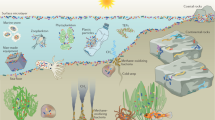
Abstract
Biofilms are a form of collective life with emergent properties that confer many advantages on their inhabitants, and they represent a much higher level of organization than single cells do. However, to date, no global analysis on biofilm abundance exists. We offer a critical discussion of the definition of biofilms and compile current estimates of global cell numbers in major microbial habitats, mindful of the associated uncertainty. Most bacteria and archaea on Earth (1.2 × 1030 cells) exist in the ‘big five’ habitats: deep oceanic subsurface (4 × 1029), upper oceanic sediment (5 × 1028), deep continental subsurface (3 × 1029), soil (3 × 1029) and oceans (1 × 1029). The remaining habitats, including groundwater, the atmosphere, the ocean surface microlayer, humans, animals and the phyllosphere, account for fewer cells by orders of magnitude. Biofilms dominate in all habitats on the surface of the Earth, except in the oceans, accounting for ~80% of bacterial and archaeal cells. In the deep subsurface, however, they cannot always be distinguished from single sessile cells; we estimate that 20–80% of cells in the subsurface exist as biofilms. Hence, overall, 40–80% of cells on Earth reside in biofilms. We conclude that biofilms drive all biogeochemical processes and represent the main way of active bacterial and archaeal life.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Costerton, J. W. et al. Bacterial biofilms in nature and disease. Ann. Rev. Microbiol. 41, 435–464 (1987).
Costerton, J. W. et al. Microbial biofilms. Ann. Rev. Microbiol. 49, 711–745 (1995).
Lappin-Scott, H. M. & Costerton, J. W. (eds) Microbial Biofilms (Cambridge Univ. Press, 1995).
Stoodley, P., Sauer, K., Davies, D. G. & Costerton, J. W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209 (2002).
Davey, M. E. & O’toole, G. A. Microbial biofilms: from ecology to molecular genetics. Microb. Mol. Biol. Rev. 64, 847–867 (2000).
Watnick, R. & Kolter, R. Biofilm, city of microbes. J. Bacteriol. 182, 2675–2679 (2000).
Donlan, R. M. & Costerton, J. W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193 (2002).
Krumbein, W. E. et al. in Fossil and Recent Biofilms (eds Krumbein, W. E., Paterson, D. M. & Zavarzin, G. A.) 1–27 (Springer Science & Business Media, 2003).
Costerton, J. W. The Biofilm Primer 169–195 (Springer Science & Business Media, 2007).
Flemming, H.-C. et al. Biofilms: an emergent form of microbial life. Nat. Rev. Microbiol. 14, 563–575 (2016).
Battin, T., Besemer, K., Bengtsson, M. M., Romani, A. & Packman, A. I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 14, 251–263 (2016).
Tuson, H. H. & Weibel, D. B. Bacteria-surface interactions. Soft Matter 9, 4368–4380 (2013). This study explains the influence of surfaces on attached cells and biofilms — revisited after ZoBell.
Woodcock, S. & Sloan, W. T. Biofilm community succession: a neutral perspective. Microbiology 63, 664–668 (2017).
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115, 6506–6511 (2018). This paper takes a comprehensive and thorough approach to assess the biomass on Earth. It is one of the few publications in which the supplementary material is a gold mine of information, particularly on methods of quantification.
Magnabosco, C. et al. The biomass and biodiversity of the continental subsurface. Nat. Geosci. 11, 707–717 (2018). This paper presents a metastudy on 200 publications on continental subsurface cell concentrations, even providing a history of biomass estimates and also providing a highly valuable and comprehensive supplement.
Conselice, J., Wilkinson, A., Duncan, K. & Mortlock, A. The evolution of galaxy number density AT z >8 and its implications. Astrophys. J. 830, 83 (2016).
Orell, A., Fröls, S. & Albers, S.-V. Archaeal biofilms: the great unexplored. Annu. Rev. Microbiol. 67, 337–354 (2013).
Fröls, S. Archaeal biofilms: widespread and complex. Biochem. Soc. Trans. 41, 393–398 (2013).
Van Wolferen, M., Orell, A. & Albers, S.-V. Archaeal biofilm formation. Nat. Rev. Microbiol. 16, 699–713 (2018).
Pedersen, K. & Ekendahl, S. Incorporation of CO2 and introduced organic compounds by bacterial populations from the deep crystalline bedrock of the Stripa mine. J. Gen. Microbiol. 138, 369–376 (1992).
McMahon, S. & Parnell, J. Weighing the deep continental biosphere. FEMS Microbiol. Ecol. 87, 113–120 (2013). This study, inspired by Whitman et al. (1998), presents an excellent approach to quantifying cells in the deep subsurface.
Schrenk, M. O., Huber, J. A. & Edwards, K. J. Microbial provinces in the subseafloor. Annu. Rev. Mar. Sci. 2, 279–304 (2010). This paper describes places where microorganisms can live in rocks.
Heberling, C., Lowell, R. P., Liu, L. & Fisk, M. R. Extent of the microbial biosphere in the oceanic crust. Geochem. Geophys. Geosys. 11, Q08003 (2010).
Menez, B., Pasini, V. & Brunelli, D. Life in the hydrated suboceanic mantle. Nat. Geosci. 5, 133–137 (2012).
Edwards, K. J., Wheat, C. G. & Sylvain, J. B. Under the sea: microbial life in the volcanic crust. Nat. Rev. Microbiol. 9, 703–712 (2011). This paper is a very well-written treatise on life below the ocean.
