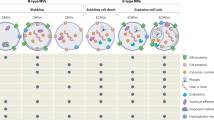
Abstract
Most bacteria release membrane vesicles (MVs) that contain specific cargo molecules and have diverse functions, including the transport of virulence factors, DNA transfer, interception of bacteriophages, antibiotics and eukaryotic host defence factors, cell detoxification and bacterial communication. MVs not only are abundant in nature but also show great promise for applications in biomedicine and nanotechnology. MVs were first discovered to originate from controlled blebbing of the outer membrane of Gram-negative bacteria and are therefore often called outer-membrane vesicles (OMVs). However, recent work has shown that Gram-positive bacteria can produce MVs, that different types of MVs besides OMVs exist and that, in addition to membrane blebbing, MVs can also be formed by endolysin-triggered cell lysis. In this Review, we provide an overview of the structures and compositions of the various vesicle types and discuss novel formation routes, which may lead to distinct vesicle types that serve particular functions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schwechheimer, C. & Kuehn, M. J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619 (2015). This comprehensive Review presents classic models of OMV formation and function.
Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadevall, A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630 (2015). This Review discusses vesicle production by organisms not belonging to the Gram-negative bacteria.
Orench-Rivera, N. & Kuehn, M. J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 18, 1525–1536 (2016).
Gujrati, V. et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 8, 1525–1537 (2014).
Kaparakis-Liaskos, M. & Ferrero, R. L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 15, 375–387 (2015).
Biller, S. J. et al. Bacterial vesicles in marine ecosystems. Science 343, 183–186 (2014). This study presents evidence that MVs are abundant in open-ocean samples and demonstrates that the DNA associated with MVs is highly enriched for viral sequences.
Grande, R. et al. Helicobacter pylori ATCC 43629/NCTC 11639 outer membrane vesicles (OMVs) from biofilm and planktonic phase associated with extracellular DNA (eDNA). Front. Microbiol. 6, 1369 (2015).
Manning, A. J. & Kuehn, M. J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 11, 258 (2011). This paper demonstrates that OMVs can function as decoys that neutralize phages and membrane-targeting antibiotics.
Toyofuku, M., Roschitzki, B., Riedel, K. & Eberl, L. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J. Proteome Res. 11, 4906–4915 (2012).
Schooling, S. R. & Beveridge, T. J. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957 (2006).
Kulp, A. & Kuehn, M. J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–184 (2010).
Beveridge, T. J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181, 4725–4733 (1999).
Mashburn-Warren, L. M. & Whiteley, M. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61, 839–846 (2006).
Turnbull, L. et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 7, 11220 (2016). This paper demonstrates for the first time that MVs can be formed as a consequence of explosive cell lysis, which is induced by the expression of a phage endolysin.
Toyofuku, M. et al. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun. 8, 481 (2017). This study shows that CMV production in the Gram-positive bacterium B. subtilis is triggered by the expression of a phage endolysin, a phenomenon named ‘bubbling cell death’.
Roier, S., Zingl, F. G., Cakar, F. & Schild, S. Bacterial outer membrane vesicle biogenesis: a new mechanism and its implications. Microb. Cell 3, 257–259 (2016).
Kulp, A. J. et al. Genome-wide assessment of outer membrane vesicle production in Escherichia coli. PLOS ONE 10, e0139200 (2015).
Elhenawy, W. et al. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. mBio 7, e00940-16 (2016).
Bager, R. J. et al. Outer membrane vesicles reflect environmental cues in Gallibacterium anatis. Vet. Microbiol. 167, 565–572 (2013).
Koeppen, K. et al. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLOS Pathog. 12, e1005672 (2016).
Sjöström, A. E., Sandblad, L., Uhlin, B. E. & Wai, S. N. Membrane vesicle-mediated release of bacterial RNA. Sci. Rep. 5, 15329 (2015).
Bitto, N. J. et al. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep. 7, 7072 (2017).
Altindis, E., Fu, Y. & Mekalanos, J. J. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc. Natl Acad. Sci. USA 111, E1548–E1556 (2014).
Wai, S. N. et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115, 25–35 (2003).
Guerrero-Mandujano, A., Hernandez-Cortez, C., Ibarra, J. A. & Castro-Escarpulli, G. The outer membrane vesicles: secretion system type zero. Traffic 18, 425–432 (2017).
Renelli, M., Matias, V., Lo, R. Y. & Beveridge, T. J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 150, 2161–2169 (2004).
