Abstract
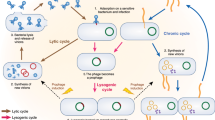
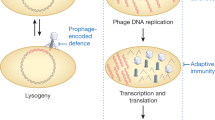
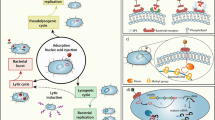
Bacteriophages (phages) are often described as obligate predators of their bacterial hosts, and phage predation is one of the leading forces controlling the density and distribution of bacterial populations. Every 48 h half of all bacteria on Earth are killed by phages. Efficient killing also forms the basis of phage therapy in humans and animals and the use of phages as food preservatives. In turn, bacteria have a plethora of resistance systems against phage attack, but very few bacterial species, if any, have entirely escaped phage predation. However, in complex communities and environments such as the human gut, this antagonistic model of attack and counter-defence does not fully describe the scope of phage–bacterium interactions. In this Review, we explore some of the more mutualistic aspects of phage–bacterium interactions in the human gut, and we suggest that the relationship between phages and their bacterial hosts in the gut is best characterized not as a fight to the death between enemies but rather as a mutualistic relationship between partners.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hendrix, R. W., Smith, M. C. M., Burns, R. N., Ford, M. E. & Hatfull, G. F. Evolutionary relationships among diverse bacteriophages and prophages: all the world’s a phage. Proc. Natl Acad. Sci. USA 96, 2192–2197 (1999).
Mushegian, A. R. Are there 1031 virus particles on earth, or more, or fewer? J. Bacteriol. 202, e00052-20 (2020).
Dennehy, J. J. & Abedon, S. T. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 1–43 (Springer International Publishing, 2020).
Hobbs, Z. & Abedon, S. T. Diversity of phage infection types and associated terminology: the problem with ‘Lytic or lysogenic’. FEMS Microbiol. Lett. 363, fnw047 (2016).
Bondy-Denomy, J. & Davidson, A. R. When a virus is not a parasite: the beneficial effects of prophages on bacterial fitness. J. Microbiol. 52, 235–242 (2014).
Harrison, E. & Brockhurst, M. A. Ecological and evolutionary benefits of temperate phage: what does or doesn’t kill you makes you stronger. BioEssays 39, 1700112 (2017).
Rakonjac, J., Bennett, N. J., Spagnuolo, J., Gagic, D. & Russel, M. Filamentous bacteriophage: biology, phage display and nanotechnology applications. Curr. Issues Mol. Biol. 13, 51–76 (2011).
Howard-Varona, C., Hargreaves, K. R., Abedon, S. T. & Sullivan, M. B. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J. 11, 1511–1520 (2017).
Proctor, L. M. & Fuhrman, J. A. Viral mortality of marine bacteria and cyanobacteria. Nature 343, 60–62 (1990).
Suttle, C. A. The significance of viruses to mortality in aquatic microbial communities. Microb. Ecol. 28, 237–243 (1994).
Touchon, M., Bernheim, A. & Rocha, E. P. Genetic and life-history traits associated with the distribution of prophages in bacteria. ISME J. 10, 2744–2754 (2016).
Breitbart, M., Bonnain, C., Malki, K. & Sawaya, N. A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 3, 754 (2018).
Zhang, R. et al. Viral control of biomass and diversity of bacterioplankton in the deep sea. Commun. Biol. 3, 1–10 (2020).
Luria, S. E. & Delbrück, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28, 491–511 (1943).
Obeng, N., Pratama, A. A. & van Elsas, J. D. The significance of mutualistic phages for bacterial ecology and evolution. Trends Microbiol. 24, 440–449 (2016).
Argov, T. et al. Temperate bacteriophages as regulators of host behavior. Curr. Opin. Microbiol. 38, 81–87 (2017).
Rodríguez-Rubio, L., Blanco-Picazo, P. & Muniesa, M. in Biocommunication of Phages (ed. Witzany, G.) 143–162 (Springer International Publishing, 2020).
Liang, G. et al. The stepwise assembly of the neonatal virome is modulated by breastfeeding. Nature 581, 470–474 (2020).
Lim, E. S. et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 21, 1228–1234 (2015).
Pannaraj, P. S. et al. Shared and distinct features of human milk and infant stool viromes. Front. Microbiol. 9, 1162 (2018).
Aggarwala, V., Liang, G. & Bushman, F. D. Viral communities of the human gut: metagenomic analysis of composition and dynamics. Mob. DNA 8, 12 (2017).
Clooney, A. G. et al. Whole-virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe 26, 764–778.e5 (2019).
Shkoporov, A. N. & Hill, C. Bacteriophages of the human gut: the “known unknown” of the microbiome. Cell Host Microbe 25, 195–209 (2019).
