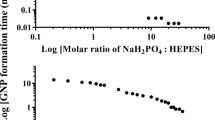
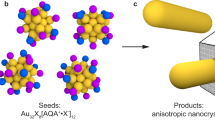
Abstract
An emerging strategy for synthesizing nanoclusters and nanoparticles involves the confinement of particle precursors within small volumes and the subsequent reduction and aggregation of those precursors into discrete particles. These spatially isolated volumes are termed nanoreactors, and they impose barriers that not only restrict the movement of metal atoms and other reactants but also provide reaction conditions that are distinct from those of the surrounding environment. Nanoreactors for particle syntheses can be prepared by various strategies, which fall generally into two categories: solution-based and substrate-confined. Solution-based nanoreactors are broadly defined as 3D capsules that can be manipulated in solution, whereas substrate-confined nanoreactors are isolated volumes on a macroscopic substrate or surface. Here, we survey and analyse the merits of different nanoreactor techniques used to synthesize clusters and nanoparticles that cannot easily be made using traditional methods. We look at how the focus in this field has expanded beyond pure synthesis to making massive and complex libraries of materials and enabling exploration of the materials genome through high-throughput screening techniques.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Davis, M. E., Chen, Z. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 7, 771–782 (2008).
Liu, J. et al. Recent advances of plasmonic nanoparticles and their applications. Materials 11, 1833 (2018).
Jin, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Murray, C. B., Kagan, C. R. & Bawendi, M. G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mat. Sci. 30, 545–610 (2000).
Jiang, Q. & Ward, M. D. Crystallization under nanoscale confinement. Chem. Soc. Rev. 43, 2066–2079 (2014).
Petrosko, S. H., Johnson, R., White, H. & Mirkin, C. A. Nanoreactors: small spaces, big implications in chemistry. J. Am. Chem. Soc. 138, 7443–7445 (2016).
Thanh, N. T. K., Maclean, N. & Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 114, 7610–7630 (2014).
Brown, K. A., Hedrick, J. L., Eichelsdoerfer, D. J. & Mirkin, C. A. Nanocombinatorics with cantilever-free scanning probe arrays. ACS Nano 13, 8–17 (2019).
Kim, K. T., Meeuwissen, S. A., Nolte, R. J. M. & van Hest, J. C. M. Smart nanocontainers and nanoreactors. Nanoscale 2, 844–858 (2010).
Li, B. et al. Organic templates for inorganic nanocrystal growth. Energy Environ. Mater. 2, 38–54 (2019).
Liu, Y., Goebl, J. & Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 42, 2610–2653 (2013).
Qiu, L., McCaffrey, R. & Zhang, W. Synthesis of metallic nanoparticles using closed-shell structures as templates. Chem. Asian J. 13, 362–372 (2018).
Vriezema, D. M. et al. Self-assembled nanoreactors. Chem. Rev. 105, 1445–1490 (2005).
Jones, M. R., Osberg, K. D., Macfarlane, R. J., Langille, M. R. & Mirkin, C. A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 111, 3736–3827 (2011).
Farrusseng, D. & Tuel, A. Perspectives on zeolite-encapsulated metal nanoparticles and their applications in catalysis. New J. Chem. 40, 3933–3949 (2016).
Jagadeesh, R. V. et al. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 358, 326–332 (2017).
McCaffrey, R. et al. Template synthesis of gold nanoparticles with an organic molecular cage. J. Am. Chem. Soc. 136, 1782–1785 (2014).
Antonietti, M., Wenz, E., Bronstein, L. & Seregina, M. Synthesis and characterization of noble metal colloids in block copolymer micelles. Adv. Mater. 7, 1000–1005 (1995).
Seregina, M. V. et al. Preparation of noble-metal colloids in block copolymer micelles and their catalytic properties in hydrogenation. Chem. Mater. 9, 923–931 (1997).
Qi, L., Cölfen, H. & Antonietti, M. Synthesis and characterization of CdS nanoparticles stabilized by double-hydrophilic block copolymers. Nano Lett. 1, 61–65 (2001).
Liu, S., Weaver, J. V. M., Save, M. & Armes, S. P. Synthesis of pH-responsive shell cross-linked micelles and their use as nanoreactors for the preparation of gold nanoparticles. Langmuir 18, 8350–8357 (2002).