Zhou, L., Srisatjaluk, R., Justus, D. E. & Doyle, R. J. On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol. Lett. 163, 223–228 (1998).
Dorward, D. W. & Garon, C. F. DNA-binding proteins in cells and membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171, 4196–4201 (1989).
Dorward, D. W. & Garon, C. F. DNA is packaged within membrane-derived vesicles of Gram-negative but not Gram-positive bacteria. Appl. Environ. Microbiol. 56, 1960–1962 (1990).
Dorward, D. W., Garon, C. F. & Judd, R. C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171, 2499–2505 (1989).
Kadurugamuwa, J. L. & Beveridge, T. J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177, 3998–4008 (1995).
Pérez-Cruz, C. et al. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Appl. Environ. Microbiol. 79, 1874–1881 (2013).
Pérez-Cruz, C., Delgado, L., López-Iglesias, C. & Mercade, E. Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLOS ONE 10, e0116896 (2015).
Li, J., Azam, F. & Zhang, S. Outer membrane vesicles containing signalling molecules and active hydrolytic enzymes released by a coral pathogen Vibrio shilonii AK1. Environ. Microbiol. 18, 3850–3866 (2016).
Hagemann, S. et al. DNA-bearing membrane vesicles produced by Ahrensia kielensis and Pseudoalteromonas marina. J. Basic Microbiol. 54, 1062–1072 (2014).
Kadurugamuwa, J. L. & Beveridge, T. J. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J. Antimicrob. Chemother. 40, 615–621 (1997).
Li, Z., Clarke, A. J. & Beveridge, T. J. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180, 5478–5483 (1998).
Devos, S. et al. Membrane vesicle secretion and prophage induction in multidrug-resistant Stenotrophomonas maltophilia in response to ciprofloxacin stress. Environ. Microbiol. 19, 3930–3937 (2017). This study shows that treatment of S. maltophilia with ciprofloxacin, which is known to induce the SOS response owing to DNA damage, stimulates the production of not only OIMVs but also large amounts of phages.
Shingaki, R., Kasahara, Y., Inoue, T., Kokeguchi, S. & Fukui, K. Chromosome DNA fragmentation and excretion caused by defective prophage gene expression in the early-exponential-phase culture of Bacillus subtilis. Can. J. Microbiol. 49, 313–325 (2003).
Küsel, K., Dorsch, T., Acker, G. & Stackebrandt, E. Microbial reduction of Fe(III) in acidic sediments: isolation of Acidiphilium cryptum JF-5 capable of coupling the reduction of Fe(III) to the oxidation of glucose. Appl. Environ. Microbiol. 65, 3633–3640 (1999).
Remis, J. P. et al. Bacterial social networks: structure and composition of Myxococcus xanthus outer membrane vesicle chains. Environ. Microbiol. 16, 598–610 (2014). This study uses several imaging techniques to visualize chains of OMVs in M. xanthus biofilms.
Wei, X., Vassallo, C. N., Pathak, D. T. & Wall, D. Myxobacteria produce outer membrane-enclosed tubes in unstructured environments. J. Bacteriol. 196, 1807–1814 (2014).
Baidya, A. K., Bhattacharya, S., Dubey, G. P., Mamou, G. & Ben-Yehuda, S. Bacterial nanotubes: a conduit for intercellular molecular trade. Curr. Opin. Microbiol. 42, 1–6 (2017).
Cao, P., Dey, A., Vassallo, C. N. & Wall, D. How Myxobacteria cooperate. J. Mol. Biol. 427, 3709–3721 (2015).
Pirbadian, S. et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl Acad. Sci. USA 111, 12883–12888 (2014).
Sure, S. K., Ackland, L. M., Torriero, A. A., Adholeya, A. & Kochar, M. Microbial nanowires: an electrifying tale. Microbiology 162, 2017–2028 (2016).
McCaig, W. D., Koller, A. & Thanassi, D. G. Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol. 195, 1120–1132 (2013).
Hampton, C. M. et al. The opportunistic pathogen Vibrio vulnificus produces outer membrane vesicles in a spatially distinct manner related to capsular polysaccharide. Front. Microbiol. 8, 2177 (2017).
Dubey, G. P. et al. Architecture and characteristics of bacterial nanotubes. Dev. Cell 36, 453–461 (2016).
Dubey, G. P. & Ben-Yehuda, S. Intercellular nanotubes mediate bacterial communication. Cell 144, 590–600 (2011).
