Abstract
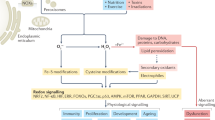
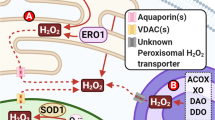
‘Reactive oxygen species’ (ROS) is an umbrella term for an array of derivatives of molecular oxygen that occur as a normal attribute of aerobic life. Elevated formation of the different ROS leads to molecular damage, denoted as ‘oxidative distress’. Here we focus on ROS at physiological levels and their central role in redox signalling via different post-translational modifications, denoted as ‘oxidative eustress’. Two species, hydrogen peroxide (H2O2) and the superoxide anion radical (O2·−), are key redox signalling agents generated under the control of growth factors and cytokines by more than 40 enzymes, prominently including NADPH oxidases and the mitochondrial electron transport chain. At the low physiological levels in the nanomolar range, H2O2 is the major agent signalling through specific protein targets, which engage in metabolic regulation and stress responses to support cellular adaptation to a changing environment and stress. In addition, several other reactive species are involved in redox signalling, for instance nitric oxide, hydrogen sulfide and oxidized lipids. Recent methodological advances permit the assessment of molecular interactions of specific ROS molecules with specific targets in redox signalling pathways. Accordingly, major advances have occurred in understanding the role of these oxidants in physiology and disease, including the nervous, cardiovascular and immune systems, skeletal muscle and metabolic regulation as well as ageing and cancer. In the past, unspecific elimination of ROS by use of low molecular mass antioxidant compounds was not successful in counteracting disease initiation and progression in clinical trials. However, controlling specific ROS-mediated signalling pathways by selective targeting offers a perspective for a future of more refined redox medicine. This includes enzymatic defence systems such as those controlled by the stress-response transcription factors NRF2 and nuclear factor-κB, the role of trace elements such as selenium, the use of redox drugs and the modulation of environmental factors collectively known as the exposome (for example, nutrition, lifestyle and irradiation).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Halliwell, B., Gutteridge & J. M. C. in Free radicals in biology and medicine (Oxford University Press, 2015).
Hawkins, C. L. & Davies, M. J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 294, 19683–19708 (2019).
Sies, H., Berndt, C. & Jones, D. P. Oxidative stress. Annu. Rev. Biochem. 86, 715–748 (2017). Concept of oxidative stress: physiological (eustress) and supraphysiological (distress).
Murphy, M. P. et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 13, 361–366 (2011).
Brandes, R. P., Rezende, F. & Schröder, K. Redox regulation beyond ROS: why ROS should not be measured as often. Circ. Res. 123, 326–328 (2018).
Rhee, S. G. Redox signaling: hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 31, 53–59 (1999).
Thannickal, V. J. & Fanburg, B. L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L1005-L1028 (2000).
Sauer, H., Wartenberg, M. & Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 11, 173–186 (2001).
Stone, J. R. & Yang, S. Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8, 243–270 (2006).
D’Autreaux, B. & Toledano, M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007). Fundamental perspectives on ROS signalling.
Forman, H. J., Maiorino, M. & Ursini, F. Signaling functions of reactive oxygen species. Biochemistry 49, 835–842 (2010).
Sies, H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J. Biol. Chem. 289, 8735–8741 (2014).
Holmström, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Reczek, C. R. & Chandel, N. S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13 (2015).
Winterbourn, C. C. Biological production, detection, and fate of hydrogen peroxide. Antioxid. Redox Signal. 29, 541–551 (2018). Comprehensive overview on H2O2 in biology.
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000).
Bagur, R. & Hajnoczky, G. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell 66, 780–788 (2017).
Sies, H. & Chance, B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 11, 172–176 (1970). First detection of H2O2 in normal aerobic eukaryotic metabolism.
Parvez, S., Long, M. J. C., Poganik, J. R. & Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 118, 8798–8888 (2018).
Jones, D. P. & Sies, H. The redox code. Antioxid. Redox Signal. 23, 734–746 (2015). The ‘redox code’ as a set of principles by which redox biology is organized.
Zhang, L. et al. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 26, 101284 (2019).
Sies, H. Oxidative stress: eustress and distress (Academic, 2020).
Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11, 613–619 (2017).
Chance, B., Sies, H. & Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605 (1979). Role and significance of hydroperoxides in metabolism.
Zeida, A. et al. Catalysis of peroxide reduction by fast reacting protein thiols. Chem. Rev. 119, 10829–10855 (2019). Comprehensive review of thiol-based redox chemistry.
Kaya, A., Lee, B. C. & Gladyshev, V. N. Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1. Antioxid. Redox Signal. 23, 814–822 (2015).
Brigelius-Flohé, R. & Flohé, L. Selenium and redox signaling. Arch. Biochem. Biophys. 617, 48–59 (2017).
Santos, C. X. et al. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. EMBO J. 35, 319–334 (2016).
Poli, G., Leonarduzzi, G., Biasi, F. & Chiarpotto, E. Oxidative stress and cell signalling. Curr. Med. Chem. 11, 1163–1182 (2004).
Sobotta, M. C. et al. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 11, 64–70 (2015). Prototypical example of redox relay in H2O2 signalling.
Tapia, P. C. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “mitohormesis” for health and vitality. Med. Hypotheses. 66, 832–843 (2006).
Ristow, M. & Zarse, K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 45, 410–418 (2010).
Ristow, M. & Schmeisser, K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 12, 288–341 (2014).
Ursini, F., Maiorino, M. & Forman, H. J. Redox homeostasis: the golden mean of healthy living. Redox Biol. 8, 205–215 (2016).
Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 25, 1058–1071 (1986).
Zhang, H. & Forman, H. J. 4-Hydroxynonenal-mediated signaling and aging. Free Radic. Biol. Med. 111, 219–225 (2017).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Kreuz, S. & Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 8, 843–862 (2016).
Poulsen, H. E. et al. Oxidatively generated modifications to nucleic acids in vivo: measurement in urine and plasma. Free Radic. Biol. Med. 145, 336–341 (2019).
Lambeth, J. D. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 43, 332–347 (2007).
Santolini, J., Wootton, S. A., Jackson, A. A. & Feelisch, M. The redox architecture of physiological function. Curr. Opin. Physiol. 9, 34–47 (2019).
Go, Y. M., Chandler, J. D. & Jones, D. P. The cysteine proteome. Free Radic. Biol. Med. 84, 227–245 (2015).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Knock, G. NADPH oxidase in the vasculature: expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic. Biol. Med. 145, 385–427 (2019).