Sakai, T. & Alexandridis, P. Mechanism of gold metal ion reduction, nanoparticle growth and size control in aqueous amphiphilic block copolymer solutions at ambient conditions. J. Phys. Chem. B 109, 7766–7777 (2005).
Vamvakaki, M. et al. Micellization in pH-sensitive amphiphilic block copolymers in aqueous media and the formation of metal nanoparticles. Faraday Discuss. 128, 129–147 (2005).
Bouyer, F., Sanson, N., Destarac, M. & Gérardin, C. Hydrophilic block copolymer-directed growth of lanthanum hydroxide nanoparticles. New J. Chem. 30, 399–408 (2006).
Chen, S. et al. Effect of hydrophobicity inside PEO–PPO–PEO block copolymer micelles on the stabilization of gold nanoparticles: experiments. Langmuir 22, 9704–9711 (2006).
Bakshi, M. S. Engineered nanomaterials growth control by monomers and micelles: from surfactants to surface active polymers. Adv. Colloid Interface Sci. 256, 101–110 (2018).
Podhorska, L. et al. Mechanisms of polymer-templated nanoparticle synthesis: contrasting ZnS and Au. Langmuir 32, 9216–9222 (2016).
Evers, M. V., Bernal, M., Roldan Cuenya, B. & Tschulik, K. Piece by piece — electrochemical synthesis of individual nanoparticles and their performance in ORR electrocatalysis. Angew. Chem. Int. Ed. 58, 8221–8225 (2019).
Behafarid, F. et al. Structural and electronic properties of micellar Au nanoparticles: size and ligand effects. ACS Nano 8, 6671–6681 (2014).
Zhang, J., Gao, Y., Alvarez-Puebla, R. A., Buriak, J. M. & Fenniri, H. Synthesis and SERS properties of nanocrystalline gold octahedra generated from thermal decomposition of HAuCl4 in block copolymers. Adv. Mater. 18, 3233–3237 (2006).
Menezes, W. G. et al. Synthesis of stable AuAg bimetallic nanoparticles encapsulated by diblock copolymer micelles. Nanoscale 4, 1658–1664 (2012).
Li, X., Fu, X. & Yang, H. Preparation and photocatalytic activity of eccentric Au–titania core–shell nanoparticles by block copolymer templates. Phys. Chem. Chem. Phys. 13, 2809–2814 (2011).
Dunlop, I. E. et al. Direct synthesis of PEG-encapsulated gold nanoparticles using branched copolymer nanoreactors. RSC Adv. 4, 27702–27707 (2014).
Seo, E. et al. Highly stable Au nanoparticles with double hydrophilic block copolymer templates: correlation between structure and stability. Polym. Chem. 8, 4528–4537 (2017).
Black, K. C. L., Liu, Z. & Messersmith, P. B. Catechol redox induced formation of metal core–polymer shell nanoparticles. Chem. Mater. 23, 1130–1135 (2011).
Bastakoti, B. P., Guragain, S., Yusa, S.-I. & Nakashima, K. Novel synthesis route for Ag@SiO2 core–shell nanoparticles via micelle template of double hydrophilic block copolymer. RSC Adv. 2, 5938–5940 (2012).
Xu, H. et al. An unconventional route to monodisperse and intimately contacted semiconducting organic–inorganic nanocomposites. Angew. Chem. Int. Ed. 54, 4636–4640 (2015).
Iocozzia, J. & Lin, Z. Solution-stable colloidal gold nanoparticles via surfactant-free, hyperbranched polyglycerol-b-polystyrene unimolecular templates. Langmuir 32, 7180–7188 (2016).
Lin, W. et al. Facile in situ preparation and in vitro antibacterial activity of PDMAEMA-based silver-bearing copolymer micelles. Nanoscale Res. Lett. 14, 256 (2019).
Dellinger, T. M. & Braun, P. V. Lyotropic liquid crystals as nanoreactors for nanoparticle synthesis. Chem. Mater. 16, 2201–2207 (2004).
Pena dos Santos, E. et al. Existence and stability of new nanoreactors: highly swollen hexagonal liquid crystals. Langmuir 21, 4362–4369 (2005).
Dutt, S., Siril, P. F. & Remita, S. Swollen liquid crystals (SLCs): a versatile template for the synthesis of nano structured materials. RSC Adv. 7, 5733–5750 (2017).
Lu, Y., Lin, J., Wang, L., Zhang, L. & Cai, C. Self-assembly of copolymer micelles: higher-level assembly for constructing hierarchical structure. Chem. Rev. 120, 4111–4140 (2020).