Grice, E. A. & Segre, J. A. The human microbiome: our second genome. Annu. Rev. Genom. Hum. Genet. 13, 151–170 (2012).
Gregory, A. C. et al. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe 28, 724–740.e8 (2020).
Camarillo-Guerrero, L. F., Almeida, A., Rangel-Pineros, G., Finn, R. D. & Lawley, T. D. Massive expansion of human gut bacteriophage diversity. Cell 184, 1098–1109.e9 (2021).
Nayfach, S. et al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat. Microbiol. 6, 960–970 (2021).
Roux, S. et al. IMG/VR v3: an integrated ecological and evolutionary framework for interrogating genomes of uncultivated viruses. Nucleic Acids Res. 49, D764–D775 (2021).
Benler, S. et al. Thousands of previously unknown phages discovered in whole-community human gut metagenomes. Microbiome 9, 78 (2021).
Fujimoto, K. et al. Metagenome data on intestinal phage-bacteria associations aids the development of phage therapy against pathobionts. Cell Host Microbe 28, 380–389.e9 (2020).
Jang, H. B. et al. Taxonomic assignment of uncultivated prokaryotic virus genomes is enabled by gene-sharing networks. Nat. Biotechnol. https://doi.org/10.1038/s41587-019-0100-8 (2019).
Adriaenssens, E. M. Phage diversity in the human gut microbiome: a taxonomist’s perspective. mSystems 6, e00799-21 (2021).
Zablocki, O. et al. VirION2: a short- and long-read sequencing and informatics workflow to study the genomic diversity of viruses in nature. PeerJ 9, e11088 (2021).
Yahara, K. et al. Long-read metagenomics using PromethION uncovers oral bacteriophages and their interaction with host bacteria. Nat. Commun. 12, 27 (2021).
Marbouty, M., Baudry, L., Cournac, A. & Koszul, R. Scaffolding bacterial genomes and probing host-virus interactions in gut microbiome by proximity ligation (chromosome capture) assay. Sci. Adv. 3, e1602105 (2017).
Marbouty, M., Thierry, A., Millot, G. A. & Koszul, R. MetaHiC phage-bacteria infection network reveals active cycling phages of the healthy human gut. eLife 10, e60608 (2021).
Yaffe, E. & Relman, D. A. Tracking microbial evolution in the human gut using Hi-C reveals extensive horizontal gene transfer, persistence and adaptation. Nat. Microbiol. 5, 343–353 (2020).
Zheng, W. et al. High-throughput single-microbe genomics with strain resolution applied to a human gut microbiome. Science https://doi.org/10.1126/science.abm1483 (2022).
Fitzgerald, C. B. et al. Probing the “dark matter” of the human gut phageome: culture assisted metagenomics enables rapid discovery and host-linking for novel bacteriophages. Front. Cell. Infect. Microbiol. 11, 100 (2021).
de Jonge, P. A. et al. Adsorption sequencing as a rapid method to link environmental bacteriophages to hosts. iScience 23, 101439 (2020).
Hsu, B. B. et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 25, 803–814.e5 (2019).
Reyes, A., Wu, M., McNulty, N. P., Rohwer, F. L. & Gordon, J. I. Gnotobiotic mouse model of phage–bacterial host dynamics in the human gut. Proc. Natl Acad. Sci. USA 110, 20236–20241 (2013).
Hargreaves, K. R., Kropinski, A. M. & Clokie, M. R. Bacteriophage behavioral ecology. Bacteriophage 4, e29866 (2014).
Williams, H. T. Phage-induced diversification improves host evolvability. BMC Evol. Biol. 13, 17 (2013).
Goyal, A., Wang, T., Dubinkina, V. & Maslov, S. Ecology-guided prediction of cross-feeding interactions in the human gut microbiome. Nat. Commun. 12, 1335 (2021).
Wang, T., Goyal, A., Dubinkina, V. & Maslov, S. Evidence for a multi-level trophic organization of the human gut microbiome. PLoS Comput. Biol. 15, e1007524 (2019).
Bohannan, B. J. M. & Lenski, R. E. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol. Lett. 3, 362–377 (2000).
Maura, D. et al. Intestinal colonization by enteroaggregative Escherichia coli supports long-term bacteriophage replication in mice. Environ. Microbiol. 14, 1844–1854 (2012).
Weiss, M. et al. In vivo replication of T4 and T7 bacteriophages in germ-free mice colonized with Escherichia coli. Virology 393, 16–23 (2009).
De Sordi, L., Lourenço, M. & Debarbieux, L. “I will survive”: a tale of bacteriophage-bacteria coevolution in the gut. Gut Microbes 10, 92–99 (2018).