Parascandolo, A. & Laukkanen, M. O. Carcinogenesis and reactive oxygen species signaling: interaction of the NADPH oxidase NOX1-5 and superoxide dismutase 1-3 signal transduction pathways. Antioxid. Redox Signal. 30, 443–486 (2019).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009). A comprehensive account of mitochondrial ROS production.
Spencer, N. Y. & Engelhardt, J. F. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry 53, 1551–1564 (2014).
Mishina, N. M. et al. Imaging H2O2 microdomains in receptor tyrosine kinases signaling. Methods Enzymol. 526, 175–187 (2013).
Bleier, L. et al. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic. Biol. Med. 78, 1–10 (2015).
Brand, M. D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 100, 14–31 (2016).
Higdon, A., Diers, A. R., Oh, J. Y., Landar, A. & Darley-Usmar, V. M. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem. J. 442, 453–464 (2012).
Hitzel, J. et al. Oxidized phospholipids regulate amino acid metabolism through MTHFD2 to facilitate nucleotide release in endothelial cells. Nat. Commun. 9, 2292–04602 (2018).
Kagan, V. E. et al. Redox phospholipidomics of enzymatically generated oxygenated phospholipids as specific signals of programmed cell death. Free Radic. Biol. Med. 147, 231–241 (2020).
Spickett, C. M. & Pitt, A. R. Oxidative lipidomics coming of age: advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid. Redox Signal. 22, 1646–1666 (2015).
Tyurina, Y. Y. et al. “Only a life lived for others is worth living”: redox signaling by oxygenated phospholipids in cell fate decisions. Antioxid. Redox Signal. 29, 1333–1358 (2018).
Czapski, G. A., Czubowicz, K., Strosznajder, J. B. & Strosznajder, R. P. The lipoxygenases: their regulation and implication in Alzheimer’s disease. Neurochem. Res. 41, 243–257 (2016).
Wong, H. S., Benoit, B. & Brand, M. D. Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts. Free Radic. Biol. Med. 130, 140–150 (2019).
Niedzwiecki, M. M. et al. The exposome: molecules to populations. Annu. Rev. Pharmacol. Toxicol. 59, 107–127 (2019).
Rhee, S. G. & Kil, I. S. Multiple functions and regulation of mammalian peroxiredoxins. Annu. Rev. Biochem. 86, 749–775 (2017).
Brigelius-Flohé, R. & Flohé,L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid. Redox Signal. https://doi.org/10.1089/ars.2019.7905 (2019).
Winterbourn, C. C., Kettle, A. J. & Hampton, M. B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 85, 765–792 (2016).
Barata, A. G. & Dick, T. P. A role for peroxiredoxins in H2O2- and MEKK-dependent activation of the p38 signaling pathway. Redox Biol. 28, 101340 (2019).
Mailloux, R. J. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid. Med. Cell Longev. 2018, 7857251 (2018).
Hanschmann, E. M., Godoy, J. R., Berndt, C., Hudemann, C. & Lillig, C. H. Thioredoxins, glutaredoxins, and peroxiredoxins–molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 19, 1539–1605 (2013).
Lyublinskaya, O. & Antunes, F. Measuring intracellular concentration of hydrogen peroxide with the use of genetically encoded H2O2 biosensor HyPer. Redox Biol. 24, 101200 (2019).
Lim, J. B., Huang, B. K., Deen, W. M. & Sikes, H. D. Analysis of the lifetime and spatial localization of hydrogen peroxide generated in the cytosol using a reduced kinetic model. Free Radic. Biol. Med. 89, 47–53 (2015).
Gao, C., Tian, Y., Zhang, R., Jing, J. & Zhang, X. Endoplasmic reticulum-directed ratiometric fluorescent probe for quantitive detection of basal H2O2. Anal. Chem. 89, 12945–12950 (2017).
Forman, H. J., Bernardo, A. & Davies, K. J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 603, 48–53 (2016).
Mishina, N. M. et al. Which antioxidant system shapes intracellular H2O2 gradients? Antioxid. Redox Signal. 31, 664–670 (2019).
Fransen, M. & Lismont, C. Redox signaling from and to peroxisomes: progress, challenges, and prospects. Antioxid. Redox Signal. 30, 95–112 (2019).
Lismont, C., Revenco, I. & Fransen, M. Peroxisomal hydrogen peroxide metabolism and signaling in health and disease. Int. J. Mol. Sci. 20, ijms20153673 (2019).
Appenzeller-Herzog, C. et al. Transit of H2O2 across the endoplasmic reticulum membrane is not sluggish. Free Radic. Biol. Med. 94, 157–160 (2016).
Bestetti, S. et al. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 28, 101326 (2019).
Yoboue, E. D., Sitia, R. & Simmen, T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death. Dis. 9, 331 (2018).
Dooley, C. T. et al. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 279, 22284–22293 (2004).
Belousov, V. V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 (2006). First genetically encoded H2O2 probe using the OxyR domain.
Bilan, D. S. & Belousov, V. V. In vivo imaging of hydrogen peroxide with HyPer probes. Antioxid. Redox Signal. 29, 569–584 (2018).
Morgan, B. et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 12, 437–443 (2016).
Roma, L. P., Deponte, M., Riemer, J. & Morgan, B. Mechanisms and applications of redox-sensitive green fluorescent protein-based hydrogen peroxide probes. Antioxid. Redox Signal. 29, 552–568 (2018).
Fernandez-Puente, E. et al. Expression and functional analysis of the hydrogen peroxide biosensors HyPer and HyPer2 in C2C12 myoblasts/myotubes and single skeletal muscle fibres. Sci. Rep. 10, 871–57821 (2020).
Huang, B. K., Ali, S., Stein, K. T. & Sikes, H. D. Interpreting heterogeneity in response of cells expressing a fluorescent hydrogen peroxide biosensor. Biophys. J. 109, 2148–2158 (2015).
Henzler, T. & Steudle, E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J. Exp. Bot. 51, 2053–2066 (2000). Discovery of H2O2 transport across membranes by aquaporins.
Bienert, G. P. & Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596–1604 (2014). Groundbreaking work on the role of some aquaporins as peroxiporins.
Bestetti, S. et al. A persulfidation-based mechanism controls aquaporin-8 conductance. Sci. Adv. 4, eaar5770 (2018).