Zhao, Z. et al. General synthesis of ultrafine monodispersed hybrid nanoparticles from highly stable monomicelles. Adv. Mater. 33, 2100820 (2021).
Wang, H. et al. Rhodium nanoparticles inside well-defined unimolecular amphiphilic polymeric nanoreactors: synthesis and biphasic hydrogenation catalysis. Nanoscale Adv. 3, 2554–2566 (2021).
Li, Z., Peng, J. & Lin, Z. One-dimensional hairy CNT/polymer/Au nanocomposites via ligating with amphiphilic crosslinkable block copolymers. Giant 5, 100048 (2021).
Wang, Y. & Zhu, X. Nanofabrication within unimolecular nanoreactors. Nanoscale 12, 12698–12711 (2020).
Myers, V. S. et al. Dendrimer-encapsulated nanoparticles: new synthetic and characterization methods and catalytic applications. Chem. Sci. 2, 1632–1646 (2011).
Bronstein, L. M. & Shifrina, Z. B. Dendrimers as encapsulating, stabilizing, or directing agents for inorganic nanoparticles. Chem. Rev. 111, 5301–5344 (2011).
Yamamoto, K., Imaoka, T., Tanabe, M. & Kambe, T. New horizon of nanoparticle and cluster catalysis with dendrimers. Chem. Rev. 120, 1397–1437 (2020).
Crooks, R. M., Zhao, M., Sun, L., Chechik, V. & Yeung, L. K. Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 34, 181–190 (2001).
Scott, R. W. J., Wilson, O. M. & Crooks, R. M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 109, 692–704 (2005).
Zhang, L., Iyyamperumal, R., Yancey, D. F., Crooks, R. M. & Henkelman, G. Design of Pt-shell nanoparticles with alloy cores for the oxygen reduction reaction. ACS Nano 7, 9168–9172 (2013).
Ballauff, M. & Likos, C. N. Dendrimers in solution: insight from theory and simulation. Angew. Chem. Int. Ed. 43, 2998–3020 (2004).
Yamamoto, K. & Imaoka, T. Precision synthesis of subnanoparticles using dendrimers as a superatom synthesizer. Acc. Chem. Res. 47, 1127–1136 (2014).
Tsukamoto, T., Kambe, T., Nakao, A., Imaoka, T. & Yamamoto, K. Atom-hybridization for synthesis of polymetallic clusters. Nat. Commun. 9, 3873 (2018).
Imaoka, T. & Yamamoto, K. Wet-chemical strategy for atom-precise metal cluster catalysts. Bull. Chem. Soc. Jpn 92, 941–948 (2019).
Albrecht, K., Sakane, N., Inomata, Y. & Yamamoto, K. Effect of the core structure on the sequential coordination of phenylazomethine dendrimer. J. Inorg. Organomet. Polym. Mater. 25, 133–139 (2015).
Yamamoto, K., Higuchi, M., Shiki, S., Tsuruta, M. & Chiba, H. Stepwise radial complexation of imine groups in phenylazomethine dendrimers. Nature 415, 509–511 (2002).
Imaoka, T. et al. Probing stepwise complexation in phenylazomethine dendrimers by a metallo-porphyrin core. J. Am. Chem. Soc. 127, 13896–13905 (2005).
Imaoka, T., Horiguchi, H. & Yamamoto, K. Metal assembly in novel dendrimers with porphyrin cores. J. Am. Chem. Soc. 125, 340–341 (2003).
Imaoka, T., Tanaka, R. & Yamamoto, K. Investigation of a molecular morphology effect on polyphenylazomethine dendrimers; physical properties and metal-assembling processes. Chem. Eur. J. 12, 7328–7336 (2006).
Tsukamoto, T., Kuzume, A., Nagasaka, M., Kambe, T. & Yamamoto, K. Quantum materials exploration by sequential screening technique of heteroatomicity. J. Am. Chem. Soc. 142, 19078–19084 (2020).
Pang, X., Zhao, L., Han, W., Xin, X. & Lin, Z. A general and robust strategy for the synthesis of nearly monodisperse colloidal nanocrystals. Nat. Nanotechnol. 8, 426–431 (2013).
Chen, Y. et al. Precisely size-tunable monodisperse hairy plasmonic nanoparticles via amphiphilic star-like block copolymers. Small 12, 6714–6723 (2016).
