Abstract
In the ocean’s major oxygen minimum zones (OMZs), oxygen is effectively absent from sea water and life is dominated by microorganisms that use chemicals other than oxygen for respiration. Recent studies that combine advanced genomic and chemical detection methods are delineating the different metabolic niches that microorganisms can occupy in OMZs. Understanding these niches, the microorganisms that inhabit them, and their influence on marine biogeochemical cycles is crucial as OMZs expand with increasing seawater temperatures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

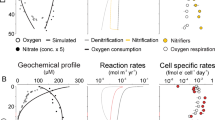
Reproduced from Frank J. Stewart and Osvaldo Ulloa, from: Metagenomics of the Microbial Nitrogen Cycle: Theory, Methods and Applications (Edited by: Diana Marco). Caister Academic Press, UK (2014)82.


Similar content being viewed by others
Change history
08 October 2018
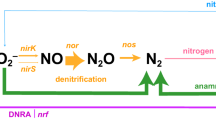
In Figure 3, ‘Candidatus Scalindua’ and Thaumarchaeota were erroneously shown to produce nitrous oxide (N2O). As neither group directly produces N2O, the arrows and products have been removed both online and in the pdf. The authors apologize for any confusion caused.
References
Thamdrup, B., Dalsgaard, T. & Revsbech, N. P. Widespread functional anoxia in the oxygen minimum zone of the Eastern South Pacific. Deep Sea Res. Part I Oceanogr. Res. Pap. 65, 36–45 (2012).
Wishner, K. F., Outram, D. M., Seibel, B. A., Daly, K. L. & Williams, R. L. Zooplankton in the eastern tropical north Pacific: boundary effects of oxygen minimum zone expansion. Deep Sea Res. Part I Oceanogr. Res. Pap. 79, 122–140 (2013).
Wright, J. J., Konwar, K. M. & Hallam, S. J. Microbial ecology of expanding oxygen minimum zones. Nat. Rev. Microbiol. 10, 381–394 (2012).
Loginova, A. N., Thomsen, S. & Engel, A. Chromophoric and fluorescent dissolved organic matter in and above the oxygen minimum zone off Peru. J. Geophys. Res. Oceans. 121, 7973–7990 (2016).
Cavan, E. L., Trimmer, M., Shelley, F. & Sanders, R. Remineralization of particulate organic carbon in an ocean oxygen minimum zone. Nat. Commun. 8, 14847 (2017).
Aldunate, M., De la Iglesia, R., Bertagnolli, A. D. & Ulloa, O. Oxygen modulates bacterial community composition in the coastal upwelling waters off central Chile. Deep Sea Res. Part II Top. Stud. Oceanogr. https://doi.org/10.1016/j.dsr2.2018.02.001 (2018).
Loscher, C. R. et al. Water column biogeochemistry of oxygen minimum zones in the eastern tropical North Atlantic and eastern tropical South Pacific oceans. Biogeosciences 13, 3585–3606 (2016).
Ulloa, O., Canfield, D. E., DeLong, E. F., Letelier, R. M. & Stewart, F. J. Microbial oceanography of anoxic oxygen minimum zones. Proc. Natl Acad. Sci. USA 109, 15996–16003 (2012).
Schmidtko, S., Stramma, L. & Visbeck, M. Decline in global oceanic oxygen content during the past five decades. Nature 542, 335–339 (2017).
Revsbech, N. P. et al. Determination of ultra-low oxygen concentrations in oxygen minimum zones by the STOX sensor. Limnol. Oceanogr. Meth. 7, 371–381 (2009).
Revsbech, N. P., Thamdrup, B., Dalsgaard, T. & Canfield, D. E. Construction of stox oxygen sensors and their application for determination of O2 concentrations in oxygen minimum zones. Methods Enzymol. 486, 325–341 (2011).
Larsen, M. et al. In situ quantification of ultra-low O2 concentrations in oxygen minimum zones: application of novel optodes. Limnol. Oceanogr. Meth. 14, 784–800 (2016).
Kalvelage, T. et al. Oxygen sensitivity of anammox and coupled N-cycle orocesses in oxygen minimum zones. PLOS ONE 6, e29299 (2011).
Tiano, L. et al. Oxygen distribution and aerobic respiration in the north and south eastern tropical Pacific oxygen minimum zones. Deep Sea Res. Part I Oceanogr. Res. Pap. 94, 173–183 (2014).
Dalsgaard, T. et al. Oxygen at nanomolar levels reversibly suppresses process rates and gene expression in anammox and denitrification in the oxygen minimum zone off Northern Chile. mBio 5, e01966 (2014).
Ganesh, S. et al. Size-fraction partitioning of community gene transcription and nitrogen metabolism in a marine oxygen minimum zone. ISME J. 9, 2682–2696 (2015).
