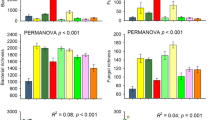
Abstract
The soil microbiome governs biogeochemical cycling of macronutrients, micronutrients and other elements vital for the growth of plants and animal life. Understanding and predicting the impact of climate change on soil microbiomes and the ecosystem services they provide present a grand challenge and major opportunity as we direct our research efforts towards one of the most pressing problems facing our planet. In this Review, we explore the current state of knowledge about the impacts of climate change on soil microorganisms in different climate-sensitive soil ecosystems, as well as potential ways that soil microorganisms can be harnessed to help mitigate the negative consequences of climate change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
U.S. Global Change Research Program. Impacts, risks, and adaptation in the United States: Fourth National Climate Assessment Vol. II (USGCRP, 2018).
Friedlingstein, P. et al. Climate–carbon cycle feedback analysis: results from the C4MIP model intercomparison. J. Clim. 19, 3337–3353 (2006).
Wang, K. et al. Modeling global soil carbon and soil microbial carbon by integrating microbial processes into the ecosystem process model TRIPLEX-GHG. J. Adv. Modeling Earth Syst. 9, 2368–2384 (2017).
Wieder, W. R., Bonan, G. B. & Allison, S. D. Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Change 3, 909–912 (2013).
Wahl T. et al. When environmental forces collide. Eos.org https://eos.org/features/when-environmental-forces-collide (2018).
Berard, A., Pierre, R. & Kaisermann, A. Soil microbial community responses to heat wave components: drought and high temperature. Clim. Res. 3, 243–264 (2014).
Sheik, C. et al. Effect of warming and drought on grassland microbial communities. ISME J. 5, 1692–1700 (2011).
Tarnocai, C. et al. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 23, GB2023 (2009).
Bruhwiler, L. et al. in Second State of the Carbon Cycle Report (SOCCR2): a sustained assessment report (eds Cavallaro, N. et al.) 42–70 (U.S. Global Change Research Program, 2018).
Bradford, M. A. et al. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol. Lett. 11, 1316–1327 (2008).
Bond-Lamberty, B., Bailey, V., Chen, M., Gough, C. & Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 560, 80–83 (2018). Based on existing data, this study determines trends towards increased soil microbial mineralization of SOC, leading to increased CO 2 emissions as a result of climate change.
Rustad, L. et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126, 543–562 (2001).
Nottingham, A. T. et al. Climate warming and soil carbon in tropical forests: insights from an elevation gradient in the Peruvian Andes. Bioscience 65, 906–921 (2015).
Vitousek, P. M. et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750 (1997).
Syakila, A. & Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 1, 17–26 (2011).
Regan, K. et al. Spatial and temporal dynamics of nitrogen fixing, nitrifying and denitrifying microbes in an unfertilized grassland soil. Soil Biol. Biochem. 109, 214–226 (2017).
Groffman, P. M. et al. Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol. Appl. 16, 2091–2122 (2006).
Butterbach-Bahl, K., Baggs Elizabeth, M., Dannenmann, M., Kiese, R. & Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Phil. Trans. R. Soc. B 368, 20130122 (2013).
van Kessel, M. A. H. J. et al. Complete nitrification by a single microorganism. Nature 528, 555–559 (2015).
Xia, F. et al. Ubiquity and diversity of complete ammonia oxidizers (comammox). Appl. Environ. Microb. 84, e01390-18 (2018).
Kuzyakov, Y. & Blagodatskaya, E. Microbial hotspots and hot moments in soil: concept & review. Soil Biol. Biochem. 83, 184–199 (2015).
Norby, R. J. et al. Model–data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytol. 209, 17–28 (2016).
Schimel, J., Balser, T. C. & Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 88, 1386–1394 (2007).
Evans, S. E. & Wallenstein, M. D. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 17, 155–164 (2014). This study assesses the phylogenetic conservation of ecological strategies in response to drying–re-wetting in incubation studies on soils from the Rainfall Manipulation Plot Study (RaMPS) in the US tallgrass prairie.