Scanlan, P. D. Bacteria–bacteriophage coevolution in the human gut: implications for microbial diversity and functionality. Trends Microbiol. 25, 614–623 (2017).
Mangalea, M. R. & Duerkop, B. A. Fitness trade-offs resulting from bacteriophage resistance potentiate synergistic antibacterial strategies. Infect. Immun. 88, e00926-19 (2020).
Burmeister, A. R. & Turner, P. E. Trading-off and trading-up in the world of bacteria–phage evolution. Curr. Biol. 30, R1120–R1124 (2020).
Lourenço, M. et al. The spatial heterogeneity of the gut limits predation and fosters coexistence of bacteria and bacteriophages. Cell Host Microbe 28, 390–401.e5 (2020).
Shkoporov, A. et al. Viral biogeography of gastrointestinal tract and parenchymal organs in two representative species of mammals. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-803286/v1 (2021).
Lee, S. M. et al. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501, 426–429 (2013).
Barr, J. J. et al. Bacteriophage adhering to mucus provide a non–host-derived immunity. Proc. Natl Acad. Sci. USA 110, 10771–10776 (2013).
Green, S. I. et al. Targeting of mammalian glycans enhances phage predation in the gastrointestinal tract. mBio 12, e03474-20 (2021).
Almeida, A. et al. A new genomic blueprint of the human gut microbiota. Nature 568, 499–504 (2019).
Zou, Y. et al. 1,520 reference genomes from cultivated human gut bacteria enable functional microbiome analyses. Nat. Biotechnol. 37, 179–185 (2019).
Thomas, A. M. & Segata, N. Multiple levels of the unknown in microbiome research. BMC Biol. 17, 48 (2019).
Carrow, H. C., Batachari, L. E. & Chu, H. Strain diversity in the microbiome: lessons from Bacteroides fragilis. PLoS Pathog. 16, e1009056 (2020).
Lloyd-Price, J. et al. Strains, functions and dynamics in the expanded human microbiome project. Nature 550, 61 (2017).
Yan, Y., Nguyen, L. H., Franzosa, E. A. & Huttenhower, C. Strain-level epidemiology of microbial communities and the human microbiome. Genome Med. 12, 71 (2020).
Kelsen, J. R. & Wu, G. D. The gut microbiota, environment and diseases of modern society. Gut Microbes 3, 374–382 (2012).
Rainey, P. B. & Quistad, S. D. Toward a dynamical understanding of microbial communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190248 (2020).
Berg, G. et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103 (2020).
Lourenço, M., De Sordi, L. & Debarbieux, L. The diversity of bacterial lifestyles hampers bacteriophage tenacity. Viruses 10, 327 (2018).
Silveira, C. B. & Rohwer, F. L. Piggyback-the-Winner in host-associated microbial communities. npj Biofilms Microbiomes 2, 16010 (2016).
Koonin, E. V. & Yutin, N. The crAss-like phage group: how metagenomics reshaped the human virome. Trends Microbiol. 28, 349–359 (2020).
Dutilh, B. E. et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat. Commun. 5, 4498 (2014).
Guerin, E. et al. Biology and taxonomy of crass-like bacteriophages, the most abundant virus in the human gut. Cell Host Microbe 24, 653–664.e6 (2018).
Yutin, N. et al. Analysis of metagenome-assembled viral genomes from the human gut reveals diverse putative CrAss-like phages with unique genomic features. Nat. Commun. 12, 1044 (2021).
Yutin, N. et al. Discovery of an expansive bacteriophage family that includes the most abundant viruses from the human gut. Nat. Microbiol. 3, 38–46 (2018).
Edwards, R. A. et al. Global phylogeography and ancient evolution of the widespread human gut virus crAssphage. Nat. Microbiol. 4, 1727–1736 (2019).
Shkoporov, A. N. et al. Long-term persistence of crAss-like phage crAss001 is associated with phase variation in Bacteroides intestinalis. BMC Biol. 19, 163 (2021).
Guerin, E. et al. Isolation and characterisation of ΦcrAss002, a crAss-like phage from the human gut that infects Bacteroides xylanisolvens. Microbiome 9, 89 (2021).
Gulyaeva, A. et al. Discovery, diversity, and functional associations of crAss-like phages in human gut metagenomes from four Dutch cohorts. Cell Rep. 38, 110204 (2022).
Porter, N. T. et al. Phase-variable capsular polysaccharides and lipoproteins modify bacteriophage susceptibility in Bacteroides thetaiotaomicron. Nat. Microbiol. 5, 1170–1181 (2020).
Hryckowian, A. J. et al. Bacteroides thetaiotaomicron-infecting bacteriophage isolates inform sequence-based host range predictions. Cell Host Microbe 28, 371–379.e5 (2020).