Johnson, H. P. & Pruis, M. J. Fluxes of fluid and heat from the oceanic crustal reservoir. Earth Planet. Sci. Lett. 216, 565–574 (2003).
Lloyd, K. G., May, M. K., Kevorkian, R. T. & Steen, A. D. Meta-analysis of quantification methods shows that archaea and bacteria have similar abundances in the subseafloor. Appl. Environ. Microbiol. 79, 7790–7799 (2013).
Danovaro, R., Corinaldesi, C., Rastelli, E. & Dell Anno, A. Towards a better quantitative assessment of the relevance of deep-sea viruses, Bacteria and Archaea in the functioning of the ocean seafloor. Aquat. Microb. Ecol. 75, (81–90 (2015).
Lipp, J. S., Morono, Y., Inagaki, F. & Hinrichs, K.-U. Significant contribution of Archaea to extent biomass in marine subsurface sediments. Nature 454, 991–994 (2008).
Justice, N. B. et al. Heterotrophic archaea contribute to carbon cycling in low-pH, suboxic biofilm communities. Appl. Environ. Microbiol. 78, 8321–8330 (2012).
Xie, S., Lipp, J. S., Wegener, G., Ferdelman, T. G. & Hinrichs, K.-U. Turnover of microbial lipids in the deep biosphere and growth of benthic archaeal populations. Proc. Natl Acad. Sci. USA 110, 6010–6014 (2013).
Griebler, C., Mindl, B., Slezak, D. & Geiger-Kaiser, M. Distribution patterns of attached and suspended bacteria in pristine and contaminated shallow aquifers studied with an in situ sediment exposure microcosm. Aquat. Microb. Ecol. 28, 117–129 (2002).
Biddle, J., Jungbluth, S. P., Lever, M. A. & Rappe, M. S. in Microbial Life of the Deep Biosphere (eds Kallmeyer, J. & Wagner, D.) 29–62 (De Gruyter, Berlin, 2014).
Morono, Y. & Inagaki, F. Analysis of low-biomass microbial communities in the deep biosphere. Adv. Appl. Microbiol. 95, 149–178 (2016). This paper presents a comprehensive overview of methods to determine biomass in the subsurface.
Jørgensen, B. B. & Boetius, A. Feast and famine — microbial life in the deep-sea bed. Nat. Rev. Microbiol. 5, 770–781 (2007).
Santelli, C. M. et al. Abundance and diversity of microbial life in the ocean crust. Nature 453, 653–656 (2008).
Whitman, W. B., Coleman, D. C. & Wiebe, W. J. Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. USA 95, 6578–6583 (1998). This paper, the classic on the global census of microorganisms, reflects the fun the authors must have had while writing it.
Kallmeyer, J., Pockalny, R., Adhikari, R. R., Smith, D. C. & D’Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc. Natl Acad. Sci. USA 109, 16213–16216 (2012). This study is a thoughtful and complex revisit of numbers from Whitman et al. (1998) that is based on their own measurements.
Parkes, R. J. et al. A review of prokaryotic populations and processes in sub-seafloor sediments, including biosphere:geosphere interactions. Mar. Geol. 352, 409–425 (2014). This paper presents an excellent review on how deeply buried prokaryotes influence the surface processes on Earth.
Colwell, F. S. & D’Hondt, S. Nature and extent of the deep biosphere. Rev. Miner. Geochem. 75, 547–574 (2013).
Pedersen, K. Exploration of deep intraterrestrial microbial life: current perspectives. FEMS Microbiol. Lett. 185, 9–16 (2000).
Thorseth, I. H. et al. Diversity of life in ocean floor basalt. Earth Planet. Sci. Lett. 194, 31–37 (2001).
Boetius, A. et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626 (2001).
Trembath-Reichert, E. et al. Methyl-compound use and slow growth characterize microbial life in 2-km-deep subseafloor coal and shale beds. Prod. Natl Acad. Sci. USA 114, E9206–E9215 (2017).
Gerbersdorf, S. & Wieprecht, S. Biostabilization of cohesive sediments: revisiting the role of abiotic conditions, physiology and diversity of microbes, polymeric secretion, and biofilm architecture. Geobiology 15, 68–97 (2017).
Schippers, A. et al. Prokaryotic cells of the deep sub-seafloor biosphere identified as living bacteria. Nature 433, 861–864 (2017).
Orsi, W. D. Ecology and evolution of seafloor and subseafloor microbial communities. Nat. Rev. Microbiol. 16, 671–683 (2018). This paper presents a valuable resource about various paths of microbial metabolism in the subsurface.
Ramirez-Llodra, E. et al. Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences 7, 2851–2899 (2010).
Hoehler, T. & Jørgensen, B. B. Microbial life under extreme energy limitation. Nat. Rev. Microbiol. 11, 83–94 (2013). This paper presents in-depth considerations of the ways of life and survival in the deep subsurface.
Røy, H. et al. Aerobic microbial respiration in 86-million-year-old deep-sea red clay. Science 336, 922–925 (2012).
Edwards, K. J., Bach, W. & McCollom, T. M. Geomicrobiology in oceanography: microbe-mineral interactions at and below the seafloor. Trends Microbiol. 13, 449–456 (2005). This paper presents a view on the microbial traffic between the deep subsurface and the sea floor.