Yang, K., Feng, L. & Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 105, 228–241 (2016).
Chen, Y. et al. Hairy uniform permanently ligated hollow nanoparticles with precise dimension control and tunable optical properties. J. Am. Chem. Soc. 139, 12956–12967 (2017).
Bai, J. et al. Highly water-dispersed superparamagnetic magnetite colloidal nanocrystal clusters from multifunctional polymeric nanoreactors: synthesis and properties. RSC Adv. 6, 9429–9435 (2016).
Chen, M. et al. Cyclodextrin-based polymer-assisted Ru nanoparticles for the aqueous hydrogenation of biomass-derived platform molecules. ChemistrySelect 2, 10537–10545 (2017).
Liang, T. et al. Unconventional approach to fabricating a TiO2 nanoring with precise dimension control based on starlike polymeric nanoreactors. J. Phys. Chem. Lett. 12, 3456–3463 (2021).
Pang, X., He, Y., Jung, J. & Lin, Z. 1D nanocrystals with precisely controlled dimensions, compositions, and architectures. Science 353, 1268–1272 (2016).
Xing, H. et al. Bottom-up strategy to prepare nanoparticles with a single DNA strand. J. Am. Chem. Soc. 139, 3623–3626 (2017).
Bai, Y. et al. Independent control over size, valence, and elemental composition in the synthesis of DNA–nanoparticle conjugates. Chem. Sci. 11, 1564–1572 (2020).
Morita, T. et al. Impact of temperature on the fusion growth of bimetallic Au–Pt nanoparticles from each nanocluster conjugated with a thermoresponsive polymer. Cryst. Growth Des. 19, 6199–6206 (2019).
Eastoe, J., Hollamby, M. J. & Hudson, L. Recent advances in nanoparticle synthesis with reversed micelles. Adv. Colloid Interface Sci. 128-130, 5–15 (2006).
Ganguli, A. K., Ganguly, A. & Vaidya, S. Microemulsion-based synthesis of nanocrystalline materials. Chem. Soc. Rev. 39, 474–485 (2010).
Wolf, S. & Feldmann, C. Microemulsions: options to expand the synthesis of inorganic nanoparticles. Angew. Chem. Int. Ed. 55, 15728–15752 (2016).
Boutonnet, M. & Sanchez-Dominguez, M. Microemulsion droplets to catalytically active nanoparticles. How the application of colloidal tools in catalysis aims to well designed and efficient catalysts. Catal. Today 285, 89–103 (2017).
Das, A., Yadav, N., Manchala, S., Bungla, M. & Ganguli, A. K. Mechanistic investigations of growth of anisotropic nanostructures in reverse micelles. ACS Omega 6, 1007–1029 (2021).
Richard, B., Lemyre, J.-L. & Ritcey, A. M. Nanoparticle size control in microemulsion synthesis. Langmuir 33, 4748–4757 (2017).
Bryant, K., Ibrahim, G. & Saunders, S. R. Switchable surfactants for the preparation of monodisperse, supported nanoparticle catalysts. Langmuir 33, 12982–12988 (2017).
Yadav, N., Chowdhury, P. K. & Ganguli, A. K. Mechanistic insights into the growth of anisotropic nanostructures inside reverse micelles: a solvation perspective. J. Phys. Chem. B 123, 5324–5336 (2019).
Sharma, S., Yadav, N., Chowdhury, P. K. & Ganguli, A. K. Controlling the microstructure of reverse micelles and their templating effect on shaping nanostructures. J. Phys. Chem. B 119, 11295–11306 (2015).
Kaur, R. & Mehta, S. K. Metallomicelle templated transition metal nanostructures: synthesis, characterization, DFT study and catalytic activity. Phys. Chem. Chem. Phys. 19, 18372–18382 (2017).
Koninti, R. K., Satpathi, S. & Hazra, P. Ultrafast fluorescence dynamics of highly stable copper nanoclusters synthesized inside the aqueous nanopool of reverse micelles. J. Phys. Chem. C 122, 5742–5752 (2018).
Glasscott, M. W., Pendergast, A. D. & Dick, J. E. A universal platform for the electrodeposition of ligand-free metal nanoparticles from a water-in-oil emulsion system. ACS Appl. Nano Mater. 1, 5702–5711 (2018).