Sutterlin, H. A. et al. Disruption of lipid homeostasis in the Gram-negative cell envelope activates a novel cell death pathway. Proc. Natl Acad. Sci. USA 113, E1565–E1574 (2016).
Hoekstra, D., van der Laan, J. W., de Leij, L. & Witholt, B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim. Biophys. Acta 455, 889–899 (1976).
Burdett, I. D. & Murray, R. G. Electron microscope study of septum formation in Escherichia coli strains B and B/r during synchronous growth. J. Bacteriol. 119, 1039–1056 (1974).
Deatherage, B. L. et al. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72, 1395–1407 (2009).
Bernadac, A., Gavioli, M., Lazzaroni, J. C., Raina, S. & Lloubés, R. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180, 4872–4878 (1998).
Sonntag, I., Schwarz, H., Hirota, Y. & Henning, U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J. Bacteriol. 136, 280–285 (1978).
Yem, D. W. & Wu, H. C. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J. Bacteriol. 133, 1419–1426 (1978).
Murata, M., Noor, R., Nagamitsu, H., Tanaka, S. & Yamada, M. Novel pathway directed by sigma E to cause cell lysis in Escherichia coli. Genes Cells 17, 234–247 (2012).
McBroom, A. J., Johnson, A. P., Vemulapalli, S. & Kuehn, M. J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188, 5385–5392 (2006).
Schwechheimer, C., Rodriguez, D. L. & Kuehn, M. J. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. MicrobiologyOpen 4, 375–389 (2015).
Roier, S. et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 7, 10515 (2016). This study demonstrates that iron limitation controls OMV formation by affecting expression of phospholipid transporter genes in H. influenzae.
McBroom, A. J. & Kuehn, M. J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63, 545–558 (2007).
Tashiro, Y. et al. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. J. Bacteriol. 191, 7509–7519 (2009).
Florez, C., Raab, J. E., Cooke, A. C. & Schertzer, J. W. Membrane distribution of the Pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa. mBio 8, e01034-17 (2017).
Mashburn, L. M. & Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437, 422–425 (2005).
Schertzer, J. W. & Whiteley, M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio 3, e00297-11 (2012).
Mashburn-Warren, L. et al. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69, 491–502 (2008).
Kadurugamuwa, J. L. & Beveridge, T. J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178, 2767–2774 (1996).
Fulsundar, S. et al. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl. Environ. Microbiol. 80, 3469–3483 (2014).
Brennan, C. A. et al. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. eLife 3, e01579 (2014).
Aschtgen, M. S. et al. Rotation of Vibrio fischeri flagella produces outer membrane vesicles that induce host development. J. Bacteriol. 198, 2156–2165 (2016). This study identifies a unique mechanism of OMV formation that is based on the rotation of membrane-sheathed flagella.
Aschtgen, M. S., Wetzel, K., Goldman, W., McFall-Ngai, M. & Ruby, E. Vibrio fischeri-derived outer membrane vesicles trigger host development. Cell. Microbiol. 18, 488–499 (2016).
Geis, G., Suerbaum, S., Forsthoff, B., Leying, H. & Opferkuch, W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 38, 371–377 (1993).
Qin, Z., Lin, W. T., Zhu, S., Franco, A. T. & Liu, J. Imaging the motility and chemotaxis machineries in Helicobacter pylori by cryo-electron tomography. J. Bacteriol. 199, e00695–16 (2016).
Toyofuku, M. et al. Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1. Environ. Microbiol. 16, 2927–2938 (2014).
Wang, X., Thompson, C. D., Weidenmaier, C. & Lee, J. C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 9, 1379 (2018).
Hayashi, J., Hamada, N. & Kuramitsu, H. K. The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbiol. Lett. 216, 217–222 (2002).
Koning, R. I. et al. Cryo-electron tomography analysis of membrane vesicles from Acinetobacter baumannii ATCC19606 T. Res. Microbiol. 164, 397–405 (2013).
Shetty, A. & Hickey, W. J. Effects of outer membrane vesicle formation, surface-layer production and nanopod development on the metabolism of phenanthrene by Delftia acidovorans Cs1-4. PLOS ONE 9, e92143 (2014).
Borneleit, P., Hermsdorf, T., Claus, R., Walther, P. & Kleber, H. P. Effect of hexadecane-induced vesiculation on the outer membrane of Acinetobacter calcoaceticus. J. Gen. Microbiol. 134, 1983–1992 (1988).