Colwell, F. S. & Smith, R. P. in The Subseafloor Biosphere at Mid-Ocean Ridges Vol. 144 (eds Wilcock, W. S. D., Delong, E. F., Kelley, D. S., Baross, J. A. & Cary, S. C.) 355–367 (2013).
Sharma, A. et al. Microbial activity at gigapascal pressures. Science 295, 1514–1516 (2002).
Siliakus, M. F., van der Oost, J. & Kengen, S. W. M. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 21, 651–670 (2017).
Fang, J. et al. Predominance of viable spore-forming piezophilic bacteria in high-pressure enrichment cultures from ~1.5 to 2.4 km-deep coal-bearing sediments below the ocean floor. Front. Microbiol. 8, 137 (2017).
Starnawski, P. et al. Microbial community assembly and evolution in subseafloor sediment. Proc. Natl Acad. Sci. USA 114, 2940–2945 (2017).
Brazelton, W. J., Morrill, P. L., Szponar, N. & Schrenk, M. O. Bacterial communities associated with subsurface geochemical processes in continental serpentinite springs. Appl. Environ. Microbiol. 79, 3906–3916 (2013).
D’Hondt, S. et al. Distribution of microbial activities in deep seafloor sediments. Science 306, 2216–2221 (2004).
Parkes, R. J. et al. Deep sub-seafloor prokaryotes stimulated at interfaces over geological time. Nature 436, 390–394 (2005).
Ciobanu, M.-C. et al. Microorganisms persist at record depths in the subseafloor of the Canterbury Basin. ISME J. 8, 1370–1380 (2014).
Morono, Y. et al. Carbon and nitrogen assimilation in deep subseafloor microbial cells. Proc. Natl Acad. Sci. USA 108, 18925–18300 (2011).
Johnson, S. S. et al. Ancient bacteria show evidence of DNA repair. Proc. Natl Acad. Sci. USA 104, 14401–14405 (2007).
Marshall, K. C. Bacterial adhesion in oligotrophic habitats. Microbiol. Sci. 2, 321–326 (1985).
Lever, M. A. et al. Life under extreme energy limitation: a synthesis of laboratory and field-based investigations. FEMS Microbiol. Rev. 39, 688–728 (2015).
Dang, H. & Lovell, C. R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 80, 91–138 (2016). This paper presents an excellent overview of interfaces with cell aggregates in the ocean.
Orcutt, B. N. et al. Microbial activity in the marine deep biosphere: progress and prospects. Front. Microbiol. 4, 189 (2013). This paper presents a compilation of ample evidence that the deep marine biosphere is alive.
Orcutt, B. N., Sylvan, J. B., Knab, N. J. & Edwards, K. J. Microbial ecology of the dark ocean above, at, and below the seafloor. Microb. Mol. Biol. Rev. 75, 361–422 (2011). This paper presents a comprehensive overview of life in the deep ocean biosphere.
Teske, A. P. The deep subsurface biosphere is alive and well. Trends Microbiol. 13, 402–404 (2005).
Gold, T. The deep, hot biosphere. Proc. Natl Acad. Sci. USA 89, 6045–6049 (1992). This seminal, strongly argued and visionary paper turns the attention to the borders of life in the deep depths.
Kieft, T. L. in Their World: A Diversity of Microbial Environments (ed. Hurst, C. J.) 225–253 (Springer Int. Publ., Switzerland, 2016).
Ehrlich, H. L., Newman, D. K. & Kappler, A. (eds) Ehrlich’s Geomicrobiology 6th edn (CRC Press, Boca Raton, 2015).
Beatty, J. T. et al. An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proc. Natl Acad. Sci. USA 102, 9306–9310 (2005).
Osburn, M. R., LaRowe, D. E., Momper, L. M. & Amend, J. P. Chemolithotrophy in the continental deep subsurface: Sanford Underground Research Facility (SURF), USA. Front. Microbiol. 5, 610 (2014).
Ehrlich, H. L. Microbes as geologic agents: their role in mineral formation. Geomicrobiol. J. 16, 135–153 (1999).
Pedersen, K. Microbial life in deep granitic rock. FEMS Microbiol. Rev. 20, 399–414 (1997).
Douglas, S. Mineralogical footprints of microbial life. Am. J. Sci. 305, 503–525 (2005).
Tuck, V. A. et al. Biologically induced clay formation in subsurface granitic environments. J. Geochem. Explor. 90, 123–133 (2006).
Anderson, C., James, R. E., Fru, E. C., Kennedy, C. B. & Pedersen, K. In-situ ecological development of a bacteriogenic iron oxide producing microbial community from a subsurface granitic rock environment. Geobiology 4, 29–42 (2006).
Stevens, T. O. & McKinley, J. P. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 270, 450–454 (1995).
Fredrickson, J. K. & Balkwill, D. L. Geomicrobial processes and biodiversity in the deep terrestrial subsurface. Geomicrobiol. J. 23, 345–356 (2006).
Albrechtsen, H.-J. Distribution of bacteria, estimated by a viable count method, and heterotrophic activity in different size fractions of aquifer sediment. Geomicrobiol. J. 12, 253–264 (1994).
Jägevall, S., Rabe, L. & Pedersen, K. Abundance and diversity of biofilms in natural and artificial aquifers of the Äspö Hard Rock Laboratory, Sweden. Microb. Ecol. 61, 410–422 (2011).
Itävaraa, M. et al. Characterization of bacterial diversity to a depth of 1500m in the Outokumpu deep borehole, Fennoscandian Shield. FEMS Microbiol. Ecol. 77, 295–309 (2011).