Siringan, P., Connerton, P. L., Cummings, N. J. & Connerton, I. F. Alternative bacteriophage life cycles: the carrier state of Campylobacter jejuni. Open Biol. 4, 130200 (2014).
Devoto, A. E. et al. Megaphages infect Prevotella and variants are widespread in gut microbiomes. Nat. Microbiol. 4, 693 (2019).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460 (2015).
Shkoporov, A. N. et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe 26, 527–541.e5 (2019).
Minot, S. et al. Rapid evolution of the human gut virome. Proc. Natl Acad. Sci. USA 110, 12450–12455 (2013).
Chen, L. et al. The long-term genetic stability and individual specificity of the human gut microbiome. Cell 184, 2302–2315.e12 (2021).
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Moreno-Gallego, J. L. et al. Virome diversity correlates with intestinal microbiome diversity in adult monozygotic twins. Cell Host Microbe 25, 261–272.e5 (2019).
Roux, S., Krupovic, M., Poulet, A., Debroas, D. & Enault, F. Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads. PLoS ONE 7, e40418 (2012).
Diard, M. et al. Inflammation boosts bacteriophage transfer between Salmonella spp. Science 355, 1211–1215 (2017).
De Sordi, L., Lourenço, M. & Debarbieux, L. The battle within: interactions of bacteriophages and bacteria in the gastrointestinal tract. Cell Host Microbe 25, 210–218 (2019).
Minot, S., Grunberg, S., Wu, G. D., Lewis, J. D. & Bushman, F. D. Hypervariable loci in the human gut virome. Proc. Natl Acad. Sci. USA 109, 3962–3966 (2012).
Morozova, V. et al. First crAss-like phage genome encoding the diversity-generating retroelement (DGR). Viruses 12, 573 (2020).
Mills, S. et al. Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes 4, 4–16 (2013).
Turkington, C. J. R., Morozov, A., Clokie, M. R. J. & Bayliss, C. D. Phage-resistant phase-variant sub-populations mediate herd immunity against bacteriophage invasion of bacterial meta-populations. Front. Microbiol. 10, 1473 (2019).
Eriksen, R. S., Svenningsen, S. L., Sneppen, K. & Mitarai, N. A growing microcolony can survive and support persistent propagation of virulent phages. Proc. Natl Acad. Sci. USA 115, 337–342 (2018).
De Sordi, L., Khanna, V. & Debarbieux, L. The gut microbiota facilitates drifts in the genetic diversity and infectivity of bacterial viruses. Cell Host Microbe 22, 801–808.e3 (2017).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 (2010).
Samson, J. E., Magadán, A. H., Sabri, M. & Moineau, S. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–687 (2013).
van Houte, S., Buckling, A. & Westra, E. R. Evolutionary ecology of prokaryotic immune mechanisms. Microbiol. Mol. Biol. Rev. 80, 745–763 (2016).
Ofir, G. & Sorek, R. Contemporary phage biology: from classic models to new insights. Cell 172, 1260–1270 (2018).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
Burmeister, A. R. et al. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc. Natl Acad. Sci. USA 117, 11207–11216 (2020).
Rigottier-Gois, L. et al. The surface rhamnopolysaccharide epa of Enterococcus faecalis is a key determinant of intestinal colonization. J. Infect. Dis. 211, 62–71 (2015).
Chatterjee, A. et al. Bacteriophage resistance alters antibiotic-mediated intestinal expansion of enterococci. Infect. Immun. 87, e00085-19 (2019).
Moulton-Brown, C. E. & Friman, V.-P. Rapid evolution of generalized resistance mechanisms can constrain the efficacy of phage–antibiotic treatments. Evol. Appl. 11, 1630–1641 (2018).
Hosseinidoust, Z., van de Ven, T. G. M. & Tufenkji, N. Evolution of Pseudomonas aeruginosa virulence as a result of phage predation. Appl. Environ. Microbiol. 79, 6110–6116 (2013).
Alseth, E. O. et al. Bacterial biodiversity drives the evolution of CRISPR-based phage resistance. Nature 574, 549–552 (2019).
Christen, M. et al. Quantitative selection analysis of bacteriophage φCbK susceptibility in Caulobacter crescentus. J. Mol. Biol. 428, 419–430 (2016).
Pal, C., Maciá, M. D., Oliver, A., Schachar, I. & Buckling, A. Coevolution with viruses drives the evolution of bacterial mutation rates. Nature 450, 1079–1081 (2007).
Bayliss, C. D. Determinants of phase variation rate and the fitness implications of differing rates for bacterial pathogens and commensals. FEMS Microbiol. Rev. 33, 504–520 (2009).
Moxon, R., Bayliss, C. & Hood, D. Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Annu. Rev. Genet. 40, 307–333 (2006).