Medrano-Fernandez, I. et al. Stress regulates aquaporin-8 permeability to impact cell growth and survival. Antioxid. Redox Signal. 24, 1031–1044 (2016).
Pak, V. V. et al. Ultrasensitive genetically encoded indicator for intracellular hydrogen peroxide identifies novel roles for cellular oxidants in cell migration and mitochondrial function. Cell Metab. 31, 642–653 (2020).
Tamma, G. et al. Aquaporin membrane channels in oxidative stress, cell signaling, and aging: recent advances and research trends. Oxid. Med. Cell Longev. 2018, 1501847 (2018).
Rajasekaran, N. S. et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 130, 427–439 (2007).
Marinho, H. S., Real, C., Cyrne, L., Soares, H. & Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2, 535–562 (2014).
Jones, D. P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol 295, C849-C868 (2008).
Bak, D. W., Bechtel, T. J., Falco, J. A. & Weerapana, E. Cysteine reactivity across the subcellular universe. Curr. Opin. Chem. Biol. 48, 96–105 (2019).
Go, Y. M. & Jones, D. P. The redox proteome. J. Biol. Chem. 288, 26512–26520 (2013).
Behring, J. B. et al. Spatial and temporal alterations in protein structure by EGF regulate cryptic cysteine oxidation. Sci. Signal. 13, 13–615 (2020).
Brigelius-Flohé, R. & Flohé, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 15, 2335–2381 (2011).
Young, D. et al. Protein promiscuity in H2O2 signaling. Antioxid. Redox Signal. 30, 1285–1324 (2019).
Xiao, H., et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983 (2020).
Itoh, K. et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322 (1997). Discovery of the NRF2–KEAP1 system.
Yamamoto, M., Kensler, T. W. & Motohashi, H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 98, 1169–1203 (2018).
Fourquet, S., Guerois, R., Biard, D. & Toledano, M. B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 285, 8463–8471 (2010).
Kobayashi, M. et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell Biol. 29, 493–502 (2009).
Cebula, M., Schmidt, E. E. & Arner, E. S. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox Signal. 23, 823–853 (2015).
Singh, C. K. et al. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 28, 643–661 (2018).
Cheng, X., Ku, C. H. & Siow, R. C. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic. Biol. Med. 64, 4–11 (2013).
Karin, M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 1, a000141 (2009).
Pahl, H. L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 18, 6853–6866 (1999).
Oliveira-Marques, V., Marinho, H. S., Cyrne, L. & Antunes, F. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxid. Redox Signal. 11, 2223–2243 (2009).
Schreck, R., Rieber, P. & Baeuerle, P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappaB transcription factor and HIV-1. EMBO J. 10, 2247–2258 (1991). Description of the role of H2O2 in NF-κB activation.
Halvey, P. J. et al. Selective oxidative stress in cell nuclei by nuclear-targeted D-amino acid oxidase. Antioxid. Redox Signal. 9, 807–816 (2007).
Kaelin, W. G. Jr. & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Jiang, B. H., Rue, E., Wang, G. L., Roe, R. & Semenza, G. L. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 271, 17771–17778 (1996). Seminal article on HIF.
Hernansanz-Agustin, P. et al. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol. 12, 1040–1051 (2017).
Prabhakar, N. R., Kumar, G. K., Nanduri, J. & Semenza, G. L. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid. Redox Signal. 9, 1397–1403 (2007).
Waypa, G. B., Smith, K. A. & Schumacker, P. T. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol. Asp. Med. 47-48, 76–89 (2016).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000).
Pouyssegur, J. & Mechta-Grigoriou, F. Redox regulation of the hypoxia-inducible factor. Biol. Chem. 387, 1337–1346 (2006).
Acker, T., Fandrey, J. & Acker, H. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovasc. Res. 71, 195–207 (2006).
Eijkelenboom, A. & Burgering, B. M. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Klotz, L. O. & Steinbrenner, H. Cellular adaptation to xenobiotics: interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 13, 646–654 (2017).
Liu, B., Chen, Y. & St Clair, D. K. ROS and p53: a versatile partnership. Free Radic. Biol. Med. 44, 1529–1535 (2008).
Uehara, I. & Tanaka, N. Role of p53 in the regulation of the inflammatory tumor microenvironment and tumor suppression. Cancers (Basel). 10, 219 (2018).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Hinchy, E. C. et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J. Biol. Chem. 293, 17208–17217 (2018).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 10-0199 (2020).
Schmeisser, K. & Parker, J. A. Pleiotropic effects of mTOR and autophagy during development and aging. Front. Cell Dev. Biol. 7, 192 (2019).
Sirover, M. A. Pleiotropic effects of moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cancer progression, invasiveness, and metastases. Cancer Metastasis Rev. 37, 665–676 (2018).
Peralta, D. et al. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 11, 156–163 (2015).
Lin, C. S. & Klingenberg, M. Isolation of the uncoupling protein from brown adipose tissue mitochondria. FEBS Lett. 113, 299–303 (1980). Discovery of uncoupling protein.
Echtay, K. S. et al. Uncoupling proteins: Martin Klingenberg’s contributions for 40 years. Arch. Biochem. Biophys. 657, 41–55 (2018).
Berry, B. J., Trewin, A. J., Amitrano, A. M., Kim, M. & Wojtovich, A. P. Use the protonmotive force: mitochondrial uncoupling and reactive oxygen species. J. Mol. Biol. 430, 3873–3891 (2018).
Jezek, P., Holendova, B., Garlid, K. D. & Jaburek, M. Mitochondrial uncoupling proteins: subtle regulators of cellular redox signaling. Antioxid. Redox Signal. 29, 667–714 (2018).
Echtay, K. S. et al. Superoxide activates mitochondrial uncoupling proteins. Nature 415, 96–99 (2002).
Mailloux, R. J. & Harper, M. E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 51, 1106–1115 (2011).
Dustin, C. M., Heppner, D. E., Lin, M. J. & van der Vliet, A. Redox regulation of tyrosine kinase signaling: more than meet the eye. J, Biochem. 167, 151–163 (2020).
Truong, T. H. & Carroll, K. S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 48, 332–356 (2013).
Londhe, A. D. et al. Regulation of PTP1B activation through disruption of redox-complex formation. Nat. Chem Biol. 16, 122–125 (2020).
Truong, T. H. et al. Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem. Biol. 23, 837–848 (2016).