Berg, M. P. et al. Adapt or disperse: understanding species persistence in a changing world. Glob. Chang. Biol. 16, 587–598 (2010).
de Vries, F. T. & Shade, A. Controls on soil microbial community stability under climate change. Front. Microbiol. 4, 265 (2013).
Thompson, L. R. et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551, 457–463 (2017).
Jansson, J. K. & Hofmockel, K. S. The soil microbiome—from metagenomics to metaphenomics. Curr. Opin. Microbiol. 43, 162–168 (2018).
Waldrop, M. P. & Firestone, M. K. Response of microbial community composition and function to soil climate change. Microb. Ecol. 52, 716–724 (2006).
Fierer, N. & Jackson, R. B. The diversity and biogeography of soil bacterial communities. Proc. Natl Acad. Sci. USA 103, 626–631 (2006).
Schimel, J. & Schaeffer, S. Microbial control over carbon cycling in soil. Front. Microbiol. 3, 348 (2012).
Cordero, O. X. & Datta, M. S. Microbial interactions and community assembly at microscales. Curr. Opin. Microbiol. 31, 227–234 (2016).
Dunbar, J. et al. Common bacterial responses in six ecosystems exposed to 10 years of elevated atmospheric carbon dioxide. Environ. Microbiol. 14, 1145–1158 (2012).
Hayden, H. L. et al. Changes in the microbial community structure of bacteria, archaea and fungi in response to elevated CO2 and warming in an Australian native grassland soil. Environ. Microbiol. 14, 3081–3096 (2012).
Allison, S. D. A trait-based approach for modelling microbial litter decomposition. Ecol. Lett. 15, 1058–1070 (2012). This article describes a trait-based modelling approach that links phylogenetic and functional information to predict ecosystem processes carried out by microbial communities.
Tu, Q. et al. Metagenomic reconstruction of nitrogen cycling pathways in a CO2-enriched grassland ecosystem. Soil Biol. Biochem. 106, 99–108 (2017).
Yu, H. et al. Elevated CO2 and warming altered grassland microbial communities in soil top-layers. Front. Microbiol. 9, 1790 (2018). This study uses a gene array to measure the functional gene composition, structure and metabolic potential of soil microbial communities under warming, eCO 2 and eCO 2 with warming conditions in a semi-arid grassland.
Adair, C. E., Reich, P. B., Trost, J. J. & Hobbie, S. E. Elevated CO2 stimulates grassland soil respiration by increasing carbon inputs rather than by enhancing soil moisture. Glob. Chang. Biol. 17, 3546–3563 (2011).
Bréchet, L. M. et al. Distinct responses of soil respiration to experimental litter manipulation in temperate woodland and tropical forest. Ecol. Evol. 8, 3787–3796 (2018).
Bengtson, P., Barker, J. & Grayston, S. J. Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol. Evol. 2, 1843–1852 (2012).
Jansson, C., Vogel, J., Hazen, S., Brutnell, T. & Mockler, T. Climate-smart crops with enhanced photosynthesis. J. Exp. Bot. 69, 3801–3809 (2018).
Qiao, N. et al. Labile carbon retention compensates for CO2 released by priming in forest soils. Glob. Chang. Biol. 20, 1943–1954 (2014).
van Groenigen, K. J., Qi, X., Osenberg, C. W., Luo, Y. Q. & Hungate, B. A. Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 344, 508–509 (2014).
Drake, J. E. et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 14, 349–357 (2011).
Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627 (2004). This paper discusses different land management strategies to optimize soil carbon sequestration through soil microbial activities.
Kuzyakov, Y. Priming effects: interactions between living and dead organic matter. Soil Biol. Biochem. 42, 1363–1371 (2010).
Scharlemann, J. P. W., Tanner, E. V. J., Hiederer, R. & Kapos, V. Global soil carbon: understanding and managing the largest terrestrial carbon pool. Carbon Manag. 5, 81–91 (2014).
Melillo, J. M. et al. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 358, 101–104 (2017). This article describes the results of long-term warming field studies at the Harvard Forest field station on soil carbon flux and how the soil microorganisms acclimate to the warmer soil conditions.