Glasscott, M. W. et al. Electrosynthesis of high-entropy metallic glass nanoparticles for designer, multi-functional electrocatalysis. Nat. Commun. 10, 2650 (2019).
Jeun, Y. E., Baek, B., Lee, M. W. & Ahn, H. S. Surfactant-free electrochemical synthesis of metallic nanoparticles via stochastic collisions of aqueous nanodroplet reactors. Chem. Commun. 54, 10052–10055 (2018).
Park, J. H. & Ahn, H. S. Electrochemical synthesis of multimetallic nanoparticles and their application in alkaline oxygen reduction catalysis. Appl. Surf. Sci. 504, 144517 (2020).
Damarla, K., Rachuri, Y., Suresh, E. & Kumar, A. Nanoemulsions with all ionic liquid components as recyclable nanoreactors. Langmuir 34, 10081–10091 (2018).
Pei, Y. et al. Nanoreactors stable up to 200°C: a class of high temperature microemulsions composed solely of ionic liquids. Chem. Commun. 54, 6260–6263 (2018).
Boken, J., Soni, S. K. & Kumar, D. Microfluidic synthesis of nanoparticles and their biosensing applications. Crit. Rev. Anal. Chem. 46, 538–561 (2016).
Pan, L.-J. et al. Controllable synthesis of nanocrystals in droplet reactors. Lab Chip 18, 41–56 (2018).
Santana, J. S. & Skrabalak, S. E. Continuous flow routes toward designer metal nanocatalysts. Adv. Energy Mater. 10, 1902051 (2020).
Sui, J., Yan, J., Liu, D., Wang, K. & Luo, G. Continuous synthesis of nanocrystals via flow chemistry technology. Small 16, 1902828 (2020).
Roberts, E. J., Karadaghi, L. R., Wang, L., Malmstadt, N. & Brutchey, R. L. Continuous flow methods of fabricating catalytically active metal nanoparticles. ACS Appl. Mater. Interfaces 11, 27479–27502 (2019).
Solsona, M. et al. Microfluidics and catalyst particles. Lab Chip 19, 3575–3601 (2019).
Gao, Y., Pinho, B. & Torrente-Murciano, L. Recent progress on the manufacturing of nanoparticles in multi-phase and single-phase flow reactors. Curr. Opin. Chem. Eng. 29, 26–33 (2020).
Ding, Y., Howes, P. D. & deMello, A. J. Recent advances in droplet microfluidics. Anal. Chem. 92, 132–149 (2020).
Suea-Ngam, A., Howes, P. D., Srisa-Art, M. & deMello, A. J. Droplet microfluidics: from proof-of-concept to real-world utility? Chem. Commun. 55, 9895–9903 (2019).
Niu, G., Ruditskiy, A., Vara, M. & Xia, Y. Toward continuous and scalable production of colloidal nanocrystals by switching from batch to droplet reactors. Chem. Soc. Rev. 44, 5806–5820 (2015).
Khan, S. A., Günther, A., Schmidt, M. A. & Jensen, K. F. Microfluidic synthesis of colloidal silica. Langmuir 20, 8604–8611 (2004).
Wang, H., Nakamura, H., Uehara, M., Miyazaki, M. & Maeda, H. Preparation of titania particles utilizing the insoluble phase interface in a microchannel reactor. Chem. Commun. 2002, 1462–1463 (2002).
Takagi, M., Maki, T., Miyahara, M. & Mae, K. Production of titania nanoparticles by using a new microreactor assembled with same axle dual pipe. Chem. Eng. J. 101, 269–276 (2004).
Shestopalov, I., Tice, J. D. & Ismagilov, R. F. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip 4, 316–321 (2004).
Hung, L.-H. et al. Alternating droplet generation and controlled dynamic droplet fusion in microfluidic device for CdS nanoparticle synthesis. Lab Chip 6, 174–178 (2006).
Chan, E. M., Alivisatos, A. P. & Mathies, R. A. High-temperature microfluidic synthesis of CdSe nanocrystals in nanoliter droplets. J. Am. Chem. Soc. 127, 13854–13861 (2005).
Yen, B. K. H., Günther, A., Schmidt, M. A., Jensen, K. F. & Bawendi, M. G. A microfabricated gas–liquid segmented flow reactor for high-temperature synthesis: the case of CdSe quantum dots. Angew. Chem. Int. Ed. 44, 5447–5451 (2005).