Heppner, D. E. et al. Direct cysteine sulfenylation drives activation of the Src kinase. Nat. Commun. 9, 4522–06790 (2018).
Dagnell, M. et al. Bicarbonate is essential for protein tyrosine phosphatase 1B (PTP1B) oxidation and cellular signaling through EGF-triggered phosphorylation cascades. J. Biol. Chem. 294, 12330–12338 (2019).
Truzzi, D. R. et al. The bicarbonate/carbon dioxide pair increases hydrogen peroxide-mediated hyperoxidation of human peroxiredoxin 1. J. Biol. Chem. 294, 14055–14067 (2019).
Löwe, O. et al. BIAM switch assay coupled to mass spectrometry identifies novel redox targets of NADPH oxidase 4. Redox Biol. 21, 101125 (2019).
Bogeski, I. & Niemeyer, B. A. Redox regulation of ion channels. Antioxid. Redox Signal. 21, 859–862 (2014).
Kourie, J. I. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol. 275, C1-C24 (1998).
Sahoo, N., Hoshi, T. & Heinemann, S. H. Oxidative modulation of voltage-gated potassium channels. Antioxid. Redox Signal. 21, 933–952 (2014).
Ruppersberg, J. P. et al. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature 352, 711–714 (1991). Description of redox regulation of K+ channel.
Forrester, S. J., Kikuchi, D. S., Hernandes, M. S., Xu, Q. & Griendling, K. K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122, 877–902 (2018).
Chen, P. H., Chi, J. T. & Boyce, M. Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways. Glycobiology 28, 556–564 (2018).
Taniguchi, N. et al. Glyco-redox, a link between oxidative stress and changes of glycans: lessons from research on glutathione, reactive oxygen and nitrogen species to glycobiology. Arch. Biochem. Biophys. 595, 72–80 (2016).
Nordzieke, D. E. & Medrano-Fernandez, I. The plasma membrane: a platform for intra- and intercellular redox signaling. Antioxidants (Basel) 7, 168 (2018).
Patinen, T. et al. Regulation of stress signaling pathways by protein lipoxidation. Redox Biol. 23, 101114 (2019).
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Ingold, I. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409–422 (2018).
Somyajit, K. et al. Redox-sensitive alteration of replisome architecture safeguards genome integrity. Science 358, 797–802 (2017).
Ahmed, W. & Lingner, J. PRDX1 and MTH1 cooperate to prevent ROS-mediated inhibition of telomerase. Genes Dev. 32, 658–669 (2018).
Rhee, S. G., Woo, H. A. & Kang, D. The role of peroxiredoxins in the transduction of H2O2 signals. Antioxid. Redox Signal. 28, 537–557 (2018).
Sarsour, E. H., Kumar, M. G., Chaudhuri, L., Kalen, A. L. & Goswami, P. C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 11, 2985–3011 (2009).
Srinivas, U. S., Tan, B. W. Q., Vellayappan, B. A. & Jeyasekharan, A. D. ROS and the DNA damage response in cancer. Redox Biol. 25, 101084 (2019).
Mailloux, R. J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 4, 381–398 (2015).
Matilainen, O., Quiros, P. M. & Auwerx, J. Mitochondria and epigenetics - crosstalk in homeostasis and stress. Trends Cell Biol. 27, 453–463 (2017).
Castro, L., Tortora, V., Mansilla, S. & Radi, R. Aconitases: non-redox iron-sulfur proteins sensitive to reactive species. Acc. Chem. Res. 52, 2609–2619 (2019).
Braymer, J. J., Stumpfig, M., Thelen, S., Muhlenhoff, U. & Lill, R. Depletion of thiol reducing capacity impairs cytosolic but not mitochondrial iron-sulfur protein assembly machineries. Biochim. Biophys. Acta Mol. Cell Res. 1866, 240–251 (2019).
Bulthuis, E. P., Adjobo-Hermans, M. J. W., Willems, P. H. G. M. & Koopman, W. J. H. Mitochondrial morphofunction in mammalian cells. Antioxid. Redox Signal. 30, 2066–2109 (2019).
Kondadi, A. K., Anand, R. & Reichert, A. S. Functional interplay between cristae biogenesis, mitochondrial dynamics and mitochondrial DNA integrity. Int. J. Mol. Sci. 20, ijms20174311 (2019).
Murley, A. & Nunnari, J. The emerging network of mitochondria-organelle contacts. Mol. Cell 61, 648–653 (2016).
Frank, M. et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim. Biophys. Acta 1823, 2297–2310 (2012).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950 (2014).
Sies, H. Biochemistry of the peroxisome in the liver cell. Angew. Chem. Int. Ed. Engl. 13, 706–718 (1974).
Gebicka, L. & Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 197, 110699 (2019).
Böhm, B., Heinzelmann, S., Motz, M. & Bauer, G. Extracellular localization of catalase is associated with the transformed state of malignant cells. Biol. Chem. 396, 1339–1356 (2015).
Wang, L., Zhang, L., Niu, Y., Sitia, R. & Wang, C. C. Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1alpha to promote oxidative protein folding. Antioxid. Redox Signal. 20, 545–556 (2014).
Cenci, S. & Sitia, R. Managing and exploiting stress in the antibody factory. FEBS Lett. 581, 3652–3657 (2007).
Laporte, A., Lortz, S., Schaal, C., Lenzen, S. & Elsner, M. Hydrogen peroxide permeability of cellular membranes in insulin-producing cells. Biochim. Biophys. Acta Biomembr. 1862, 183096 (2019).
Cao, S. S. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 21, 396–413 (2014).
Eletto, D., Chevet, E., Argon, Y. & Appenzeller-Herzog, C. Redox controls UPR to control redox. J. Cell Sci. 127, 3649–3658 (2014).
Amodio, G., Moltedo, O., Faraonio, R. & Remondelli, P. Targeting the endoplasmic reticulum unfolded protein response to counteract the oxidative stress-induced endothelial dysfunction. Oxid. Med. Cell Longev. 2018, 4946289 (2018).
Görlach, A., Bertram, K., Hudecova, S. & Krizanova, O. Calcium and ROS: a mutual interplay. Redox Biol. 6, 260–271 (2015).
Hempel, N. & Trebak, M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 63, 70–96 (2017).
Feno, S., Butera, G., Vecellio, R. D., Rizzuto, R. & Raffaello, A. Crosstalk between calcium and ROS in pathophysiological conditions. Oxid. Med. Cell Longev. 2019, 9324018 (2019).