Romero-Olivares, A. L., Allison, S. D. & Treseder, K. K. Soil microbes and their response to experimental warming over time: a meta-analysis of field studies. Soil Biol. Biochem. 107, 32–40 (2017).
Schindlbacher, A. et al. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol. Biochem. 43, 1417–1425 (2011).
Clemmensen, K. E. et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1615–1618 (2013).
Allison, S. D. & Treseder, K. K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 14, 2898–2909 (2008).
Morrissey, E. M. et al. Evolutionary history constrains microbial traits across environmental variation. Nat. Ecol. Evol. 3, 1064–1069 (2019).
DeAngelis, K. M. et al. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 6, 104 (2015).
Zhang, B. et al. Responses of soil microbial communities to experimental warming in alpine grasslands on the Qinghai–Tibet Plateau. PLOS ONE 8, E103859 (2014).
Deslippe, J. R., Hartmann, M., Simard, S. W. & Mohn, W. W. Long-term warming alters the composition of Arctic soil microbial communities. FEMS Microbiol. Ecol. 82, 303–315 (2012).
Guo, X. et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Change 8, 813–818 (2018).
Frey, S. D., Drijber, R., Smith, H. & Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 40, 2904–2907 (2008).
Zhou, J. et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Change 2, 106–110 (2011).
Heimann, M. & Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451, 289–292 (2008). This article provides evidence and discusses uncertainties about terrestrial ecosystem feedback with climate change.
Turetsky, M. R. et al. Permafrost collapse is accelerating carbon release. Nature 569, 32–34 (2019).
Mackelprang, R., Saleska, S. R., Jacobsen, C. S., Jansson, J. K. & Taş, N. Permafrost meta-omics and climate change. Annu. Rev. Earth Planet. Sci. 44, 439–462 (2016).
Hultman, J. et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 521, 208–212 (2015).
Tas, N. et al. Landscape topography structures the soil microbiome in Arctic polygonal tundra. Nat. Commun. 9, 777 (2018).
Woodcroft, B. J. et al. Genome-centric view of carbon processing in thawing permafrost. Nature 560, 49–54 (2018). This study assembles thousands of genomes from soil metagenomes, corresponding to primarily uncultivated and uncharacterized microorganisms, along a permafrost thaw gradient in Sweden.
Johnston, E. R. et al. Responses of tundra soil microbial communities to half a decade of experimental warming at two critical depths. Proc. Natl Acad. Sci. USA 116, 15096–15105 (2019).
Bottos, E. M. et al. Dispersal limitation and thermodynamic constraints govern spatial structure of permafrost microbial communities. FEMS Microbiol. Ecol. 94, 1–14 (2018).
Mackelprang, R. et al. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 480, 368–371 (2011).
Müller, O. et al. Disentangling the complexity of permafrost soil by using high resolution profiling of microbial community composition, key functions and respiration rates. Environ. Microbiol. 20, 4328–4342 (2018).
Singleton, C. M. et al. Methanotrophy across a natural permafrost thaw environment. ISME J. 12, 2544–2558 (2018).
Emerson, J. B. et al. Host-linked soil viral ecology along a permafrost thaw gradient. Nat. Microbiol. 3, 870–880 (2018).
Trubl, G. et al. Soil viruses are underexplored players in ecosystem carbon processing. mSystems 3, e00076-18 (2018). This study characterizes viruses in thawing permafrost peatlands and suggests that viruses can impact the biogeochemistry of their hosts and carbon metabolism.
Penton, C. R. et al. Fungal diversity in permafrost and tallgrass prairie soils under experimental warming conditions. Appl. Environ. Microb. 79, 7063–7072 (2013).
Schütte, U. M. E. et al. Effect of permafrost thaw on plant and soil fungal community in a boreal forest: does fungal community change mediate plant productivity response? J. Ecol. 107, 1737–1752 (2019).
Cook, B. I., Ault, T. R. & Smerdon, J. E. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Science Adv. 1, e1400082 (2015).
Huang, J. P., Yu, H. P., Guan, X. D., Wang, G. Y. & Guo, R. X. Accelerated dryland expansion under climate change. Nat. Clim. Change 6, 166–171 (2016).