Panariello, L. et al. Highly reproducible, high-yield flow synthesis of gold nanoparticles based on a rational reactor design exploiting the reduction of passivated Au(iii). React. Chem. Eng. 5, 663–676 (2020).
Ahrberg, C. D., Wook Choi, J. & Geun Chung, B. Automated droplet reactor for the synthesis of iron oxide/gold core-shell nanoparticles. Sci. Rep. 10, 1737 (2020).
Santana, J. S., Gamler, J. T. L. & Skrabalak, S. E. Integration of sequential reactions in a continuous flow droplet reactor: a route to architecturally defined bimetallic nanostructures. Part. Part. Syst. Charact. 36, 1900142 (2019).
Tofighi, G. et al. Microfluidic synthesis of ultrasmall AuPd nanoparticles with a homogeneously mixed alloy structure in fast continuous flow for catalytic applications. J. Phys. Chem. C 122, 1721–1731 (2018).
Li, X. et al. Microfluidically assisted construction of hierarchical multicomponent microparticles for short intermediate diffusion paths in heterogeneous catalysis. ACS Appl. Nano Mater. 1, 6398–6406 (2018).
Kwak, C. H. et al. Customized microfluidic reactor based on droplet formation for the synthesis of monodispersed silver nanoparticles. J. Ind. Eng. Chem. 63, 405–410 (2018).
Gu, T. et al. Electrically controlled mass transport into microfluidic droplets from nanodroplet carriers with application in controlled nanoparticle flow synthesis. Lab Chip 18, 1330–1340 (2018).
Abalde-Cela, S., Taladriz-Blanco, P., de Oliveira, M. G. & Abell, C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci. Rep. 8, 2440 (2018).
Wang, Z. et al. Microfluidic synthesis and characterization of FePtSn/C catalysts with enhanced electro-catalytic performance for direct methanol fuel cells. Electrochim. Acta 230, 245–254 (2017).
Tao, S., Yang, M., Chen, H., Ren, M. & Chen, G. Microfluidic synthesis of Ag@Cu2O core-shell nanoparticles with enhanced photocatalytic activity. J. Colloid Interface Sci. 486, 16–26 (2017).
Santana, J. S., Koczkur, K. M. & Skrabalak, S. E. Synthesis of core@shell nanostructures in a continuous flow droplet reactor: controlling structure through relative flow rates. Langmuir 33, 6054–6061 (2017).
Maguire, P. et al. Continuous in-flight synthesis for on-demand delivery of ligand-free colloidal gold nanoparticles. Nano Lett. 17, 1336–1343 (2017).
Li, X. et al. Hierarchically structured particles for micro flow catalysis. Chem. Eng. J. 326, 1058–1065 (2017).
Kunal, P. et al. Continuous flow synthesis of Rh and RhAg alloy nanoparticle catalysts enables scalable production and improved morphological control. Chem. Mater. 29, 4341–4350 (2017).
Kulkarni, A. A. & Sebastian Cabeza, V. Insights in the diffusion controlled interfacial flow synthesis of Au nanostructures in a microfluidic system. Langmuir 33, 14315–14324 (2017).
He, X. et al. Overview of the application of flow microreactors in the synthesis of silver nanomaterials. Nano 12, 1730002 (2017).
Xu, L., Peng, J., Yan, M., Zhang, D. & Shen, A. Q. Droplet synthesis of silver nanoparticles by a microfluidic device. Chem. Eng. Process. 102, 186–193 (2016).
Sebastian, V., Smith, C. D. & Jensen, K. F. Shape-controlled continuous synthesis of metal nanostructures. Nanoscale 8, 7534–7543 (2016).
Riche, C. T., Roberts, E. J., Gupta, M., Brutchey, R. L. & Malmstadt, N. Flow invariant droplet formation for stable parallel microreactors. Nat. Commun. 7, 10780 (2016).
Niu, G. et al. Synthesis of Pt–Ni octahedra in continuous-flow droplet reactors for the scalable production of highly active catalysts toward oxygen reduction. Nano Lett. 16, 3850–3857 (2016).
Zhang, D. et al. One-step, facile and ultrafast synthesis of phase- and size-controlled Pt–Bi intermetallic nanocatalysts through continuous-flow microfluidics. J. Am. Chem. Soc. 137, 6263–6269 (2015).
Hafermann, L. & Köhler, J. M. Photochemical micro continuous-flow synthesis of noble metal nanoparticles of the platinum group. Chem. Eng. Tehcnol. 38, 1138–1143 (2015).