Cota, I., Blanc-Potard, A. B. & Casadesús, J. STM2209-STM2208 (opvAB): a phase variation locus of Salmonella enterica involved in control of O-antigen chain length. PLoS ONE 7, e36863 (2012).
Sekulovic, O., Ospina Bedoya, M., Fivian-Hughes, A. S., Fairweather, N. F. & Fortier, L.-C. The Clostridium difficile cell wall protein CwpV confers phase-variable phage resistance. Mol. Microbiol. 98, 329–342 (2015).
Gencay, Y. E., Sørensen, M. C. H., Wenzel, C. Q., Szymanski, C. M. & Brøndsted, L. Phase variable expression of a single phage receptor in Campylobacter jejuni NCTC12662 influences sensitivity toward several diverse CPS-dependent phages. Front. Microbiol. 9, 82 (2018).
Neff, C. P. et al. Diverse intestinal bacteria contain putative zwitterionic capsular polysaccharides with anti-inflammatory properties. Cell Host Microbe 20, 535–547 (2016).
Porter, N. T., Canales, P., Peterson, D. A. & Martens, E. C. A subset of polysaccharide capsules in the human symbiont bacteroides thetaiotaomicron promote increased competitive fitness in the mouse gut. Cell Host Microbe 22, 494–506.e8 (2017).
Sørensen, M. C. H. et al. Campylobacter phages use hypermutable polyG tracts to create phenotypic diversity and evade bacterial resistance. Cell Rep. 35, 109214 (2021).
Bossi, L., Fuentes, J. A., Mora, G. & Figueroa-Bossi, N. Prophage contribution to bacterial population dynamics. J. Bacteriol. 185, 6467–6471 (2003).
Barr, J. J. et al. Subdiffusive motion of bacteriophage in mucosal surfaces increases the frequency of bacterial encounters. Proc. Natl Acad. Sci. USA 112, 13675–13680 (2015).
Tzipilevich, E., Habusha, M. & Ben-Yehuda, S. Acquisition of phage sensitivity by bacteria through exchange of phage receptors. Cell 168, 186–199.e12 (2017).
Lu, H.-P. et al. Spatial heterogeneity of gut microbiota reveals multiple bacterial communities with distinct characteristics. Sci. Rep. 4, 6185 (2014).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 (2016).
Paterson, S. et al. Antagonistic coevolution accelerates molecular evolution. Nature 464, 275–278 (2010).
Scanlan, P. D., Hall, A. R., Lopez-Pascua, L. D. C. & Buckling, A. Genetic basis of infectivity evolution in a bacteriophage. Mol. Ecol. 20, 981–989 (2011).
Hyman, P. & Abedon, S. T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 70, 217–248 (2010).
Betts, A., Gray, C., Zelek, M., MacLean, R. C. & King, K. C. High parasite diversity accelerates host adaptation and diversification. Science 360, 907–911 (2018).
Brown, B. P. et al. crAssphage genomes identified in fecal samples of an adult and infants with evidence of positive genomic selective pressure within tail protein genes. Virus Res. 292, 198219 (2021).
Stewart, F. M. & Levin, B. R. The population biology of bacterial viruses: why be temperate. Theor. Popul. Biol. 26, 93–117 (1984).
Maslov, S. & Sneppen, K. Well-temperate phage: optimal bet-hedging against local environmental collapses. Sci. Rep. 5, 10523 (2015).
Weitz, J. S., Li, G., Gulbudak, H., Cortez, M. H. & Whitaker, R. J. Viral invasion fitness across a continuum from lysis to latency. Virus Evol. 5, vez006 (2019).
Wahl, A., Battesti, A. & Ansaldi, M. Prophages in Salmonella enterica: a driving force in reshaping the genome and physiology of their bacterial host? Mol. Microbiol. 111, 303–316 (2019).
Calero-Cáceres, W., Ye, M. & Balcázar, J. L. Bacteriophages as environmental reservoirs of antibiotic resistance. Trends Microbiol. 27, 570–577 (2019).
Wang, X. et al. Cryptic prophages help bacteria cope with adverse environments. Nat. Commun. 1, 147 (2010).
Arnold, J. W. & Koudelka, G. B. The Trojan horse of the microbiological arms race: phage-encoded toxins as a defence against eukaryotic predators. Environ. Microbiol. 16, 454–466 (2014).
Dragoš, A. et al. Pervasive prophage recombination occurs during evolution of spore-forming bacilli. ISME J. 15, 1344–1358 (2021).
Brown, E. M. et al. Gut microbiome ADP-ribosyltransferases are widespread phage-encoded fitness factors. Cell Host Microbe 29, 1351–1365.e11 (2021).