McHugh, T. A. et al. Climate controls prokaryotic community composition in desert soils of the southwestern United States. FEMS Microbiol. Ecol. 93, fix116 (2017).
Schimmel, J. P. Life in dry soils: effects of drought on soil microbial communities and processes. Ann. Rev. Ecol. Evol. Syst. 49, 409–432 (2018). This article reviews current knowledge of microbial community dynamics and physiological responses to drought.
Hyvonen, R. et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol. 173, 463–480 (2007).
Pointing, S. B. & Belnap, J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 10, 551–562 (2012).
Garcia-Pichel, F., Johnson, S. L., Youngkin, D. & Belnap, J. Small-scale vertical distribution of bacterial biomass and diversity in biological soil crusts from arid lands in the Colorado Plateau. Microb. Ecol. 46, 312–321 (2003).
Steven, B., Kuske, C. R., Gallegos-Graves, L. V., Reed, S. C. & Belnap, J. Climate change and physical disturbance manipulations result in distinct biological soil crust communities. Appl. Environ. Microb. 81, 7448–7459 (2015).
Reed, S. C. et al. in Biological Soil Crusts: An Organizing Princincipal in Drylands (eds Weber, B., Büdel, B. & Belnap, J.) 451–476 (Springer, 2016).
Upton, R. N., Bach, E. M. & Hofmockel, K. S. Belowground response of prairie restoration and resiliency to drought. Agric. Ecosyst. Environ. 266, 122–132 (2018).
de Vries, F. T. et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9, 3033 (2018). This study uses network analyses to show that bacterial networks are less resilient to drought than fungal networks, suggesting that bacteria are less stable to environmental change than fungi.
Treseder, K. K., Berlemont, R., Allison, S. D. & Martiny, A. C. Drought increases the frequencies of fungal functional genes related to carbon and nitrogen acquisition. PLOS ONE 13, e0206441 (2018).
Carson, J. K. et al. Low pore connectivity increases bacterial diversity in soil. Appl. Environ. Microb. 76, 3936–3942 (2010).
Dechesne, A., Wang, G., Gülez, G., Or, D. & Smets, B. F. Hydration-controlled bacterial motility and dispersal on surfaces. Proc. Natl Acad. Sci. USA 107, 14369–14372 (2010).
Guhr, A., Borken, W., Spohn, M. & Matzner, E. Redistribution of soil water by a saprotrophic fungus enhances carbon mineralization. Proc. Natl Acad. Sci. USA 112, 14647–14651 (2015).
Barnard, R. L., Osborne, C. A. & Firestone, M. K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 7, 2229–2241 (2013). This study finds different responses of potentially active bacterial and fungal communities to desiccation and re-wetting across three grassland sites in California, United States.
Boot, C. M., Schaeffer, S. M. & Schimel, J. P. Static osmolyte concentrations in microbial biomass during seasonal drought in a California grassland. Soil Biol. Biochem. 57, 356–361 (2013).
Kakumanu, M. L., Cantrell, C. L. & Williams, M. A. Microbial community response to varying magnitudes of desiccation in soil: a test of the osmolyte accumulation hypothesis. Soil Biol. Biochem. 57, 644–653 (2013).
Meisner, A., Leizeaga, A., Rousk, J. & Bååth, E. Partial drying accelerates bacterial growth recovery to rewetting. Soil Biol. Biochem. 112, 269–276 (2017). Based on experiments and other recent results, this article proposes a framework for microbial response patterns after drying–re-wetting, in which the harshness of drying determines the response pattern of bacteria upon re-wetting dried soils.
Bouskill, N. J. et al. Belowground response to drought in a tropical forest soil. II. Change in microbial function impacts carbon composition. Front. Microbiol. 7, 323 (2016).
Naylor, D., DeGraaf, S., Purdom, E. & Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 11, 2691–2704 (2017).
Unger, S., Maguas, C., Pereira, J., David, T. & Werner, C. The influence of precipitation pulses on soil respiration—assessing the “Birch effect” by stable carbon isotopes. Soil Biol. Biochem. 42, 1800–1810 (2010).