Kobayashi, H., Uematsu, K., Hirayama, H. & Horikoshi, K. Novel toluene elimination system in a toluene-tolerant microorganism. J. Bacteriol. 182, 6451–6455 (2000).
Feiner, R. et al. A new perspective on lysogeny: prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 13, 641–650 (2015).
Catalão, M. J., Gil, F., Moniz-Pereira, J., São-José, C. & Pimentel, M. Diversity in bacterial lysis systems: bacteriophages show the way. FEMS Microbiol. Rev. 37, 554–571 (2013).
Pennington, J. M. & Rosenberg, S. M. Spontaneous DNA breakage in single living Escherichia coli cells. Nat. Genet. 39, 797–802 (2007).
Taddei, F., Matic, I. & Radman, M. cAMP-dependent SOS induction and mutagenesis in resting bacterial populations. Proc. Natl Acad. Sci. USA 92, 11736–11740 (1995).
Bernier, S. P. et al. Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLOS Genet. 9, e1003144 (2013).
Okshevsky, M. & Meyer, R. L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 41, 341–352 (2015).
Scholl, D. Phage tail-like bacteriocins. Annu. Rev. Virol. 4, 453–467 (2017).
Domingues, S. & Nielsen, K. M. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr. Opin. Microbiol. 38, 16–21 (2017).
Ho, M. H., Chen, C. H., Goodwin, J. S., Wang, B. Y. & Xie, H. Functional advantages of Porphyromonas gingivalis vesicles. PLOS ONE 10, e0123448 (2015).
Kolling, G. L. & Matthews, K. R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 65, 1843–1848 (1999).
Yaron, S., Kolling, G. L., Simon, L. & Matthews, K. R. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 66, 4414–4420 (2000).
Rumbo, C. et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55, 3084–3090 (2011).
Blesa, A. & Berenguer, J. Contribution of vesicle-protected extracellular DNA to horizontal gene transfer in Thermus spp. Int. Microbiol. 18, 177–187 (2015).
Klieve, A. V. et al. Naturally occurring DNA transfer system associated with membrane vesicles in cellulolytic Ruminococcus spp. of ruminal origin. Appl. Environ. Microbiol. 71, 4248–4253 (2005).
Tashiro, Y. et al. Interaction of bacterial membrane vesicles with specific species and their potential for delivery to target cells. Front. Microbiol. 8, 571 (2017).
Li, Z., Clarke, A. J. & Beveridge, T. J. A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division and secretion in surface membrane vesicles. J. Bacteriol. 178, 2479–2488 (1996).
Prangishvili, D. et al. Sulfolobicins, specific proteinaceous toxins produced by strains of the extremely thermophilic archaeal genus Sulfolobus. J. Bacteriol. 182, 2985–2988 (2000).
Seccareccia, I., Kost, C. & Nett, M. Quantitative analysis of Lysobacter predation. Appl. Environ. Microbiol. 81, 7098–7105 (2015).
Casida, L. E. Minireview: Nonobligate bacterial predation of bacteria in soil. Microb. Ecol. 15, 1–8 (1988).
Vasilyeva, N. V., Tsfasman, I. M., Suzina, N. E., Stepnaya, O. A. & Kulaev, I. S. Secretion of bacteriolytic endopeptidase L5 of Lysobacter sp. XL1 into the medium by means of outer membrane vesicles. FEBS J. 275, 3827–3835 (2008).
Kudryakova, I. V., Suzina, N. E., Vinokurova, N. G., Shishkova, N. A. & Vasilyeva, N. V. Studying factors involved in biogenesis of Lysobacter sp. XL1 outer membrane vesicles. Biochemistry (Mosc.) 82, 501–509 (2017).
Kudryakova, I. V., Suzina, N. E. & Vasilyeva, N. V. Biogenesis of Lysobacter sp. XL1 vesicles. FEMS Microbiol. Lett. 362, fnv137 (2015).
Tzipilevich, E., Habusha, M. & Ben-Yehuda, S. Acquisition of phage sensitivity by bacteria through exchange of phage receptors. Cell 168, 186–199 (2017). The study demonstrates that CMVs can transmit phage receptors to phage-resistant cells, which then become phage sensitive.
Kharina, A. et al. Temperate bacteriophages collected by outer membrane vesicles in Komagataeibacter intermedius. J. Basic Microbiol. 55, 509–513 (2015).
Manning, A. J. & Kuehn, M. J. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J. Mol. Microbiol. Biotechnol. 23, 131–141 (2013).