Fogg, P. C. M., Allison, H. E., Saunders, J. R. & McCarthy, A. J. Bacteriophage lambda: a paradigm revisited. J. Virol. 84, 6876–6879 (2010).
Sausset, R., Petit, M. A., Gaboriau-Routhiau, V. & De Paepe, M. New insights into intestinal phages. Mucosal Immunol. 13, 205–215 (2020).
De Paepe, M. et al. Carriage of λ latent virus is costly for its bacterial host due to frequent reactivation in monoxenic mouse intestine. PLoS Genet. 12, e1005861 (2016).
Duerkop, B. A., Clements, C. V., Rollins, D., Rodrigues, J. L. M. & Hooper, L. V. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc. Natl Acad. Sci. USA 109, 17621–17626 (2012).
Haaber, J. et al. Bacterial viruses enable their host to acquire antibiotic resistance genes from neighbouring cells. Nat. Commun. 7, 1–8 (2016).
Wendling, C. C., Refardt, D. & Hall, A. R. Fitness benefits to bacteria of carrying prophages and prophage-encoded antibiotic-resistance genes peak in different environments. Evolution 75, 515–528 (2021).
Sweere, J. M. et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 363, eaat9691 (2019).
Chuang, C.-H. et al. Shanghai fever: a distinct Pseudomonas aeruginosa enteric disease. Gut 63, 736–743 (2014).
Khatoon, H., Iyer, R. V. & Iyer, V. N. A new filamentous bacteriophage with sex-factor specificity. Virology 48, 145–155 (1972).
Wang, Q., Kan, B. & Wang, R. Isolation and characterization of the new mosaic filamentous phage VFJ Φ of Vibrio cholerae. PLoS ONE 8, e70934 (2013).
Roux, S. et al. Cryptic inoviruses revealed as pervasive in bacteria and archaea across earth’s biomes. Nat. Microbiol. 4, 1895–1906 (2019).
Garmaeva, S. et al. Stability of the human gut virome and effect of gluten-free diet. Cell Rep. 35, 109132 (2021).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I–restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020).
Gogokhia, L. et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 25, 285–299.e8 (2019).
Van Belleghem, J. D., Clement, F., Merabishvili, M., Lavigne, R. & Vaneechoutte, M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep. 7, 8004 (2017).
Stecher, B. et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl Acad. Sci. USA 109, 1269–1274 (2012).
Smillie, C. S. et al. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480, 241–244 (2011).
Wiser, M. J., Ribeck, N. & Lenski, R. E. Long-term dynamics of adaptation in asexual populations. Science 342, 1364–1367 (2013).
Frazão, N., Sousa, A., Lässig, M. & Gordo, I. Horizontal gene transfer overrides mutation in Escherichia coli colonizing the mammalian gut. Proc. Natl Acad. Sci. USA 116, 17906–17915 (2019).
Garud, N. R., Good, B. H., Hallatschek, O. & Pollard, K. S. Evolutionary dynamics of bacteria in the gut microbiome within and across hosts. PLoS Biol. 17, e3000102 (2019).
Zhao, S. et al. Adaptive evolution within gut microbiomes of healthy people. Cell Host Microbe 25, 656–667.e8 (2019).
Lerner, A., Matthias, T. & Aminov, R. Potential effects of horizontal gene exchange in the human gut. Front. Immunol. 8, 1630 (2017).
Sitaraman, R. Prokaryotic horizontal gene transfer within the human holobiont: ecological-evolutionary inferences, implications and possibilities. Microbiome 6, 163 (2018).
Baron, S. A., Diene, S. M. & Rolain, J.-M. Human microbiomes and antibiotic resistance. Hum. Microbiome J. 10, 43–52 (2018).
Brinkac, L., Voorhies, A., Gomez, A. & Nelson, K. E. The threat of antimicrobial resistance on the human microbiome. Microb. Ecol. 74, 1001–1008 (2017).
Penders, J., Stobberingh, E. E., Savelkoul, P. H. M. & Wolffs, P. The human microbiome as a reservoir of antimicrobial resistance. Front. Microbiol. https://doi.org/10.3389/fmicb.2013.00087 (2013).
Enault, F. et al. Phages rarely encode antibiotic resistance genes: a cautionary tale for virome analyses. ISME J. 11, 237–247 (2017).
Duranti, S. et al. Prevalence of antibiotic resistance genes among human gut-derived bifidobacteria. Appl. Environ. Microbiol. 83, e02894-16 (2017).
Jeong, H., Arif, B., Caetano-Anollés, G., Kim, K. M. & Nasir, A. Horizontal gene transfer in human-associated microorganisms inferred by phylogenetic reconstruction and reconciliation. Sci. Rep. 9, 1–18 (2019).