Joseph, S. K. et al. Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. J. Biol. Chem. 293, 17464–17476 (2018).
Booth, D. M., Enyedi, B., Geiszt, M., Varnai, P. & Hajnoczky, G. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol. Cell 63, 240–248 (2016). Description of H2O2 redox nanodomains.
Csordas, G., Weaver, D. & Hajnoczky, G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 28, 523–540 (2018).
Egea, J. et al. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 13, 94–162 (2017).
Go, Y. M. & Jones, D. P. Redox theory of aging: implications for health and disease. Clin. Sci. 131, 1669–1688 (2017).
Valko, M. et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 (2007).
Milkovic, L., Cipak, G. A., Cindric, M., Mouthuy, P. A. & Zarkovic, N. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells 8, 793 (2019).
Timme-Laragy, A. R., Hahn, M. E., Hansen, J. M., Rastogi, A. & Roy, M. A. Redox stress and signaling during vertebrate embryonic development: regulation and responses. Semin. Cell Dev. Biol. 80, 17–28 (2018).
Rampon, C., Volovitch, M., Joliot, A., Vriz, S. Hydrogen peroxide and redox regulation of developments. Antioxidants (Basel) 7, 159 (2018).
Oswald, M. C. W., Garnham, N., Sweeney, S. T. & Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 592, 679–691 (2018).
Wilson, C., Munoz-Palma, E. & Gonzalez-Billault, C. From birth to death: a role for reactive oxygen species in neuronal development. Semin. Cell Dev. Biol. 80, 43–49 (2018).
Wilson, C. & Gonzalez-Billault, C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front. Cell Neurosci. 9, 381 (2015).
Tan, D. Q. & Suda, T. Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxid. Redox Signal. 29, 149–168 (2018).
Rhee, S. G. & Kil, I. S. Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm. Free Radic. Biol. Med. 100, 73–80 (2016). An account of the role of peroxiredoxins in circadian rhythms.
Nagy, A. D. & Reddy, A. B. Redox clocks: time to rethink redox interventions. Free Radic. Biol. Med. 119, 3–7 (2018).
Reinke, H. & Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 20, 227–241 (2019).
Pei, J. F. et al. Diurnal oscillations of endogenous H2O2 sustained by p66Shc regulate circadian clocks. Nat. Cell Biol. 21, 1553–1564 (2019).
Kempf, A., Song, S. M., Talbot, C. B. & Miesenbock, G. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature 568, 230–234 (2019).
Patke, A., Young, M. W. & Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020).
Nayernia, Z., Jaquet, V. & Krause, K. H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 20, 2815–2837 (2014).
Cobley, J. N., Fiorello, M. L. & Bailey, D. M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 15, 490–503 (2018).
Tarafdar, A. & Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 19, ijms19123824 (2018).
Sbodio, J. I., Snyder, S. H. & Paul, B. D. Redox mechanisms in neurodegeneration: from disease outcomes to therapeutic opportunities. Antioxid. Redox Signal. 30, 1450–1499 (2019).
Steinbrenner, H. & Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch. Biochem. Biophys. 536, 152–157 (2013).
Lepka, K. et al. Iron-sulfur glutaredoxin 2 protects oligodendrocytes against damage induced by nitric oxide release from activated microglia. Glia 65, 1521–1534 (2017).
Casas, A. I. et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl Acad. Sci. USA 114, 12315–12320 (2017).
Meda, F. et al. Nerves, H2O2 and Shh: three players in the game of regeneration. Semin. Cell Dev. Biol. 80, 65–73 (2018).
Hervera, A. et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20, 307–319 (2018).
Vicente-Gutierrez, C. et al. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat. Metab. 1, 201–211 (2019).
Bierhaus, A. et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl Acad. Sci. USA 100, 1920–1925 (2003). A report describing how psychosocial stress is translated into a cell response pattern.
Aschbacher, K. et al. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38, 1698–1708 (2013).
Aschbacher, K. & Mason, A. E. Eustress, distress and oxidative stress: promising pathways for mind-body medicine. In Oxidative Stress: Eustress and Distress (ed. Sies, H.) 583–617 (Academic, 2020).
Golbidi, S., Li, H. & Laher, I. Oxidative stress: a unifying mechanism for cell damage induced by noise, (water-pipe) smoking, and emotional stress-therapeutic strategies targeting redox imbalance. Antioxid. Redox Signal. 28, 741–759 (2018).
Münzel, T. et al. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Eur. Heart J. 38, 2838–2849 (2017).
Rousset, F., Carnesecchi, S., Senn, P. & Krause, K. H. NOX3-targeted therapies for inner ear pathologies. Curr. Pharm. Des. 21, 5977–5987 (2015).
Lugrin, J., Rosenblatt-Velin, N., Parapanov, R. & Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 395, 203–230 (2014).
Pei, L. & Wallace, D. C. Mitochondrial etiology of neuropsychiatric disorders. Biol. Psychiatry 83, 722–730 (2018).
Nathan, C. & Cunningham-Bussel, A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361 (2013). A review bridging immunology and redox biology.
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Kenny, E. F. et al. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife. 6, e24437 (2017).
Anelli, T., Sannino, S. & Sitia, R. Proteostasis and “redoxtasis” in the secretory pathway: tales of tails from ERp44 and immunoglobulins. Free Radic. Biol. Med. 83, 323–330 (2015).
Garaude, J. Reprogramming of mitochondrial metabolism by innate immunity. Curr. Opin. Immunol. 56, 17–23 (2018).
Abais, J. M., Xia, M., Zhang, Y., Boini, K. M. & Li, P. L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 22, 1111–1129 (2015).
Jones, R. M. & Neish, A. S. Redox signaling mediated by the gut microbiota. Free Radic. Biol. Med. 105, 41–47 (2017).
Aviello, G. & Knaus, U. G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 11, 1011–1023 (2018).
Cano, S. M., Lancel, S., Boulanger, E. & Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants (Basel) 7, 98 (2018).
Niethammer, P. Wound redox gradients revisited. Semin. Cell Dev. Biol. 80, 13–16 (2018).
Love, N. R. & Chen, Y. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 15, 222–228 (2013).
Levigne, D., Modarressi, A., Krause, K. H. & Pittet-Cuenod, B. NADPH oxidase 4 deficiency leads to impaired wound repair and reduced dityrosine-crosslinking, but does not affect myofibroblast formation. Free Radic. Biol. Med. 96, 374–384 (2016).