Sakurai, K. & Yoshikawa, H. Isolation and identification of bacteria able to form biofilms from deep subsurface environments. J. Nucl. Sci. Technol. 49, 287–292 (2012).
Wanger, G., Southam, G. & Onstott, T. C. Structural and chemical characterization of natural fracture surface from 2.8 km below land surface: biofilms in the deep subsurface. Geomicrobiol. J. 23, 443–452 (2006).
Escudero, C., Vera, M., Oggerin, M. & Amils, R. Active microbial biofilms in deep poor porous continental subsurface rocks. Sci. Rep. 8, 1538 (2018). This paper presents evidence from CARD–FISH and fluorescent lectin-binding assays for biofilm existence in the deep underground.
Gantner, S. et al. In-situ quantification of the spatial scale of calling distances and population density-independent N-acylhomoserine-lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol. Ecol. 56, 188–194 (2006). This elegant study determines the calling distance of microbial aggregates.
Amano, Y., Iwatsuki, T. & Naganuma, T. Characteristics of naturally grown biofilms in deep groundwaters and their heavy metal sorption property in a deep subsurface environment. Geomicrobiol. J. 34, 769–783 (2017).
Leon-Morales, C. F., Leis, A. P., Strathmann, M. & Flemming, H.-C. Interactions between laponite and microbial biofilms in porous media: implications for colloid transport and biofilm stability. Water Res. 38, 3614–3626 (2004).
Coombs, P. et al. The role of biofilms in subsurface transport processes. Q. J. Eng. Geol. Hydrogeol. 43, 131–139 (2010).
Nazina, T. N. et al. Microbiology of formation waters from the deep repository of liquid radioactive wastes Severnyi. FEMS Microbiol. Ecol. 49, 97–107 (2004).
Onstott, T. C., Colwell, F. S., Kieft, T. L., Murdoch, L. & Phelps, T. J. New horizons for deep surface microbiology. Microbe 11, 499–505 (2009).
Meyer-Reil, L. A. Microbial life in sedimentary biofilms — the challenge to microbial ecologists. Mar. Ecol. Prog. Ser. 112, 303–311 (1994).
Petro, C., Starnawski, P., Schramm, A. & Kjeldsen, K. U. Microbial community assembly in marine sediments. Aquat. Microb. Ecol. 79, 177–195 (2017).
Glud, R. et al. High rates of microbial turnover in sediments in the deepest oceanic trench on Earth. Nat. Geosci. 6, 284–288 (2013).
Bowles, M. W., Mogollon, J., Kasten, S., Zabel, M. & Hinrichs, K. U. Global rates of marine sulfate reduction and implications for sub-sea-floor metabolic activities. Science 344, 889–891 (2014).
D’Hondt, S. et al. Presence of oxygen and aerobic communities from seafloor to basement in deep-sea sediments. Nat. Geosci. 8, 299–304 (2015).
Probandt, D. et al. Microbial life on a sand grain. ISME J. 12, 623–633 (2018).
Balzer, M., Witt, N., Flemming, H.-C. & Wingender, J. Accumulation of fecal indicator bacteria in river biofilms. Water Sci. Technol. 61, 1106–1111 (2010).
Weise, W. & Rheinheimer, G. Scanning electron microscopy and epifluorescence investigation of bacterial colonization of marine sand sediments. Microb. Ecol. 4, 175–188 (1978).
Chen, X. D. et al. Stabilizing effects of biofilms: EPS penetration and re-distribution of bed stability down the sediment profile. J. Geophys. Res. Biogeosci. 122, 3113–3125 (2017).
Chen, X. D. et al. Hindered erosion: The biological mediation of noncohesive sediment behaviour. Water Resour. Res. 53, 4787–4801 (2017).
Spadafora, A., Perri, E., McKenzie, J. & Vasconcelos, C. Microbial biomineralization process forming modern Ca:Mg carbonate stromatolites. Sedimentology 57, 27–40 (2010).
Bontognali, T. R. R. et al. Dolomite formation within microbial mats in the coastal sabkha of Abu Dhabi (United Arab Emirates). Sedimentology 57, 824–844 (2010).
Fierer, N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 15, 579–590 (2017).
Raynaud, X. & Nunan, N. Spatial ecology of bacteria at the microscale in soil. PLOS ONE 9, e87217 (2014).
Wei, C.-L. et al. Global patterns and predictions of seafloor biomass using random forests. PLOS ONE 5, e15323 (2010).
Ranjard, L. & Richaume, A. Quantitative and qualitative distribution of bacteria in soil. Res. Microbiol. 152, 707–716 (2001).
Kuzyakov, Y. & Blagodatskaya, L. Microbial hotspots and hot moments in soil: concept and review. Soil Biol. Biochem. 83, 184–199 (2015).
Young, I. M., Crawford, J. W., Nunan, N., Otten, W. & Spiers, A. Microbial distribution in soils: physics and scaling. Adv. Agronom. 100, 81–121 (2008). This is an excellent overview of soil microbiology.
Chotte, J. L., Ladd, J. N. & Amato, N. Sites of microbial assimilation, and turnover of soluble and particulate 14C-labelled substrates decomposing in clay soil. Soil Biol. Biochem. 30, 205–218 (1998).
Flemming, H.-C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Davis, C. A. et al. Microbial-induced heterogeneity in the acoustic properties of porous media. Geophys. Res. Let. 36, L21405 (2009).