Birch, H. F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10, 9–31 (1958).
Blazewicz, S. J., Schwartz, E. & Firestone, M. K. Growth and death of bacteria and fungi underlie rainfall-induced carbon dioxide pulses from seasonally dried soil. Ecology 95, 1162–1172 (2014). This study uses stable isotope probing with H 2 18O combined with quantitative PCR to determine the dynamics of growing and dying bacterial and fungal populations following soil desiccation and re-wetting.
Stovicek, A., Kim, M., Or, D. & Gillor, O. Microbial community response to hydration–desiccation cycles in desert soil. Sci. Rep. 7, 45735 (2017).
Schaeffer, S. M., Homyak, P. M., Boot, C. M., Roux-Michollet, D. & Schimel, J. P. Soil carbon and nitrogen dynamics throughout the summer drought in a California annual grassland. Soil Biol. Biochem. 115, 54–62 (2017). Using a long-term field experiment in annual dry grasslands, this study demonstrates a positive relationship between drought length, microbial biomass carbon and nitrogen, extractable carbon and nitrogen, and microbial activity upon re-wetting.
Neilson, J. W. et al. Significant impacts of increasing aridity on the arid soil microbiome. mSystems 2, e00195-16 (2017).
Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Pachauri, R. & Meyer, L.) (IPCC, 2014).
Sorensen, P. O., Templer, P. H. & Finzi, A. C. Contrasting effects of winter snowpack and soil frost on growing season microbial biomass and enzyme activity in two mixed-hardwood forests. Biogeochemistry 128, 141–154 (2016).
Roy Chowdhury, T. et al. Metaphenomic responses of a native prairie soil microbiome to moisture perturbations. mSystems 4, e00061-19 (2019).
Gedney, N., Cox, P. M. & Huntingford, C. Climate feedback from wetland methane emissions. Geophys. Res. Lett. 31, L20503 (2004).
Moomaw, W. R. et al. Wetlands in a changing climate: science, policy and management. Wetlands 38, 183–205 (2018).
Chambers, L. G., Osborne, T. Z. & Reddy, K. R. Effect of salinity-altering pulsing events on soil organic carbon loss along an intertidal wetland gradient: a laboratory experiment. Biogeochemistry 115, 363–383 (2013).
Steinmuller, H. E. & Chambers, L. G. Can saltwater intrusion accelerate nutrient export from freshwater wetland soils? An experimental approach. Soil Sci. Soc. Am. J. 82, 283–29 (2018).
Sjogaard, K. S., Valdemarsen, T. B. & Treusch, A. H. Responses of an agricultural soil microbiome to flooding with seawater after managed coastal realignment. Microorganisms 6, 1–18 (2018).
Knelman, E. J., Schmidt, K. S., Garayburu-Caruso, V., Kumar, S. & Graham, B. E. Multiple, compounding disturbances in a forest ecosystem: fire increases susceptibility of soil edaphic properties, bacterial community structure, and function to change with extreme precipitation event. Soil Syst. 3, 1–16 (2019).
Sun, H. et al. Fungal community shifts in structure and function across a boreal forest fire chronosequence. Appl. Environ. Microb. 81, 7869–7880 (2015).
Hart, S. C., DeLuca, T., Newman, G., MacKenzie, D. & Boyle, S. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 220, 166–184 (2005).
Nave, L. E., Vance, E. D., Swanston, C. W. & Curtis, P. S. Fire effects on temperate forest soil C and N storage. Ecol. Appl. 21, 1189–1201 (2011).
Hinojosa, M. B., Parra, A., Laudicina, V. A. & Moreno, J. M. Post-fire soil functionality and microbial community structure in a mediterranean shrubland subjected to experimental drought. Sci. Total Environ. 573, 1178–1189 (2016).
Tas, N. et al. Impact of fire on active layer and permafrost microbial communities and metagenomes in an upland Alaskan boreal forest. ISME J. 8, 1904–1919 (2014).
Goberna, M., García, C., Insam, H., Hernández, M. T. & Verdú, M. Burning fire-prone mediterranean shrublands: immediate changes in soil microbial community structure and ecosystem functions. Microb. Ecol. 64, 242–255 (2012).
