1. The consequent proteolytic processing of pro-IL-1β and pro-IL-18 into their bioactive forms results in inflammation and proteolytic activation of gasdermin D to drive pyroptotic cell death.
- NLR (nucleotide-binding domain and leucine-rich repeat containing) family
-
Evolutionarily conserved diverse family of proteins, further classified into NLRA, NLRB, NLRP and NLRC, in accordance with their N-terminal domains and the presence or absence of CARD domains. Certain (but probably not all) NLRs function in innate immune sensing of pathogens and infection-associated cellular changes. They contribute to the protection of the infected host by instructing an antimicrobial defence, including inflammatory responses.
- Type III secretion (T3SS) apparatus
-
Complex molecular machines used by bacteria to inject effector proteins into eukaryotic host cells.
- Flagellin
-
Subunit protein of the flagellum that endows bacteria with motility.
- Gasdermin family
-
Conserved family of proteins in vertebrates, named after the restriction of gasdermin A to gut and skin epithelial cells, although it is now clear that these proteins are much more widely expressed. At least some of the gasdermins can form pores in membranes after proteolytic cleavage (for example, through processing of gasdermin D by caspase 1 or 11).
- ESCRT
-
(endosomal sorting complexes required for transport). Multiprotein machinery that enables membrane bending/budding away from the cytoplasm.
- Lipopolysaccharide
-
(LPS). Large molecules, comprising a lipid and a complex polysaccharide, found in the outer membrane of Gram-negative bacteria.
- Gram-negative bacteria
-
Diverse group of bacteria defined by their inability to retain crystal violet (or Gram) stains, because of the architecture of their cell envelope being composed of an inner cytoplasmic and outer bacterial cell membrane separated by a thin peptidoglycan cell wall. Typical examples include Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa, Chlamydia trachomatis and Yersinia pestis.
- Toll-like receptor
-
Family of transmembrane receptors that recognize PAMPs and DAMPs and, upon stimulation, can induce diverse pro-inflammatory responses.
- RIG-I-like receptors
-
Cytosolic pattern recognition receptors that respond to double-stranded RNA.
- Antigen-presenting cells
-
While all nucleated cells can present antigens, the group of professional antigen-presenting cells, which comprise macrophages, dendritic cells and B cells, are capable of priming naive T cells by the processing and presentation of antigen-derived peptides in the context of class I or class II MHC molecules and by the delivery of co-stimulatory signals.
- PB1-F2
-
A protein encoded by the influenza A virus that contributes to its pathogenicity.
- M2 protein
-
Protein encoded by the influenza A virus that is part of the viral envelope; it is capable of forming a tunnel between host cell compartments.
- Z-RNAs
-
Left-handed form of double-stranded RNA that is bound by proteins, such as ADAR, ZBP1 or their viral homologues.
- Cytotoxic lymphocytes
-
CD8+ T cells and natural killer cells, which can kill infected or malignant cells via diverse mechanisms, including the delivery of perforin and granzymes, the use of FAS ligand to activate the death receptor FAS, and the delivery of IFNγ to target cells.
- BH3-mimetic drugs
-
Small-molecule inhibitors of pro-survival BCL-2 proteins. They mimic the action of the pro-apoptotic BH3-only proteins, which as the natural cellular inhibitors of pro-survival BCL-2 proteins are critical for the initiation of the intrinsic apoptosis signalling pathway.
- Necrosome
-
Protein complex consisting of RIPK1, RIPK3 and FADD. This complex is formed in response to TNFR1 stimulation when both the activation of NF-κB and caspase 8 activity are blocked. This signalling complex causes the activation of the pseudokinase MLKL, the critical effector of necroptosis.
- Tumour lysis syndrome
-
Caused by the failure to safely remove large numbers of dying tumour cells during anticancer therapy, which can cause renal failure, cardiac abnormalities, seizures and sudden death.
- CAR (chimeric antigen receptor) T cells
-
T lymphocytes engineered to express artificial antigen receptors capable of directly recognizing proteins on cancer cells and killing these malignant cells.
- Amyloid plaques
-
Beta-amyloid protein aggregates implicated in the destruction of nerve connections, thus causing degenerative disorders, such as Alzheimer disease.
- Periodic fever syndrome
-
Group of rare genetic autoinflammatory diseases in which patients develop periodic fevers with a range of inflammatory pathologies, including stomatitis, aphtitis and adenitis.
- Serpin
-
Superfamily of proteins that share a structural homology, where many perform serine protease inhibitory activity whereas others (for example, CrmA from cowpox virus) perform cysteine protease inhibitory activity.
- Canonical and non-canonical NF-κB pathways
-
Induced by the stimulation of a variety of surface receptors (for example, TLRs, members of the TNFR superfamily, and antigen receptors), the two distinct NF-κB signalling pathways involve different members of the NF-κB/REL protein family. The classical/canonical NF-κB pathway operates via heterodimers of NF-κB1 (its cleavage product p50) with RELA or c-REL, whereas the non-canonical pathway is mediated mainly by heterodimers of NF-κB2 (its cleavage product p52) with RELB.
- cGAS
-
Intracellular DNA sensor that induces an interferon response.
- Mast cells
-
Tissue-resident cells involved in immune defence against parasitic infections and allergic responses.
- Ripoptosomes
-
Signalling platforms comprising RIPK1, RIPK3, FADD and caspase 8 that can induce either apoptosis or necroptosis, depending on the state of the cell.
- Immune checkpoint signalling
-
Signalling pathways that attenuate the activity of immune cells, mainly T lymphocytes, thereby regulating immune responses and preventing the destruction of self-tissues (contributing to self-tolerance). The inhibition of immune checkpoint regulators, such as PD1 or CTLA4, can enhance CD8+ cytotoxic T cell-mediated killing of cancer cells.
Rights and permissions
About this article
Cite this article
Bedoui, S., Herold, M.J. & Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol 21, 678–695 (2020). https://doi.org/10.1038/s41580-020-0270-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-020-0270-8
This article is cited by
-
A matter of new life and cell death: programmed cell death in the mammalian ovary
Journal of Biomedical Science (2024)
-
Herpes simplex virus infection induces necroptosis of neurons and astrocytes in human fetal organotypic brain slice cultures
Journal of Neuroinflammation (2024)
-
Identification of PANoptosis-related signature reveals immune infiltration characteristics and immunotherapy responses for renal cell carcinoma
BMC Cancer (2024)
-
Deep learning enables the discovery of a novel cuproptosis-inducing molecule for the inhibition of hepatocellular carcinoma
Acta Pharmacologica Sinica (2024)
-
Non-canonical BIM-regulated energy metabolism determines drug-induced liver necrosis
Cell Death & Differentiation (2024)