Reyes-Robles, T. et al. Vibrio cholerae outer membrane vesicles inhibit bacteriophage infection. J. Bacteriol. https://doi.org/10.1128/JB.00792-17 (2018).
Toyofuku, M. et al. Membrane vesicle-mediated bacterial communication. ISME J. 11, 1504–1509 (2017).
Devos, S. et al. The effect of imipenem and diffusible signaling factors on the secretion of outer membrane vesicles and associated Ax21 proteins in Stenotrophomonas maltophilia. Front. Microbiol. 6, 298 (2015).
Ionescu, M. et al. Xylella fastidiosa outer membrane vesicles modulate plant colonization by blocking attachment to surfaces. Proc. Natl Acad. Sci. USA 111, E3910–E3918 (2014).
Lynch, J. B. & Alegado, R. A. Spheres of hope, packets of doom: the good and bad of outer membrane vesicles in interspecies and ecological dynamics. J. Bacteriol. 199, e00012-17 (2017).
Aung, K. M. et al. Naturally occurring IgG antibodies provide innate protection against Vibrio cholerae bacteremia by recognition of the outer membrane protein U. J. Innate Immun. 8, 269–283 (2016).
Duperthuy, M. et al. Role of the Vibrio cholerae matrix protein Bap1 in cross-resistance to antimicrobial peptides. PLOS Pathog. 9, e1003620 (2013).
Codemo, M. et al. Immunomodulatory effects of pneumococcal extracellular vesicles on cellular and humoral host defenses. mBio 9, e00559-18 (2018).
Kimmitt, P. T., Harwood, C. R. & Barer, M. R. Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6, 458–465 (2000).
Quinones, M., Kimsey, H. H. & Waldor, M. K. LexA cleavage is required for CTX prophage induction. Mol. Cell 17, 291–300 (2005).
Chatterjee, D. & Chaudhuri, K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 585, 1357–1362 (2011).
Bielaszewska, M. et al. Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: intracellular delivery, trafficking and mechanisms of cell injury. PLOS Pathog. 13, e1006159 (2017).
Kunsmann, L. et al. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci. Rep. 5, 13252 (2015).
Gaudin, M. et al. Extracellular membrane vesicles harbouring viral genomes. Environ. Microbiol. 16, 1167–1175 (2014). This study shows that MVs can harbour viral genomes, suggesting a link between phage release and vesicle formation.
Biller, S. J. et al. Membrane vesicles in sea water: heterogeneous DNA content and implications for viral abundance estimates. ISME J. 11, 394–404 (2017).
Soler, N., Krupovic, M., Marguet, E. & Forterre, P. Membrane vesicles in natural environments: a major challenge in viral ecology. ISME J. 9, 793–796 (2015).
Gamalier, J. P., Silva, T. P., Zarantonello, V., Dias, F. F. & Melo, R. C. Increased production of outer membrane vesicles by cultured freshwater bacteria in response to ultraviolet radiation. Microbiol. Res. 194, 38–46 (2017).
Breitbart, M. & Rohwer, F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 13, 278–284 (2005).
Radman, M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 5A, 355–367 (1975).
Kenyon, C. J. & Walker, G. C. DNA-damaging agents stimulate gene-expression at specific loci in Escherichia coli. Proc. Natl Acad. Sci. USA 77, 2819–2823 (1980).
Fernandez De Henestrosa, A. R. et al. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 35, 1560–1572 (2000).
McPartland, A., Green, L. & Echols, H. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell 20, 731–737 (1980).
Little, J. W., Edmiston, S. H., Pacelli, L. Z. & Mount, D. W. Cleavage of the Escherichia coli LexA protein by the RecA protease. Proc. Natl Acad. Sci. USA 77, 3225–3229 (1980).
Michel, B. After 30 years of study, the bacterial SOS response still surprises us. PLOS Biol. 3, e255 (2005).
Tippin, B., Pham, P. & Goodman, M. F. Error-prone replication for better or worse. Trends Microbiol. 12, 288–295 (2004).
Fuchs, R. P. & Fujii, S. Translesion DNA synthesis and mutagenesis in prokaryotes. Cold Spring Harb. Perspect. Biol. 5, a012682 (2013).
Schoemaker, J. M., Gayda, R. C. & Markovitz, A. Regulation of cell division in Escherichia coli: SOS induction and cellular location of the SulA protein, a key to lon-associated filamentation and death. J. Bacteriol. 158, 551–561 (1984).