Suter, E. A., Pachiadaki, M., Taylor, G. T., Astor, Y. & Edgcomb, V. P. Free-living chemoautotrophic and particle-attached heterotrophic prokaryotes dominate microbial assemblages along a pelagic redox gradient. Environ. Microbiol. 20, 693–712 (2018).
Bristow, L. A. et al. Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters. Proc. Natl Acad. Sci. USA 113, 10601–10606 (2016).
Kalvelage, T. et al. Aerobic microbial respiration in oceanic oxygen minimum zones. PLOS ONE 10, e0133526 (2015).
Garcia-Robledo, E. et al. Cryptic oxygen cycling in anoxic marine zones. Proc. Natl Acad. Sci. USA. 114, 8319–8324 (2017).
Zakem, E. J. & Follows, M. J. A theoretical basis for a nanomolar critical oxygen concentration. Limnol. Oceanogr. 62, 795–805 (2017).
Bristow, L. A. et al. N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone. Nat. Geosci. 10, 24–29 (2017).
Resplandy, L. et al. Controlling factors of the oxygen balance in the Arabian Sea’s OMZ. Biogeosciences 9, 5095–5109 (2012).
Goericke, R., Olson, R. J. & Shalapyonok, A. A novel niche for Prochlorococcus sp in low-light suboxic environments in the Arabian Sea and the Eastern Tropical North Pacific. Deep Sea Res. Part I Oceanogr. Res. Pap. 47, 1183–1205 (2000).
Lavin, P., Gonzalez, B., Santibanez, J. F., Scanlan, D. J. & Ulloa, O. Novel lineages of Prochlorococcus thrive within the oxygen minimum zone of the eastern tropical South Pacific. Environ. Microbiol. Rep. 2, 728–738 (2010).
Franz, J. et al. Dynamics and stoichiometry of nutrients and phytoplankton in waters influenced by the oxygen minimum zone in the eastern tropical Pacific. Deep Sea Res. Part I Oceanogr. Res. Pap. 62, 20–31 (2012).
Astorga-Elo, M., Ramirez-Flandes, S., DeLong, E. F. & Ulloa, O. Genomic potential for nitrogen assimilation in uncultivated members of Prochlorococcus from an anoxic marine zone. ISME J. 9, 1264–1267 (2015).
Sansone, F. J., Popp, B. N., Gasc, A., Graham, A. W. & Rust, T. M. Highly elevated methane in the eastern tropical North Pacific and associated isotopically enriched fluxes to the atmosphere. Geophys. Res. Lett. 28, 4567–4570 (2001).
Naqvi, S. W. A. et al. Marine hypoxia/anoxia as a source of CH4 and N2O. Biogeosciences 7, 2159–2190 (2010).
Chronopoulou, P. M., Shelley, F., Pritchard, W. J., Maanoja, S. T. & Trimmer, M. Origin and fate of methane in the Eastern Tropical North Pacific oxygen minimum zone. ISME J. 11, 1386–1399 (2017).
Pack, M. A. et al. Methane oxidation in the eastern tropical North Pacific Ocean water column. J. Geophys. Res. Biogeo. 120, 1078–1092 (2015).
Tavormina, P. L. et al. Abundance and distribution of diverse membrane-bound monooxygenase (Cu-MMO) genes within the Costa Rica oxygen minimum zone. Environ. Microbiol. Rep. 5, 414–423 (2013).
Padilla, C. C. et al. Metagenomic binning recovers a transcriptionally active gammaproteobacterium linking methanotrophy to partial denitrification in an anoxic oxygen minimum zone. Front. Mar. Sci. https://doi.org/10.3389/fmars.2017.00023 (2017).
Torres-Beltrán, M. et al. Methanotrophic community dynamics in a seasonally anoxic fjord: Saanich Inlet, British Columbia. Front. Mar. Sci. https://doi.org/10.3389/fmars.2016.00268 (2016).
Padilla, C. C. et al. NC10 bacteria in marine oxygen minimum zones. ISME J. 10, 2067–2071 (2016).
Ettwig, K. F. et al. Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea. Environ. Microbiol. 10, 3164–3173 (2008).
Raghoebarsing, A. A. et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440, 918–921 (2006).
Reed, D. C., Algar, C. K., Huber, J. A. & Dick, G. J. Gene-centric approach to integrating environmental genomics and biogeochemical models. Proc. Natl Acad. Sci. USA 111, 1879–1884 (2014).
Louca, S. et al. Integrating biogeochemistry with multiomic sequence information in a model oxygen minimum zone. Proc. Natl Acad. Sci. USA. 113, E5925–E5933 (2016).
Walsh, D. A. et al. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326, 578–582 (2009).