DeLuca, T. H. & Sala, A. Frequent fire alters nitrogen transformations in ponderosa pine stands of the inland northwest. Ecology 87, 2511–2522 (2006).
Bowker, M. A., Belnap, J., Rosentreter, R. & Graham, B. Wildfire-resistant biological soil crusts and fire-induced loss of soil stability in Palouse prairies, USA. Appl. Soil Ecol. 26, 41–52 (2004).
Averill, C., Turner, B. L. & Finzi, A. C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505, 543–545 (2014).
Hicks, N. et al. Using prokaryotes for carbon capture storage. Trends Biotechnol. 35, 22–32 (2017).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011). This article describes a paradigm shift in understanding the composition of persistent SOCs, including determination of microbial cell macromolecules that persist in soil.
Kapilan, R., Vaziri, M. & Zwiazek, J. J. Regulation of aquaporins in plants under stress. Biol. Res. 51, 4 (2018).
Jansson, C., Tuskan, G. A., Wullschleger, S. D. & Kalluri, U. C. Phytosequestration: carbon biosequestration by plants and the prospects of genetic engineering. Bioscience 60, 685–696 (2010).
Wallenstein, M. D. Managing and manipulating the rhizosphere microbiome for plant health: a systems approach. Rhizosphere 3, 230–232 (2017). This article discusses several strategies to harness soil microorganisms in the plant rhizosphere to store soil carbon and help mitigate negative consequences of rising atmospheric CO 2 levels.
Lakshmanan, V., Ray, P. & Craven, K. D. Toward a resilient, functional microbiome: drought tolerance-alleviating microbes for sustainable agriculture. Methods Mol. Biol. 1631, 69–84 (2017).
Naylor, D. & Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 8, 2223 (2018).
Compant, S., van der Heijden, M. G. A. & Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 73, 197–214 (2010).
Vurukonda, S. S. K. P., Vardharajula, S., Shrivastava, M. & SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 184, 13–24 (2016).
Armada, E., Azcon, R., Lopez-Castillo, O. M., Calvo-Polanco, M. & Ruiz-Lozano, J. M. Autochthonous arbuscular mycorrhizal fungi and Bacillus thuringiensis from a degraded Mediterranean area can be used to improve physiological traits and performance of a plant of agronomic interest under drought conditions. Plant Physiol. Biochem. 90, 64–74 (2015).
Pereyra, M. A., García, P., Colabelli, M. N., Barassi, C. A. & Creus, C. M. A better water status in wheat seedlings induced by Azospirillum under osmotic stress is related to morphological changes in xylem vessels of the coleoptile. Appl. Soil Ecol. 53, 94–97 (2012).
Casanovas, E. M., Barassi, C. & Sueldo, R. J. Azospirillum inoculation mitigates water stress effects in maize seedlings. JSTOR 30, 343–350 (2002).
Quiroga, G., Erice, G., Aroca, R., Chaumont, F. & Ruiz-Lozano, J. M. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 8, 1056 (2017).
Itakura, M. et al. Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat. Clim. Change 3, 208–212 (2012).
Subbarao, G. V. et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl Acad. Sci. USA 106, 17302–17307 (2009).
National Academies of Sciences, Engineering and Medicine. Science Breakthroughs to Advance Food and Agricultural Research by 2030 (National Academies, 2019).
Lal, R. Soil erosion and the global carbon budget. Environ. Int. 29, 437–450 (2003).
Cavicchioli, R. et al. Scientists’ warning to humanity: microorganisms and climate change. Nat. Rev. Microbiol. 17, 569–586 (2019). This work presents a petition for a call to action to better understand and predict how climate change will impact crucial processes currently carried out by Earth’s microorganisms across a range of ecosystems.
Intergovernmental Panel on Climate Change. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri, R. K. & Reisinger, A.) (IPCC, 2007).
Schuur, E. A. G. et al. Climate change and the permafrost carbon feedback. Nature 520, 171–179 (2015). This article discusses evidence for gradual and sustained greenhouse gas emissions from permafrost as the climate warms. The authors also discuss the need for more integration of data with models to improve climate change predictions.