Little, J. W. & Harper, J. E. Identification of the lexA gene product of Escherichia coli K-12. Proc. Natl Acad. Sci. USA 76, 6147–6151 (1979).
Craig, N. L. & Roberts, J. W. E. coli RecA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature 283, 26–30 (1980).
Biagini, M. et al. The human pathogen Streptococcus pyogenes releases lipoproteins as lipoprotein-rich membrane vesicles. Mol. Cell. Proteomics 14, 2138–2149 (2015).
Wichgers Schreur, P. J., Rebel, J. M., Smits, M. A., van Putten, J. P. & Smith, H. E. Lgt processing is an essential step in Streptococcus suis lipoprotein mediated innate immune activation. PLOS ONE 6, e22299 (2011).
Maredia, R. et al. Vesiculation from Pseudomonas aeruginosa under SOS. Sci. World J. 2012, 402919 (2012).
Bauwens, A., Kunsmann, L., Karch, H., Mellmann, A. & Bielaszewska, M. Antibiotic-mediated modulations of outer membrane vesicles in enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob. Agents Chemother. 61, e00937-17 (2017).
Kadurugamuwa, J. L., Clarke, A. J. & Beveridge, T. J. Surface action of gentamicin on Pseudomonas aeruginosa. J. Bacteriol. 175, 5798–5805 (1993).
Hoefler, B. C. et al. A link between linearmycin biosynthesis and extracellular vesicle genesis connects specialized metabolism and bacterial membrane physiology. Cell Chem. Biol. 24, 1238–1249.e7 (2017).
Freedman, S. B. et al. Shiga toxin–producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clin. Infect. Dis. 62, 1251–1258 (2016).
MacDonald, K. L. & Beveridge, T. J. Bactericidal effect of gentamicin-induced membrane vesicles derived from Pseudomonas aeruginosa PAO1 on Gram-positive bacteria. Can. J. Microbiol. 48, 810–820 (2002).
Acknowledgements
M.T. was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (project 16H06189), N.N. was supported by the Japan Science and Technology Agency (JST) (ERATO project JPMJER1502), and L.E. was supported by the Swiss National Science Foundation (SNSF) (Project 31003A_169307). The authors acknowledge K. Agnoli-Antkowiak for valuable comments on the manuscript.
Reviewer information
Nature Reviews Microbiology thanks S. Schild, S. N. Wai and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
M.T. and L.E. researched data for the article and contributed substantially to discussion of the content. All authors wrote the article and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Endolysins
-
Hydrolytic enzymes that are produced by bacteriophages to degrade the cell wall of the bacterial host during the final stage of the lytic cycle.
- Autolysins
-
Peptidoglycan hydrolases that are produced by bacteria for peptidoglycan turnover and to complete cell division by separating the daughter cell from the mother cell.
- Intercalation
-
The reversible insertion of molecules into materials with layered structures such as the cellular membrane.
- Turgor pressure
-
Turgor is the force that pushes the cytoplasmic membrane against the cell wall as a result of the osmotic flow of water.
- Quorum sensing
-
A cell-to-cell communication mechanism in bacteria by which gene regulation is controlled in a population-dependent manner through the production and perception of signal molecules.
- B-band LPS
-
Pseudomonas aeruginosa synthesizes two types of lipopolysaccharide (LPS) referred to as A-band and B-band LPS. The A-band LPS contains a conserved O-polysaccharide region composed of d-rhamnose (homopolymer), whereas the B-band O-antigen (heteropolymer) structure varies among different serotypes.
- Bacteriocins
-
Protein or peptide toxins produced by bacteria to kill or inhibit growth of bacteria that are closely related to the producer.
Rights and permissions
About this article
Cite this article
Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol 17, 13–24 (2019). https://doi.org/10.1038/s41579-018-0112-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-018-0112-2
This article is cited by
-
Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields
Cell Communication and Signaling (2024)
-
Exploiting bacterial-origin immunostimulants for improved vaccination and immunotherapy: current insights and future directions
Cell & Bioscience (2024)
-
Extracellular vesicles from vaginal Gardnerella vaginalis and Mobiluncus mulieris contain distinct proteomic cargo and induce inflammatory pathways
npj Biofilms and Microbiomes (2024)
-
Stepwise assembly and release of Tc toxins from Yersinia entomophaga
Nature Microbiology (2024)
-
Characterization and immunological effect of outer membrane vesicles from Pasteurella multocida on macrophages
Applied Microbiology and Biotechnology (2024)