Hawley, A. K., Brewer, H. M., Norbeck, A. D., Pasa-Tolic, L. & Hallam, S. J. Metaproteomics reveals differential modes of metabolic coupling among ubiquitous oxygen minimum zone microbes. Proc. Natl Acad. Sci. USA 111, 11395–11400 (2014).
Murillo, A. A., Ramírez-Flandes, S., DeLong, E. F. & Ulloa, O. Enhanced metabolic versatility of planktonic sulfur-oxidizing γ-proteobacteria in an oxygen-deficient coastal ecosystem. Front. Mar. Sci. https://doi.org/10.3389/fmars.2014.00018 (2014).
Shah, V., Chang, B. X. & Morris, R. M. Cultivation of a chemoautotroph from the SUP05 clade of marine bacteria that produces nitrite and consumes ammonium. ISME J. 11, 263–271 (2017).
Callbeck, C. M. et al. Oxygen minimum zone cryptic sulfur cycling sustained by offshore transport of key sulfur oxidizing bacteria. Nat. Commun. 9, 1729 (2018).
Giovannoni, S. J. SAR11 Bacteria: the most abundant plankton in the oceans. Annu. Rev. Mar. Sci. 9, 231–255 (2017).
Ganesh, S., Parris, D. J., De Long, E. F. & Stewart, F. J. Metagenomic analysis of size-fractionated picoplankton in a marine oxygen minimum zone. ISME J. 8, 187–211 (2014).
Tsementzi, D. et al. SAR11 bacteria linked to ocean anoxia and nitrogen loss. Nature 536, 179–183 (2016).
Johnson, Z. I. et al. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311, 1737–1740 (2006).
Kashtan, N. et al. Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science 344, 416–420 (2014).
Bryant, J. A., Stewart, F. J., Eppley, J. M. & DeLong, E. F. Microbial community phylogenetic and trait diversity declines with depth in a marine oxygen minimum zone. Ecology 93, 1659–1673 (2012).
Beman, J. M. & Carolan, M. T. Deoxygenation alters bacterial diversity and community composition in the ocean’s largest oxygen minimum zone. Nat. Commun. 4, 2705 (2013).
Cassman, N. et al. Oxygen minimum zones harbour novel viral communities with low diversity. Environ. Microbiol. 14, 3043–3065 (2012).
Woebken, D. et al. A microdiversity study of anammox bacteria reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones. Environ. Microbiol. 10, 3106–3119 (2008).
Luke, C., Speth, D. R., Kox, M. A. R., Villanueva, L. & Jetten, M. S. M. Metagenomic analysis of nitrogen and methane cycling in the Arabian Sea oxygen minimum zone. PeerJ 4, e1924 (2016).
Villanueva, L., Speth, D. R., van Alen, T., Hoischen, A. & Jetten, M. S. M. Shotgun metagenomic data reveals significant abundance but low diversity of “Candidatus Scalindua” marine anammox bacteria in the Arabian Sea oxygen minimum zone. Front. Microbiol. https://doi.org/10.3389/fmicb.2014.00031 (2014).
Ganesh, S. et al. Single cell genomic and transcriptomic evidence for the use of alternative nitrogen substrates by anammox bacteria. ISME J. https://doi.org/10.1038/s41396-018-0223-9 (2018).
Babbin, A. R. et al. Multiple metabolisms constrain the anaerobic nitrite budget in the Eastern Tropical South Pacific. Global Biogeochem. Cycles 31, 258–271 (2017).
Bianchi, D., Babbin, A. R. & Galbraith, E. D. Enhancement of anammox by the excretion of diel vertical migrators. Proc. Natl Acad. Sci. USA 111, 15653–15658 (2014).
Fernandez, C., Gonzalez, M. L., Munoz, C., Molina, V. & Farias, L. Temporal and spatial variability of biological nitrogen fixation off the upwelling system of central Chile (35–38.5 °S). J. Geophys. Res. Oceans 120, 3330–3349 (2015).
Bonnet, S. et al. Dynamics of N-2 fixation and fate of diazotroph-derived nitrogen in a low-nutrient, low-chlorophyll ecosystem: results from the VAHINE mesocosm experiment (New Caledonia). Biogeosciences 13, 2653–2673 (2016).
Jayakumar, A. et al. Biological nitrogen fixation in the oxygen-minimum region of the eastern tropical North Pacific ocean. ISME J. 11, 2356–2367 (2017).
Loescher, C. R. et al. Facets of diazotrophy in the oxygen minimum zone waters off Peru. ISME J. 8, 2180–2192 (2014).
Martinez-Perez, C. et al. Metabolic versatility of a novel N2-fixing Alphaproteobacterium isolated from a marine oxygen minimum zone. Environ. Microbiol. 20, 755–768 (2018).
Delmont, T. O. et al. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat. Microbiol. 3, 804–813 (2018).
Thrash, J. C. et al. Metabolic roles of uncultivated bacterioplankton lineages in the northern Gulf of Mexico “dead zone”. mBio 8, e01017–17 (2017).