Stocker, T. F. et al. Climate Change 2013. The Physical Science Basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge Univ. Press, 2014).
Llado, S., Lopez-Mondejar, R. & Baldrian, P. Forest soil bacteria: diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 81, e00063 (2017).
Kirschbaum, M. U. F. Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry 48, 21–51 (2000).
Ramankutty, N., Evan, A. T., Monfreda, C. & Foley, J. A. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Glob. Biogeochem. Cycles 22, GB1003 (2008).
Jones, D. L., Nguyen, C. & Finlay, R. D. Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321, 5–33 (2009).
Tian, H. et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 531, 225–228 (2016).
Zhang, Z. et al. Emerging role of wetland methane emissions in driving 21st century climate change. Proc. Natl Acad. Sci. USA 36, 9647–9652 (2017). This study determines the potential sensitivities of terrestrial wetlands to rising temperatures, and emphasizes the importance of including CH 4 feedback from wetlands in climate models.
Makhalanyane, T. P. et al. Microbial ecology of hot desert edaphic systems. FEMS Microbiol. Rev. 39, 203–221 (2015). This article reviews the microbial communities of hot desert terrestrial biotopes, the processes that govern their assembly, the possible effects of global climate change on hot desert microbial communities and the resulting feedback and directions for future research.
Acknowledgements
This research was supported by the US Department of Energy Office of Biological and Environmental Research (BER) and is a contribution to the Scientific Focus Area ‘Phenotypic response of the soil microbiome to environmental perturbations’. Pacific Northwest National Laboratory is operated for the Department of Energy by Battelle Memorial Institute under contract DE-AC05-76RLO1830.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Permafrost
-
Soil that has been frozen for at least 2 consecutive years.
- Carbon use efficiency
-
The difference between the amount of carbon respired as CO2 and that incorporated into the cellular biomass.
- r-Strategists
-
Species that typically have high growth rates and are able to respond quickly to resources as they become available.
- K-Strategists
-
Species that typically are slow growing and adapted to utilize minimal resources.
- Metaphenome
-
A community phenotype that is the product of genomic potential encoded in metagenomes and the environmental conditions that govern which genes are expressed.
- C4 plants
-
Plants that fix CO2 into a four-carbon compound (in addition to a three-carbon compound) and that have high photosynthetic efficiency due to an absence of photorespiration.
- C3 plants
-
Plants that fix CO2 into a three-carbon compound and that have a lower photosynthetic efficiency than C4 plants.
- Metagenome-assembled genomes
-
(MAGs). Genomes that are derived from assembled metagenome data; often using a process called ‘binning’.
- Auxiliary metabolic genes
-
Genes on viral sequences (genomes or contigs) that represent non-viral metabolic genes, such as genes involved in carbon metabolism.
- Matric potentials
-
The potential energy of water that is due to adhesion of water molecules to soil particles.
- Albedo
-
The amount of light or radiation that is reflected from a surface.
- Necromass
-
Residue mixtures of molecules derived from microorganisms, including biomass, intracellular and extracellular biomolecules/aggregations.
- Biochar
-
Fire-derived (pyrolysed) carbon (also known as black carbon) that has been proposed as a soil carbon-storage amendment.
Rights and permissions
About this article
Cite this article
Jansson, J.K., Hofmockel, K.S. Soil microbiomes and climate change. Nat Rev Microbiol 18, 35–46 (2020). https://doi.org/10.1038/s41579-019-0265-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-019-0265-7
This article is cited by
-
Response mechanism of soil microorganisms to simulated precipitation in the source wetland of Qinghai Lake
Ecological Processes (2024)
-
Programmed microalgae-gel promotes chronic wound healing in diabetes
Nature Communications (2024)
-
River water quality shaped by land–river connectivity in a changing climate
Nature Climate Change (2024)
-
The interplay between microbial communities and soil properties
Nature Reviews Microbiology (2024)
-
Microbially mediated mechanisms underlie soil carbon accrual by conservation agriculture under decade-long warming
Nature Communications (2024)