Tang, J., Mo, Y., Zhang, J. & Zhang, R. Influence of biological aggregating agents associated with microbial population on soil aggregate stability. Appl. Soil. Ecol. 47, 153–159 (2011).
Cunliffe, M. et al. Sea surface microlayers: a unified physicochemical and biological perspective of the ocean-air interface. Prog. Oceanogr. 109, 104–116 (2013).
Engel, A. et al. The ocean’s vital skin: toward an integrated understanding of the sea surface microlayer. Front. Mar. Sci. 4, 165 (2017).
Aller, J. Y., Kusnetzova, M. R., Jahns, C. J. & Kemp, P. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 36, 801–812 (2005).
Wurl, O. & Cunliffe, M. in The Perfect Slime: Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R., Wingender, J.) 249–268 (IWA Publishing, London, 2016).
Franklin, R. B. et al. Bacterial diversity in the bacterioneuston (sea surface microlayer): the bacterioneuston through the looking glass. Environ. Microbiol. 7, 723–736 (2005).
Wurl, O. & Holmes, M. The gelatinous nature of the sea-surface microlayer. Mar. Chem. 110, 89–97 (2008).
Rahlff, J. et al. High wind speeds prevent formation of a distinct bacterioneuston community in the sea-surface microlayer. FEMS Microb. Ecol. 93, fix041 (2017).
Wurl, O., Ekau, W., Landing, W. M. & Zappa, C. J. Sea surface layer in a changing ocean — a perspective. Elem. Sci. Anth. 5, 31 (2017). This paper presents a very good overview of the neuston microbial community.
Barberàn, A., Henley, J., Fierer, N. & Casamayor, E. O. Structure, inter-annual recurrence and global-scale connectivity of airborne microbial communities. Sci. Total Environ. 487, 187–195 (2014).
Hardy, J. T. The sea surface microlayer: biology, chemistry and anthropogenic enrichment. Prog. Oceanog. 11, 307–328 (1982).
Upstill-Goddard, R. C., Frost, T. & Henry, G. R. Bacterioneuston control of air-water methane exchanged determined with a laboratory gas exchange tank. Glob. Biogeochem. Cycles 17, 1108 (2003).
Hörtnagl, P., Pérez, M. R. & Sommaruga, P. Living at the border: a community and single-cell assessment of lake bacterioneuston activity. Limnol. Oceanogr. 55, 1134–1144 (2010).
Arístegui, J., Gasol, J. M., Duarte, C. M. & Herndl, G. J. Microbial Oceanography of the dark ocean’s pelagic realm. Limnol. Oceanogr. 54, 1501–1529 (2009).
Buitenhuis, E. T. et al. Picoheterotroph (Bacteria and Archaea) biomass distribution in the global ocean. Earth Syst. Sci. Data 4, 101–106 (2012).
Buitenhuis, E. T. et al. Picophytoplankton biomass distribution in the global ocean. Earth Syst. Sci. Data 4, 37–46 (2012). References 128 and 129 present gold mines of marine microbiological data.
Morris, R. M. et al. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420, 806–809 (2002).
Giovannoni, S. J. SAR11 bacteria: the most abundant plankton in the Oceans. Annu. Rev. Mar. Sci. 9, 231–255 (2017).
Flombaum, P. et al. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl Acad. Sci. USA 110, 9824–9829 (2013).
Giovannoni, S. J. et al. Genome streamlining in a cosmopolitan oeanic bacterium. Science 309, 1242–1245 (2005).
Lauro, F. et al. The genomic basis of trophic strategy in marine bacteria. Proc. Natl Acad. Sci. USA 106, 15527–15533 (2009).
Morris, J. J., Johnson, Z. I., Szul, M. J., Keller, M. & Zinser, E. R. Dependence of the cyanobacterium Prochlorococcus on hydrogen peroxide scavenging microbes for growth at the Ocean’s surface. PLOS ONE 6, e16805 (2011).
Becker, S., Singh, A. K., Postius, C., Böger, P. & Ernst, A. Genetic diversity and distribution of periphytic Synechococcus spp. in biofilms and picoplankton of Lake Constance. FEMS Microbiol. Ecol. 49, 181–190 (2004).
Ghiglione, J.-F., Conan, P. & Pujo-Pay, M. Diversity of total and active free-living versus particle-attached bacteria in the euphotic zone of the NW Mediterranean Sea. FEMS Microbiol. Lett. 299, 9–21 (2009).
Moeseneder, M. M., Winter, C. & Herndl, G. J. Horizontal and vertical complexity of attached and free-living bacteria of the eastern Mediterranean Sea, determined by 16s rDNA and 16s rRNA fingerprints. Limnol. Oceanogr. 46, 95–107 (2001).
Lee, S. W., Lee, C. W., Bong, C. W., Narayanan, K. & Sim, E. U. The dynamics of attached and free-living bacterial population in tropical coastal waters. Mar. Freshw. Res. 66, 701–710 (2015).
Milici, M. et al. Diversity and community composition of particle-associated and free-living bacteria in mesopelagic and bathypelagic Southern Ocean water masses: Evidence of dispersal limitation in the Bransfield Strait. Limnol. Oceanogr. 62, 1080–1095 (2017).
Mestre, M., Borrull, E., Sala, M. M. & Gasol, J. M. Patterns of bacterial diversity in the marine planktonic particulate matter continuum. ISME J. 11, 999–1010 (2017).
Zettler, E. R., Mincer, T. J. & Amaral-Zettler, L. A. Life in the ‘plastisphere’: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7148 (2013).