Hawley, A. K. et al. Diverse Marinimicrobia bacteria may mediate coupled biogeochemical cycles along eco-thermodynamic gradients. Nat. Commun. 8, 1507 (2017).
Georges, A. A., El-Swais, H., Craig, S. E., Li, W. K. W. & Walsh, D. A. Metaproteomic analysis of a winter to spring succession in coastal northwest Atlantic Ocean microbial plankton. ISME J. 8, 1301–1313 (2014).
Bertagnolli, A. D., Padilla, C. C., Glass, J. B., Thamdrup, B. & Stewart, F. J. Metabolic potential and in situ activity of marine Marinimicrobia bacteria in an anoxic water column. Environ. Microbiol. 19, 4392–4416 (2017).
Sheik, C. S., Jain, S. & Dick, G. J. Metabolic flexibility of enigmatic SAR324 revealed through metagenomics and metatranscriptomics. Environ. Microbiol. 16, 304–317 (2014).
Marshall, K. T. & Morris, R. M. Genome sequence of “Candidatus Thioglobus singularis” strain PS1, a mixotroph from the SUP05 clade of marine gammaproteobacteria. Genome Announc. 3, e01155–15 (2015).
Canfield, D. E. et al. A cryptic sulfur cycle in oxygen minimum zone waters off the Chilean coast. Science 330, 1375–1378 (2010).
Widner, B. & Mulholland, M. R. Cyanate distribution and uptake in North Atlantic coastal waters. Limnol. Oceanogr. 62, 2538–2549 (2017).
Glass, J. B. et al. Meta-omic signatures of microbial metal and nitrogen cycling in marine oxygen minimum zones. Front. Microbiol. https://doi.org/10.3389/fmicb.2015.00998 (2015).
Ohnemus, D. C. et al. Elevated trace metal content of prokaryotic communities associated with marine oxygen deficient zones. Limnol. Oceanogr. 62, 3–25 (2017).
Hawco, N. J., Ohnemus, D. C., Resing, J. A., Twining, B. S. & Saito, M. A. A dissolved cobalt plume in the oxygen minimum zone of the eastern tropical South Pacific. Biogeosciences 13, 5697–5717 (2016).
Qin, W. et al. Influence of oxygen availability on the activities of ammonia-oxidizing archaea. Environ. Microbiol. Rep. 9, 250–256 (2017).
Grote, J. et al. Genome and physiology of a model Epsilonproteobacterium responsible for sulfide detoxification in marine oxygen depletion zones. Proc. Natl Acad. Sci. USA 109, 506–510 (2012).
Henson, M. W. et al. Artificial seawater media facilitate cultivating members of the microbial majority from the Gulf of Mexico. mSphere https://doi.org/10.1128/mSphere.00028-16 (2016).
Mok, J. K. et al. Iodate reduction by Shewanella oneidensis does not involve nitrate reductase. Geomicrobiol. J. 35, 570–579 (2018).
Fussel, J. et al. Nitrite oxidation in the Namibian oxygen minimum zone. ISME J. 6, 1200–1209 (2012).
Fussel, J. et al. Adaptability as the key to success for the ubiquitous marine nitrite oxidizer Nitrococcus. Sci. Adv. 3, e1700807 (2017).
Stewart, F. J. & Ulloa, O. in Metagenomics of the Microbial Nitrogen Cycle: Theory, Methods and Applications (ed. Marco, D.) 17–31 (Caister Academic Press, 2014).
Peng, X. F. et al. Ammonia and nitrite oxidation in the Eastern Tropical North Pacific. Global. Biogeochem. Cy. 29, 2034–2049 (2015).
Acknowledgements
The authors thank the National Science Foundation (grants 1151698, 1558916 and 1564559 to F.J.S.) for generous and valuable support of OMZ research.
Reviewer information
Nature Reviews Microbiology thanks A. Babbin, M. Kuypers and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bertagnolli, A.D., Stewart, F.J. Microbial niches in marine oxygen minimum zones. Nat Rev Microbiol 16, 723–729 (2018). https://doi.org/10.1038/s41579-018-0087-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-018-0087-z
This article is cited by
-
Methane-dependent complete denitrification by a single Methylomirabilis bacterium
Nature Microbiology (2024)
-
A compendium of bacterial and archaeal single-cell amplified genomes from oxygen deficient marine waters
Scientific Data (2023)
-
A miniaturized bionic ocean-battery mimicking the structure of marine microbial ecosystems
Nature Communications (2022)
-
Magnetotactic bacteria and magnetofossils: ecology, evolution and environmental implications
npj Biofilms and Microbiomes (2022)
-
Viral community analysis in a marine oxygen minimum zone indicates increased potential for viral manipulation of microbial physiological state
The ISME Journal (2022)