Kunkemoeller, B. & Kyriakides, T. R. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid. Redox Signal. 27, 823–838 (2017).
Handy, D. E. & Loscalzo, J. Responses to reductive stress in the cardiovascular system. Free Radic. Biol. Med. 109, 114–124 (2017).
Incalza, M. A. et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 100, 1–19 (2018).
Münzel, T. et al. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J. Am. Coll. Cardiol. 70, 212–229 (2017).
Schröder, K. Redox control of angiogenesis. Antioxid. Redox Signal. 30, 960–971 (2019).
Förstermann, U., Xia, N. & Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120, 713–735 (2017).
Siu, K. L. et al. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol. 11, 118–125 (2017).
Oikonomou, E. K. & Antoniades, C. Immunometabolic regulation of vascular redox state: the role of adipose tissue. Antioxid. Redox Signal. 29, 313–336 (2018).
Stocker, R. & Keaney, J. F. Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84, 1381–1478 (2004).
Trinity, J. D., Broxterman, R. M. & Richardson, R. S. Regulation of exercise blood flow: role of free radicals. Free Radic. Biol. Med. 98, 90–102 (2016).
Jackson, M. J. Redox regulation of muscle adaptations to contractile activity and aging. J. Appl. Physiol. 119, 163–171 (2015).
Le Moal, E. et al. Redox control of skeletal muscle regeneration. Antioxid. Redox Signal. 27, 276–310 (2017).
El Assar, M., Angulo, J. & Rodriguez-Manas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med.10 (2019).
McArdle, A., Pollock, N., Staunton, C. A. & Jackson, M. J. Aberrant redox signalling and stress response in age-related muscle decline: role in inter- and intra-cellular signalling. Free Radic. Biol. Med. 132, 50–57 (2019).
Cobley, J. N., Close, G. L., Bailey, D. M. & Davison, G. W. Exercise redox biochemistry: conceptual, methodological and technical recommendations. Redox Biol. 12, 540–548 (2017).
Hancock, M. et al. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. eLife 7, 41044 (2018).
Jackson, M. J. Mechanistic models to guide redox investigations and interventions in musculoskeletal ageing. Free Radic. Biol. Med. 149, 2–7 (2020).
Watson, J. D. Type 2 diabetes as a redox disease. Lancet 383, 841–843 (2014).
Haeusler, R. A., McGraw, T. E. & Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 19, 31–44 (2018).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223 (2018).
Onyango, A. N. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Oxid. Med. Cell Longev. 2018, 4321714 (2018).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956). Seminal article on the free radical theory of ageing.
Golubev, A., Hanson, A. D. & Gladyshev, V. N. Non-enzymatic molecular damage as a prototypic driver of aging. J. Biol. Chem. 292, 6029–6038 (2017).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013). Comprehensive overview of the hallmarks of ageing.
Pomatto, L. C. D. & Davies, K. J. A. The role of declining adaptive homeostasis in ageing. J. Physiol. 595, 7275–7309 (2017).
Schmidlin, C. J., Dodson, M. B., Madhavan, L. & Zhang, D. D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 134, 702–707 (2019).
Taetzsch, T., Benusa, S., Levesque, S., Mumaw, C. L. & Block, M. L. Loss of NF-kappaB p50 function synergistically augments microglial priming in the middle-aged brain. J. Neuroinflammation 16, 60–1446 (2019).
Salminen, A., Kauppinen, A. & Kaarniranta, K. AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): impact on cancer and aging. J. Mol. Med. 97, 1049–1064 (2019).
Rose, G., Crocco, P., De, R. F., Montesanto, A. & Passarino, G. Further support to the uncoupling-to-survive theory: the genetic variation of human UCP genes is associated with longevity. PLoS One 6, e29650 (2011).
Kirstein, J. et al. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. EMBO J. 34, 2334–2349 (2015).
Höhn, A. et al. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 11, 482–501 (2017).
Hipp, M. S., Kasturi, P. & Hartl, F. U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435 (2019).
Akbari, M., Kirkwood, T. B. L. & Bohr, V. A. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Rev. 54, 100940 (2019).
Campisi, J. et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192 (2019).
Labunskyy, V. M. & Gladyshev, V. N. Role of reactive oxygen species-mediated signaling in aging. Antioxid. Redox Signal. 19, 1362–1372 (2013).
Palmeira, C. M. et al. Mitohormesis and metabolic health: the interplay between ROS, cAMP and sirtuins. Free Radic. Biol. Med. 141, 483–491 (2019).
Bazopoulou, D. et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature 576, 301–306 (2019).
Golubev, A., Hanson, A. D. & Gladyshev, V. N. A tale of two concepts: harmonizing the free radical and antagonistic pleiotropy theories of aging. Antioxid. Redox Signal. 29, 1003–1017 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). Comprehensive overview of the hallmarks of cancer.
Hornsveld, M. & Dansen, T. B. The hallmarks of cancer from a redox perspective. Antioxid. Redox Signal. 25, 300–325 (2016).
Moloney, J. N. & Cotter, T. G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 80, 50–64 (2018).
Kalyanaraman, B. et al. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 15, 347–362 (2018).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Kim, J., Kim, J. & Bae, J. S. ROS homeostasis and metabolism: a critical liaison for cancer therapy. Exp. Mol. Med. 48, e269 (2016).
Steinbrenner, H., Speckmann, B. & Sies, H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxid. Redox Signal. 19, 181–191 (2013).
Yang, H. et al. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 37, 266–0909 (2018).
Chaiswing, L., St Clair, W. H. & St Clair, D. K. Redox paradox: a novel approach to therapeutics-resistant cancer. Antioxid. Redox Signal. 29, 1237–1272 (2018).
Panieri, E. & Santoro, M. M. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death. Dis. 7, e2253 (2016).
Allen, B. G. et al. First-in-human phase I clinical trial of pharmacologic ascorbate combined with radiation and temozolomide for newly diagnosed glioblastoma. Clin. Cancer Res. 25, 6590–6597 (2019).
Schoenfeld, J. D. et al. Pharmacological ascorbate as a means of sensitizing cancer cells to radio-chemotherapy while protecting normal tissue. Semin. Radiat. Oncol. 29, 25–32 (2019).
Elbatreek, M. H., Pachado, M. P., Cuadrado, A., Jandeleit-Dahm, K. & Schmidt, H. H. H. W. Reactive oxygen comes of age: mechanism-based therapy of diabetic end-organ damage. Trends Endocrinol. Metab. 30, 312–327 (2019).