Harrison, J. P., Schratzberger, M., Sapp, M. & Osborn, A. M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 14, 232 (2014).
Lapoussière, A., Michel, C., Starr, M., Gosselin, M. & Poulin, M. Role of free-living and particle-attached bacteria in the recycling and export of organic material in the Hudson Bay system. J. Mar. Syst. 88, 434–455 (2011).
Bruckner, C. G., Bahulikar, R., Rahalkar, M., Schink, B. & Kroth, P. Bacteria associated with benthic diatoms from Lake Constance: phylogeny and influences on diatom growth and secretion of extracellular polymeric substances. Appl. Environ. Microbiol. 74, 7740–7749 (2008).
Wahl, M., Goecke, F., Labes, A., Dobretsov, S. & Weinberger, F. The second skin: ecological role of epibiotic biofilms on marine organisms. Front. Microbiol. 3, 292 (2012). This study comprehensively highlights the role of eukaryotes as substrata for marine biofilms.
Eriksen, M. et al. Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLOS ONE 9, e111913 (2014).
Cózar, A. et al. Plastic debris in the open ocean. Proc. Nat. Acad. Sci. USA 111, 10239–10244 (2014).
Kooi, M., van Nes, E. H., Scheffer, M. & Koelmans, A. A. Ups and downs in the oceans: effect of biofouling on vertical transport of microplastics. Environ. Sci. Technol. 51, 7963–7971 (2017).
Ahmed, T. et al. Biodegradation of plastics: current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 25, 7287–7298 (2018).
Galloway, T. S., Cole, M. & Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 1, 0116 (2017).
Kirstein, I. V. et al. Dangerous hitchhikers? Evidence for pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 120, 1–8 (2016).
Alldredge, A. L. & Silver, M. W. Characteristics, dynamics and significance of marine snow. Prog. Oceanog. 20, 41–82 (1988).
Neu, T. In situ cell and glycoconjugate distribution in river snow studied by confocal laser scanning microscopy. Aquat. Microb. Ecol. 21, 85–95 (2000).
Azam, F. & Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 5, 782–791 (2007). This paper presents a visionary look at microorganisms and oceans.
Thiele, S., Fuchs, B. M., Ammann, R. & Iversen, M. H. Colonization in the photic zone and subsequent changes during sinking determine bacerial community composition in marine snow. Appl. Environ. Microbiol. 81, 1463–1471 (2015).
Gram, L., Grossart, H.-P., Schlinghoff, A. & Kiorboe, T. Possible quorum sensing in marine snow bacteria: production of acylated homoserine lactones by Roseobacter strains isolated from marine snow. Appl. Environ. Microbiol. 68, 4111–4116 (2002).
Eloe, E. A. et al. Compositional differences in particle-associated and free-living microbial assemblages from an extreme deep-ocean environment. Environ. Microbiol. Rep. 3, 449–458 (2011).
Yooseph, S. et al. Genomic and functional adaptation in surface ocean planktonic prokaryotes. Nature 468, 60–67 (2010).
Busch, K. et al. Bacterial colonization and vertical distribution of marine gel particles (TEP and CSP) in the Arctic Fram strait. Front. Microbiol. 4, 166 (2017).
Zäncker, B., Cunliffe, M. & Engel, A. Bacterial community composition in the sea surface microlayer off the Peruvian coast. Front. Microbiol. 9, 2699 (2018).
Decho, A. & Guiterrez, T. Microbial extracellular substances (EPSs) in Ocean systems. Front. Microbiol. 8, 922 (2017). This study sheds light on the importance of the biofilm matrix in ocean processes.
Oberbeckmann, S., Löder, M. G. & Labrenz, M. Marine microplastic-associated biofilms — a review. Environ. Chem. 12, 551–562 (2015). This paper provides an excellent overview on microplastic as an emerging habitat for microbial biofilms.
DeLeon-Rodriguez, N. et al. Microbiome of the upper troposphere: species comoposition and prevalence, effects of tropical storms and atmospheric implications. Prod. Natl Acad. Sci. USA 110, 2575–2580 (2013).
Jones, A. M. & Harrison, R. M. The effects of meteorological factors on atmospheric bioaerosol concentrations — a review. Sci. Total Environ. 326, 151–180 (2004).
Marks, R., Kruczalak, K., Jankowska, K. & Michalska, M. Bacteria and fungi in air over the Gulf of Gdansk and Baltic sea. J. Aerosol Sci. 32, 237–250 (2001).
Morris, C. E., Georgakopoulos, D. & Sands, D. Ice nucleation active bacteria and their potential role in precipitation. J. Phys. IV France 121, 87–103 (2004).
Carotenuto, F. et al. Measurements and modelling of surface-atmosphere exchange of microorganism in Mediterranean grassland. Atmos. Chem. Phys. 17, 14919–14936 (2017).
Després, V. et al. Primary biological aerosol particles in the atmosphere: a review. Tellus B Chem. Phys. Meteorol. 64, 15598 (2012).
Amato, P. et al. Microbial population in cloud water at the Puy de Drôme: Implications for the chemistry of clouds. Atmos. Environ. 39, 4143–4153 (2005).
Deguillaume, L. et al. Microbiology and atmospheric processes: Chemical interactions of primary biological aerosols. Biogeosci. Discuss. 5, 1073–1084 (2008).
Sattler, B., Puxbaum, H. & Psenner, R. Bacterial growth in supercooled cloud droplets. Geophys. Res. Let. 28, 239–242 (2001).