Chiang, Y. N., Penadés, J. R. & Chen, J. Genetic transduction by phages and chromosomal islands: the new and noncanonical. PLoS Pathog. 15, e1007878 (2019).
Chen, J. et al. Genome hypermobility by lateral transduction. Science 362, 207–212 (2018).
Humphrey, S. et al. Bacterial chromosomal mobility via lateral transduction exceeds that of classical mobile genetic elements. Nat. Commun. 12, 6509 (2021).
Fillol-Salom, A. et al. Lateral transduction is inherent to the life cycle of the archetypical Salmonella phage P22. Nat. Commun. 12, 6510 (2021).
Touchon, M., Moura de Sousa, J. A. & Rocha, E. P. Embracing the enemy: the diversification of microbial gene repertoires by phage-mediated horizontal gene transfer. Curr. Opin. Microbiol. 38, 66–73 (2017).
Kenzaka, T., Tani, K. & Nasu, M. High-frequency phage-mediated gene transfer in freshwater environments determined at single-cell level. ISME J. 4, 648–659 (2010).
Bárdy, P. et al. Structure and mechanism of DNA delivery of a gene transfer agent. Nat. Commun. 11, 3034 (2020).
Kleiner, M., Bushnell, B., Sanderson, K. E., Hooper, L. V. & Duerkop, B. A. Transductomics: sequencing-based detection and analysis of transduced DNA in pure cultures and microbial communities. Microbiome 8, 158 (2020).
Minot, S. et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011).
Zolfo, M. et al. Detecting contamination in viromes using ViromeQC. Nat. Biotechnol. 37, 1408–1412 (2019).
Modi, S. R., Lee, H. H., Spina, C. S. & Collins, J. J. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499, 219–222 (2013).
Quistad, S. D., Doulcier, G. & Rainey, P. B. Experimental manipulation of selfish genetic elements links genes to microbial community function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190681 (2020).
Morris, J. J., Lenski, R. E. & Zinser, E. R. The black queen hypothesis: evolution of dependencies through adaptive gene loss. mBio 3, e00036-12 (2012).
Langille, M. G. I., Meehan, C. J. & Beiko, R. G. Human microbiome: a genetic bazaar for microbes? Curr. Biol. 22, R20–R22 (2012).
Fullmer, M., Soucy, S. & Gogarten, J. P. The pan-genome as a shared genomic resource: mutual cheating, cooperation and the black queen hypothesis. Front. Microbiol. 6, 728 (2015).
Fitzgerald, C. B. et al. Comparative analysis of Faecalibacterium prausnitzii genomes shows a high level of genome plasticity and warrants separation into new species-level taxa. BMC Genomics 19, 931 (2018).
Zhu, A., Sunagawa, S., Mende, D. R. & Bork, P. Inter-individual differences in the gene content of human gut bacterial species. Genome Biol. 16, 82 (2015).
Sarker, S. A. et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 4, 124–137 (2016).
Jault, P. et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45 (2019).
Leitner, L. et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 21, 427–436 (2021).
Ott, S. J. et al. Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology 152, 799–811.e7 (2017).
Draper, L. A. et al. Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Biol. 18, 173 (2020).
Draper, L. A. et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome 6, 220 (2018).
Hsu, B. B. et al. In situ reprogramming of gut bacteria by oral delivery. Nat. Commun. 11, 5030 (2020).
Hevroni, G., Flores-Uribe, J., Béjà, O. & Philosof, A. Seasonal and diel patterns of abundance and activity of viruses in the Red Sea. Proc. Natl Acad. Sci. USA 117, 29738–29747 (2020).
Brum, J. R., Hurwitz, B. L., Schofield, O., Ducklow, H. W. & Sullivan, M. B. Seasonal time bombs: dominant temperate viruses affect Southern Ocean microbial dynamics. ISME J. 10, 437–449 (2016).
Wigington, C. H. et al. Re-examination of the relationship between marine virus and microbial cell abundances. Nat. Microbiol. 1, 1–9 (2016).
Džunková, M. et al. Defining the human gut host–phage network through single-cell viral tagging. Nat. Microbiol. 4, 2192–2203 (2019).
Carding, S. R., Davis, N. & Hoyles, L. Review article: the human intestinal virome in health and disease. Aliment. Pharmacol. Ther. 46, 800–815 (2017).
Maslov, S. & Sneppen, K. Population cycles and species diversity in dynamic Kill-the-Winner model of microbial ecosystems. Sci. Rep. 7, 1–8 (2017).
Zuo, T. et al. Human-gut-DNA virome variations across geography, ethnicity, and urbanization. Cell Host Microbe 28, 741–751.e4 (2020).
Lee, C. Z. et al. The gut virome in two indigenous populations from Malaysia. Sci. Rep. 12, 1824 (2022).