Keleku-Lukwete, N., Suzuki, M. & Yamamoto, M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid. Redox Signal. 29, 1746–1755 (2018).
Ames, B. N. Prolonging healthy aging: longevity vitamins and proteins. Proc. Natl Acad. Sci. USA 115, 10836–10844 (2018). An overview of healthy ageing and the role of micronutrients.
Banba, A., Tsuji, A., Kimura, H., Murai, M. & Miyoshi, H. Defining the mechanism of action of S1QELs, specific suppressors of superoxide production in the quinone-reaction site in mitochondrial complex I. J. Biol. Chem. 294, 6550–6561 (2019).
Brand, M. D. et al. Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 24, 582–592 (2016).
Galaris, D., Barbouti, A. & Pantopoulos, K. Iron homeostasis and oxidative stress: an intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 1866, 118535 (2019).
Koppenol, W. H. & Hider, R. H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 133, 3–10 (2019).
Pugh, C. W. & Ratcliffe, P. J. New horizons in hypoxia signaling pathways. Exp. Cell Res. 356, 116–121 (2017).
Jain, I. H. et al. Hypoxia as a therapy for mitochondrial disease. Science 352, 54–61 (2016).
Gorrini, C., Harris, I. S. & Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug. Discov. 12, 931–947 (2013).
von Woedtke, T., Schmidt, A., Bekeschus, S., Wende, K. & Weltmann, K. D. Plasma medicine: a field of applied redox biology. Vivo 33, 1011–1026 (2019).
Chacko, B. K., Zhi, D., Darley-Usmar, V. M. & Mitchell, T. The bioenergetic health index is a sensitive measure of oxidative stress in human monocytes. Redox Biol. 8, 43–50 (2016).
Hill, B. G., Shiva, S., Ballinger, S., Zhang, J. & Darley-Usmar, V. M. Bioenergetics and translational metabolism: implications for genetics, physiology and precision medicine. Biol. Chem. 401, 3–29 (2019).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug. Discov. 8, 579–591 (2009).
Kirkpatrick, D. L. & Powis, G. Clinically evaluated cancer drugs inhibiting redox signaling. Antioxid. Redox Signal. 26, 262–273 (2017).
Adhikari, A., Mondal, S., Darbar, S. & Kumar, P. S. Role of nanomedicine in redox mediated healing at molecular level. Biomol. Concepts 10, 160–174 (2019).
Yang, B., Chen, Y. & Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 119, 4881–4985 (2019).
Barabasi, A. L., Gulbahce, N. & Loscalzo, J. Network medicine: a network-based approach to human disease. Nat. Rev. Genet. 12, 56–68 (2011).
Go, Y. M., Fernandes, J., Hu, X., Uppal, K. & Jones, D. P. Mitochondrial network responses in oxidative physiology and disease. Free Radic. Biol. Med. 116, 31–40 (2018).
Di Mascio, P. et al. Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins. Chem. Rev. 119, 2043–2086 (2019).
Mano, C. M. et al. Excited singlet molecular O2(1Δg) is generated enzymatically from excited carbonyls in the dark. Sci. Rep. 4, 5938 (2014).
Brash, D. E., Goncalves, L. C. P. & Bechara, E. J. H. Chemiexcitation and its implications for disease. Trends Mol. Med. 24, 527–541 (2018).
Poole, L. B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 80, 148–157 (2015).
Leisegang, M. S., Schröder, K. & Brandes, R. P. Redox regulation and noncoding RNAs. Antioxid. Redox Signal. 29, 793–812 (2018).
Kalinina, E. V., Ivanova-Radkevich, V. I. & Chernov, N. N. Role of microRNAs in the regulation of redox-dependent processes. Biochemistry (Mosc,) 84, 1233–1246 (2019).
Bartesaghi, S. & Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 14, 618–625 (2018).
MacMillan-Crow, L. A., Crow, J. P., Kerby, J. D., Beckman, J. S. & Thompson, J. A. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. U. S. A. 93, 11853–11858 (1996).
Filipovic, M. R., Zivanovic, J., Alvarez, B. & Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 118, 1253–1337 (2018). Chemistry of H2S signaling.
Paul, B. D. & Snyder, S. H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 13, 499–507 (2012).
Biteau, B., Labarre, J. & Toledano, M. B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425, 980–984 (2003).
Akter, S. et al. Chemical proteomics reveals new targets of cysteine sulfinic acid reductase. Nat. Chem. Biol. 14, 995–1004 (2018).
Watson, W. H. et al. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J. Biol. Chem. 278, 33408–33415 (2003).
Acknowledgements
The authors thank the many colleagues whose research work contributed to this rapidly expanding field, and we apologize to the many authors whose interesting work could not be referred to explicitly. Fruitful discussions with W. Stahl and C. Berndt are gratefully acknowledged. Generous research support was provided by the Deutsche Forschungsgemeinschaft, Bonn, and the US National Foundation for Cancer Research, Bethesda, to H.S. and by the US National Institutes of Health to D.P.J.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks F. Ursini, M. Davies and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Pleiotropic
-
From Greek pleion, meaning ‘more’, and tropos, meaning ‘way’, in biology originally denoting that one gene can influence two or more seemingly unrelated phenotypic traits. In current usage, the term describes multiple actions exerted by a given agent. If the actions generate opposing effects (for example, both harmful and beneficial to an organism), it is antagonistic pleiotropy.
- Redox signalling
-
Response of a cell to an oxidant or reductant, or to an alteration in redox status of a cellular component, that leads to a variety of downstream effects on cell state directly or via an essential redox relay from a source to a target.
- Oxidative eustress
-
Term describing the physiological oxidative challenge (Greek eu, meaning ‘good’, ‘well’, ‘positive’), essential in redox signalling. Supraphysiological oxidative challenge is denoted as ‘oxidative distress’.
- Thiolate
-
Anion formed from thiol by dissociation of a proton (RSH → RS− + H+).
- Selenoproteins
-
Proteins with selenocysteine, the 21st amino acid, in the primary structure. Selenomethionine can sometimes substitute for methionine during protein synthesis, but unlike selenocysteine, this is not encoded within the RNA message and is generally without functional significance.
- Hormesis
-
From Greek hormesis ‘rapid motion, eagerness’, describing a biphasic dose-response phenomenon: low-dose exposure (stimulation, preconditioning) and high-dose exposure (inhibition), J-shaped or inverted U-shaped dose-response curve.