Carpenter, E. J., Lin, S. & Capone, D. G. Bacterial activity in South Pole snow. Appl. Environ. Microbiol. 66, 4514–4517 (2000).
Wainwright, M., Wickramasinghe, N. C., Narlikar, J. V. & Rajaratnam, P. Microorganisms cultured from stratospheric air samples obtained at 41 km. FEMS Microbiol. Lett. 218, 161–165 (2003).
Imshenetsky, A. A., Lysenko, S. V. & Kazakov, G. A. Upper boundary of the biosphere. Appl. Environ. Microbiol. 35, 1–5 (1978).
Burrows, S. M., Elbert, W., Lawrence, M. G. & Pöschl, U. Bacteria in the global atmosphere – part 1: review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 9, 9263–9280 (2009).
Morris, C. E. & Monier, J.-M. The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 41, 429–453 (2003).
Ruinen, J. The phyllosphere: I. An ecologically neglected milieu. Plant Soil 15, 81–109 (1961).
Bringel, F. & Couée, I. Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front. Microbiol. 6, 486 (2015).
Baldotto, L. E. B. & Olivares, F. L. Phylloepiphytic interaction between bacteria and different plant species in a tropical agricultural system. Can. J. Microbiol. 54, 918–931 (2008).
Vorholt, J. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840 (2012).
Vacher, C. et al. The phyllosphere: microbial jungle at the plant-climate interface. Ann. Rev. Ecol. Evol. Syst. 47, 1–24 (2016). This paper is a comprehensive and well-written overview of the complex interactions between plant surfaces and the atmosphere.
Lebeis, S. Greater than the sum of their parts: characterizing plant microbiomes at the community level. Curr. Opin. Plant Biol. 24, 82–86 (2015).
Morris, C. E. & Kinkel, L. L. in Phyllosphere Microbiology (eds Lindow, S. E., Hecht-Poinar, E. I. & Elliott, V. J.) 365–375 (APS Press, 2002).
De Nys, R. et al. Broad spectrum effects of secondary metabolites from the red alga delisea pulchra in antifouling assays. Biofouling 8, 259–271 (1995).
De Vos, W. Microbial biofilms and the human intestinal microbiome. NPJ Biofilms Microbiomes 1, 15005 (2015). This study explains why the human gut can be considered a biofilm.
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 14, e1002533 (2016).
Luckey, T. Introduction to intestinal microecology. Am. J. Clin. Nutr. 25, 1292–1294 (1972).
Ma, L. et al. Rapid quantification of bacteria and viruses in influent, settled water, activated sludge and effluent from a wastewater treatment plant using flow cytometry. Water Sci. Technol. 68, 1763–1769 (2013).
Mateo-Sagasta, J., Raschid-Sally, L. & Thebo, A. in Wastewater: Economic Asset in Urbanizing World (eds Drechsel, P., Qadir, M. & Wichelns, D.) 15–24 (Springer, Netherlands, 2015).
Schultz, J. E. & Breznak, J. A. Heterotrophic bacteria present in hindguts of wood-eating Termites [Reticulitermes flavipes (Kollar)]. Appl. Environ. Microbiol. 35, 930–936 (1978).
Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12, 168–180 (2014).
ZoBell, C. The effect of solid surfaces upon bacterial activity. J. Bact. 46, 39–56 (1943).
Marshall, K. C., Stout, R. & Mitchell, R. Mechanisms of the initial events in the sorption of marine bacteria to surfaces. J. Gen. Microbiol. 68, 337–348 (1971).
Fletcher, M. M. & Floodgate, G. D. An electron-microscopic demonstration of an acidic polysaccahride involved in the adhesion of a marine bacterium to solid surfaces. J. Gen. Microbiol. 74, 325–334 (1973).
Characklis, W. G. Attached microbial growths – I. Attachment and growth. Water Res. 7, 1113–1127 (1973).
Geesey, G. G., Mutch, R. & Costerton, J. W. Sessile bacteria: an important component of the microbial population in small mountain streams. Limnol. Oceanogr. 23, 1214–1224 (1978). This paper introduces the view that biofilms are the place where most bacteria live.
Corning, P. A. The re-emergence of “emergence”: a venerable concept in search of a theory. Complexity 7, 18–30 (2002).
Characklis, W. G. & Wilderer, P. A. (eds) Structure and Function of Biofilms (John Wiley, New York, 1989).
Characklis, W. G. & Marshall, K. C. (eds) Biofilms 3–15 (John Wiley & Sons, New York, NY, 1990).
Vert, M. et al. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 84, 377–410 (2012).
Fisk, M. R. et al. Evidence of biological activity in Hawaiian subsurface basalts. Geochem. Geophys. Geosyst. 4, 1103 (2003).
Fry, J. C. et al. Prokaryotic populations and activities in an interbedded coal deposit, including a previously deeply buried section (1.6–2.3 km) above ~150 Ma basement rock. Geomicrobiol. J. 26, 163–178 (2009).
United Nations. UN World Water Development Report 2017: Wastewater: the Untapped Resource. United Nations Educational, Scientific and Cultural Organization, Paris, France. http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/2017-wastewater-the-untapped-resource/ (2017).