Yan, Q. et al. Characterization of the gut DNA and RNA viromes in a cohort of Chinese residents and visiting Pakistanis. Virus Evol. 7, veab022 (2021).
Oh, J.-H. et al. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont Lactobacillus reuteri. Cell Host Microbe 25, 273–284.e6 (2019).
Boling, L. et al. Dietary prophage inducers and antimicrobials: toward landscaping the human gut microbiome. Gut Microbes 11, 721–734 (2020).
Zuo, T. et al. Gut mucosal virome alterations in ulcerative colitis. Gut 68, 1169–1179 (2019).
Khan Mirzaei, M. et al. Bacteriophages isolated from stunted children can regulate gut bacterial communities in an age-specific manner. Cell Host Microbe 27, 199–212.e5 (2020).
Monaco, C. L. et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe 19, 311–322 (2016).
Tomofuji, Y. et al. Whole gut virome analysis of 476 Japanese revealed a link between phage and autoimmune disease. Ann. Rheum. Dis. 81, 278–288 (2022).
Roach, D. R. et al. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe 22, 38–47.e4 (2017).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Sutcliffe, S. G., Shamash, M., Hynes, A. P. & Maurice, C. F. Common oral medications lead to prophage induction in bacterial isolates from the human gut. Viruses 13, 455 (2021).
Acknowledgements
A.N.S. was supported by a Wellcome Trust Research Career Development Fellowship (220646/Z/20/Z) and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101001684). C.J.T. and C.H. were supported by Science Foundation Ireland under grant no. SFI/12/RC/2273.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Luisa De Sordi, Corinne Maurice and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Abortive infection systems
-
Bacterial toxin–antitoxin systems that prevent the completion of the viral infection through facilitating suicide of the infected bacterial cell.
- Lysogenic conversion
-
Alteration in bacterial phenotypes following temperate bacteriophage infection via expression of genes encoded within the bacteriophage genome.
- Horizontal gene transfer
-
(HGT). The transfer of genes between organisms that does not involve their direct passing to progeny through replication.
- Chemostats
-
Vessels used for the continuous culture of microorganisms via constant maintenance of conditions required for growth (for example, nutrient levels).
- Gnotobiotic animals
-
Animals free of microbial colonization.
- Phase variation
-
Genetic phenomenon characterized by the stochastic, high-frequency, reversible alteration in gene expression.
- Arms race dynamics
-
The continuous and reciprocal co-evolutionary adaptions that occur between bacteriophages and their bacterial hosts. Bacteria develop means to prevent infection (such as removal or alteration of receptors), whereas bacteriophages adapt to overcome bacterial defences (such as targeting different bacterial receptors).
- Fluctuating selection
-
Transient oscillations in genotype frequencies within phage and bacterial communities via negative frequency-dependent selection. Here, bacteriophages evolve to infect common bacterial genotypes, resulting in a selective advantage for low-frequency bacterial resistance alleles and therefore enabling those rare alleles to increase in frequency. As bacterial subpopulations with an allele rise in abundance, they become the focus of bacteriophage infectivity evolution, driving diversification of bacteriophage infectivity, and again providing a selective advantage for rare bacterial genotypes, causing the cycle to continue.
- Diversity-generating retroelements
-
Genetic elements able to accelerate mutation rates within specific genomic regions through error-prone reverse transcription.
- Piggyback-the-winner model
-
A model that describes a cooperative relationship between bacteria and temperate bacteriophages, via lysogeny, that is believed to dominate when bacterial densities are high. Temperate bacteriophages ‘piggyback’ on the success of a high-density bacterial host through lysogenic infection so that the bacteriophage replicates together with the host genome and is maintained in the population. At the same time, lysogenic infection of this already ‘winning’ bacterial host can enable the bacterium to receive further competitive advantages via lysogenic conversion and superinfection exclusion.
- Superinfection immunity
-
Bacterial resistance to secondary bacteriophage infection that results from an existing bacteriophage infection.
- Peyer patches
-
Lymphoid follicles in the small intestine involved in organizing immune responses to luminal antigens.
- Gene transfer agents
-
Gene delivery systems that can package random sections of host DNA and transfer them to another cell.
- Black Queen hypothesis
-
A hypothesis proposing that reductive genome evolution is acceptable through a ‘leaky’ common good function, wherein reductions in each organism’s genome can be offset by the presence of corresponding regions within the genomes of other members of a wider population.
Rights and permissions
About this article
Cite this article
Shkoporov, A.N., Turkington, C.J. & Hill, C. Mutualistic interplay between bacteriophages and bacteria in the human gut. Nat Rev Microbiol 20, 737–749 (2022). https://doi.org/10.1038/s41579-022-00755-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-022-00755-4