- 4-Hydroxynonenal
-
A reactive aldehyde produced during free radical chain reaction of polyunsaturated fatty acids.
- Lipid peroxidation
-
Oxidative free radical chain reaction of polyunsaturated fatty acids.
- Mitochondrial electron transport chain
-
(ETC). Series of electron transfer complexes in the mitochondrial inner membrane that support oxidation of metabolic fuels to generate an electrochemical proton gradient for ATP synthesis from ADP and inorganic phosphate. Complexes within this chain are also sources of O2·– and its product, H2O2.
- Cristae
-
Folds of the mitochondrial inner membrane.
- Peroxiredoxins
-
Enzymes that catalyse reduction of H2O2 with a thioredoxin as the electron donor.
- Glutathione
-
Tripeptide (γ-glutamylcysteinylglycine) that is widespread in biology and, among other functions, supports antioxidant reactions.
- Peroxidases
-
Enzymes which catalyse the reduction of H2O2 and other hydroperoxides.
- Catalatic reaction
-
One of the modes of catalysis for catalase, along with peroxidatic reaction. In the overall reaction cycle, the intermediate formed by reaction of catalase haem iron with H2O2, termed ‘compound I’, can be reduced by a second molecule of H2O2 (catalatic reaction) or by an alternative hydrogen donor (peroxidatic reaction).
- Thioredoxin system
-
A thiol antioxidant system consisting of NADPH, thioredoxin reductase and thioredoxin that supports maintenance of protein thiols and reduction of hydroperoxides.
- Glutathione system
-
A thiol antioxidant system consisting of glutathione peroxidases, glutathione disulfide reductase, glutathione S-transferases, glutaredoxin, glutathione synthesis enzymes and glutathione which supports maintenance of protein thiols and reduction of hydroperoxides.
- Iron–sulfur (Fe–S) clusters
-
Redox centres in proteins in which iron is coordinately bonded between cysteinyl residues (thiolates) in protein and sulfide (S2−), for example, Fe2S2 and Fe4S4.
- Sirtuin family
-
Enzymes that remove acetyl (that is, functioning as deacetylases) or other acyl groups (that is, functioning as desuccinylases, demalonylases, demyristoylases or depalmitoylases) from proteins.
- Autophagy
-
A process for removal of damaged cellular structures that contributes to maintenance of cellular homeostasis.
- Glutathionylation
-
A post-translational protein modification functioning in redox signalling comprising the reversible formation of an S-glutathione adduct of a cysteinyl residue in proteins. Glutaredoxins are enzymes that catalyse the formation and removal of S-glutathionylated proteins.
- Src kinase
-
A member of a family of non-receptor tyrosine kinases with many protein targets and functions in differentiation and regulation of cell growth.
- Xanthine oxidase
-
A form of xanthine dehydrogenase that generates a relatively high proportion of O2·– instead of H2O2 during oxidation of hypoxanthine to xanthine or xanthine to uric acid.
- Aconitase
-
An enzyme in the citric acid cycle that has an Fe–S cluster and is sensitive to inactivation by O2·–.
- Mitochondrial permeability transition pore
-
A pore formed from structural changes in mitochondrial inner membrane proteins that allow influx of solutes into the mitochondrial matrix. Activation of the pore causes high-amplitude swelling of the mitochondria and activates cell death mechanisms.
- Unfolded protein response
-
(UPR). A cell stress response triggered by disruption of protein processing within the endoplasmic reticulum.
- ER stress
-
Perturbation of functions of the endoplasmic reticulum (ER), for example abnormal folding and processing of proteins.
- Axonal growth cone
-
Site of growth at the tip of a dendrite or an axon of neurons, containing cytoplasm and actin formed in the leading edge of the cell during neuronal development and regeneration.
- Cell senescence
-
A state of cells with arrested cell division and resistance to cell proliferation signals and uncontrolled cell growth. Accumulation of senescent cells is a hallmark of ageing; it contributes to decline in organ function and some age-related diseases.
- Exosomes
-
Nanometre-sized vesicles released by cells, a means for cell–cell communication. Exosomes possess characteristics of cells of origin and can be used as biomarkers of disease.
- Neutrophil extracellular traps
-
Extracellular fibre networks created by neutrophils to trap and kill invading microbes. They contain DNA from extruded chromatin and antimicrobial proteins such as neutrophil elastase and cathepsin G.
- NLRP3 inflammasome
-
NLRP3 is protein complex in cells that activates inflammatory processes. ‘NLR’ refers to ‘nucleotide-binding oligomerization domain, leucine-rich repeat’. The complex senses a broad range of microbial and environmental stressors.
- Schiff base
-
A chemical structure with a double bond between nitrogen and carbon formed by reaction of an amine with a carbonyl group.
- Michael addition
-
An addition reaction of a nucleophilic chemical with a chemical having an electrophilic α,β-conjugated carbonyl structure. The reaction can be important in pathological processes because DNA and other macromolecules contain nucleophiles, and α,β-conjugated carbonyls are major products of lipid peroxidation of polyunsaturated fatty acids.
- Redox cycling agents
-
Chemicals that undergo enzymatic one-electron reduction, generating a transient radical, which is reoxidized by molecular oxygen, reducing it to O2·–.
- Photodynamic therapy
-
A therapy using photoexcitable agents and light to generate toxic reactive oxygen species, predominantly singlet molecular oxygen.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sies, H., Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21, 363–383 (2020). https://doi.org/10.1038/s41580-020-0230-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-020-0230-3
This article is cited by
-
Total iridoid glycoside extract of Lamiophlomis rotata (Benth) Kudo accelerates diabetic wound healing by the NRF2/COX2 axis
Chinese Medicine (2024)
-
Antioxidant and acetylcholinesterase inhibitory activities, in silico analyses, and anti-Alzheimer’s disease potential of leaf extracts of three Nigerian endemic medicinal plants (Spondias mombin, Carica papaya and Kalanchoe crenata)
Future Journal of Pharmaceutical Sciences (2024)
-
NRF2 attenuation aggravates detrimental consequences of metabolic stress on cultured porcine parthenote embryos
Scientific Reports (2024)
-
FOXO transcription factors as mediators of stress adaptation
Nature Reviews Molecular Cell Biology (2024)
-
Oxidative stress and the role of redox signalling in chronic kidney disease
Nature Reviews Nephrology (2024)