Acknowledgements
Many colleagues responded to requests for bacterial numbers or were ready to discuss them, which was highly appreciated: A. Boetius, R. Colwell, A. Decho, R. Gerlach, R. Glud, B. B. Jørgensen, K. Kjeldsen, S. Kjelleberg, F. Lauro, H. Lesch, R. Meckenstock, L. Melo, L. A. Meyer-Reil, J. Parkes, K. Pedersen, H. Peter, P. Rettberg, B. Schink, U. Schreiber, S. Schuster, P. Stoodley, W. Streit, S. Swarup, U. Szewzyk, M. Vera, G. Wolfaardt, O. Wurl and, above all, J. Wingender. Special thanks to K. Peter for help with figure drafts. Furthermore, the authors are very thankful to the reviewers whose thorough work helped to improve this Analysis.
Reviewer information
Nature Reviews Microbiology thanks Y. M. Bar-On, R. Milo, P. Stoodley and I. Wagner-Döbler for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
H.-C.F. did the data research and drafted the manuscript and figures; S.W. provided the idea to this work, substantially contributed to discussion of content and performed and edited the calculations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41579_2019_158_MOESM1_ESM.pdf
Supplementary Box 1
Glossary
- Basalt
-
The most common type of solidified lava; a fine-grained igneous rock.
- Dike
-
A rock sheet formed in crevices and fractures of an already existing rock body.
- Gabbroid rock
-
A compact, dark, coarse-grained magmatic rock, chemically equivalent to basalt, that forms when molten magma is trapped in the subsurface, slowly cools and forms a crystalline mass.
- Gyre
-
A large system of circulating ocean currents, caused by the Coriolis effect, involved with large wind movements. The five most notable gyres are the Indian Ocean gyre, the North Atlantic gyre, the North Pacific gyre, the South Atlantic gyre and the South Pacific gyre.
- Emergent properties
-
The characteristics of a community not identifiable by analysing the component organisms in isolation, including novel and coherent structures, patterns and properties arising during the process of self-organization in complex systems — the whole is more than the sum of its parts.
- Substratum
-
A solid surface on which organisms adhere and grow.
- Diagenesis
-
All processes that happen during the transformation of a sediment to its final lithification. It is a low-pressure, low-temperature process that can involve microbial biofilms, owing to their extracellular polymeric substances, as opposed to metamorphism, a rock alteration process that occurs at high temperatures and pressures.
- Igneous
-
A type of crystalline rock that forms directly from the cooling of magma.
- Serpentinization
-
The hydrothermal transformation of primary ferromagnesian minerals producing fluids rich in hydrogen and various secondary minerals. The hydrogen can reduce carbon dioxide and initiate an inorganic pathway for organic compounds.
- Canterbury basin
-
The sedimentary basin around the South Island of New Zealand.
- Stable isotope incubation
-
The exposure of microbial communities to stable isotopes (for example, 13C- or 15N-labelled glucose, pyruvate and amino acids) to determine the incorporation and thus the metabolic activity of microorganisms.
- Nanometre-scale secondary ion mass spectroscopy
-
(Nano-SIMS). A type of imaging with secondary ion mass spectroscopy with nanoscopic-scale resolution.
- CARD–FISH
-
Fluorescence in situ hybridization (FISH) with horseradish-peroxidase-labelled oligonucleotide probes and tyramide signal amplification, also known as catalysed reporter deposition (CARD).
- Chasmoendoliths
-
Organisms growing in fissures of rocks.
- Cryptoendoliths
-
Organisms growing in deep cavities or crevices within rock.
- Euendoliths
-
Organisms growing in cracks and pits actively penetrating the mineral material.
- Extracellular polymeric substances
-
(EPS). Mainly polysaccharides, proteins, nucleic acids and lipids; they provide the mechanical stability of biofilms, mediate adhesion to surfaces and form a cohesive, 3D polymer network that interconnects and transiently immobilizes biofilm cells. In addition, the biofilm matrix functions as an external digestive system by retaining extracellular enzymes in close proximity to the cells that solubilize colloidal and solid biopolymers and thus make them bioavailable.
- Quorum sensing
-
The sensing of microbial population density. This mechanism can regulate gene expression in response to fluctuations of cell-population density. It is based on the production and release of small soluble molecules named ‘autoinducers’ because they act not only on other cells but also on the producing ones once a threshold concentration is reached.
- Conchoidal breakage sites
-
The locations of breakages that are characteristic of the way in which brittle materials break or fracture if they do not follow any natural planes of separation. Quartz, flint, quartzite, jasper and other fine-grained or amorphous materials, such as pure silica, obsidian and window glass, are among the materials that break in this way.
- Plastisphere
-
The microbial community of heterotrophs, autotrophs, predators and symbionts living on plastic debris in oceans, fresh water, soils and sediments.
- Troposphere
-
The lowest and densest part of the atmosphere, which extends up to ~11 km in altitude. It is where most of the weather changes occur and where the vast majority of microbial and abiotic aerosols are found.
Rights and permissions
About this article
Cite this article
Flemming, HC., Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol 17, 247–260 (2019). https://doi.org/10.1038/s41579-019-0158-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-019-0158-9
This article is cited by
-
Bacterial c-di-GMP signaling gene affects mussel larval metamorphosis through outer membrane vesicles and lipopolysaccharides
npj Biofilms and Microbiomes (2024)
-
Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era
Nature Reviews Microbiology (2024)
-
Cell-lysis sensing drives biofilm formation in Vibrio cholerae
Nature Communications (2024)
-
Beneficial applications of biofilms
Nature Reviews Microbiology (2024)
-
Electrochemically coupled CH4 and CO2 consumption driven by microbial processes
Nature Communications (2024)