Hafermann, L. & Michael Köhler, J. Small gold nanoparticles formed by rapid photochemical flow-through synthesis using microfluid segment technique. J. Nanopart. Res. 17, 99 (2015).
Larrea, A., Sebastian, V., Ibarra, A., Arruebo, M. & Santamaria, J. Gas slug microfluidics: a unique tool for ultrafast, highly controlled growth of iron oxide nanostructures. Chem. Mater. 27, 4254–4260 (2015).
Zukas, B. G. & Gupta, N. R. Interphase synthesis of zinc oxide nanoparticles in a droplet flow reactor. Ind. Eng. Chem. Res. 56, 7184–7191 (2017).
Yashina, A., Lignos, I., Stavrakis, S., Choo, J. & deMello, A. J. Scalable production of CuInS2/ZnS quantum dots in a two-step droplet-based microfluidic platform. J. Mater. Chem. C 4, 6401–6408 (2016).
Wang, J., Zhao, H., Zhu, Y. & Song, Y. Shape-controlled synthesis of CdSe nanocrystals via a programmed microfluidic process. J. Phys. Chem. C 121, 3567–3572 (2017).
Lignos, I., Maceiczyk, R. M., Kovalenko, M. V. & Stavrakis, S. Tracking the fluorescence lifetimes of cesium lead halide perovskite nanocrystals during their synthesis using a fully automated optofluidic platform. Chem. Mater. 32, 27–37 (2020).
Abdel-Latif, K. et al. Facile room-temperature anion exchange reactions of inorganic perovskite quantum dots enabled by a modular microfluidic platform. Adv. Funct. Mater. 29, 1900712 (2019).
Lignos, I. et al. Exploration of near-infrared-emissive colloidal multinary lead halide perovskite nanocrystals using an automated microfluidic platform. ACS Nano 12, 5504–5517 (2018).
Bezinge, L., Maceiczyk, R. M., Lignos, I., Kovalenko, M. V. & deMello, A. J. Pick a color MARIA: adaptive sampling enables the rapid identification of complex perovskite nanocrystal compositions with defined emission characteristics. ACS Appl. Mater. Interfaces 10, 18869–18878 (2018).
Maceiczyk, R. M. et al. Microfluidic reactors provide preparative and mechanistic insights into the synthesis of formamidinium lead halide perovskite nanocrystals. Chem. Mater. 29, 8433–8439 (2017).
Lignos, I. et al. Synthesis of cesium lead halide perovskite nanocrystals in a droplet-based microfluidic platform: fast parametric space mapping. Nano Lett. 16, 1869–1877 (2016).
Abdel-Latif, K., Bateni, F., Crouse, S. & Abolhasani, M. Flow synthesis of metal halide perovskite quantum dots: from rapid parameter space mapping to AI-guided modular manufacturing. Matter 3, 1053–1086 (2020).
Wang, L., Karadaghi, L. R., Brutchey, R. L. & Malmstadt, N. Self-optimizing parallel millifluidic reactor for scaling nanoparticle synthesis. Chem. Commun. 56, 3745–3748 (2020).
Ma, H., Pan, L., Wang, J., Zhang, L. & Zhang, Z. Synthesis of AgInS2 QDs in droplet microreactors: online fluorescence regulating through temperature control. Chin. Chem. Lett. 30, 79–82 (2019).
Guidelli, E. J. et al. Mechanistic insights and controlled synthesis of radioluminescent ZnSe quantum dots using a microfluidic reactor. Chem. Mater. 30, 8562–8570 (2018).
Swain, B. et al. Optimization of CdSe nanocrystals synthesis with a microfluidic reactor and development of combinatorial synthesis process for industrial production. Chem. Eng. J. 308, 311–321 (2017).
Lignos, I., Maceiczyk, R. & deMello, A. J. Microfluidic technology: uncovering the mechanisms of nanocrystal nucleation and growth. Acc. Chem. Res. 50, 1248–1257 (2017).
Tian, Z.-H., Wang, Y.-J., Xu, J.-H. & Luo, G.-S. Intensification of nucleation stage for synthesizing high quality CdSe quantum dots by using preheated precursors in microfluidic devices. Chem. Eng. J. 302, 498–502 (2016).
Tian, Z.-H., Xu, J.-H., Wang, Y.-J. & Luo, G.-S. Microfluidic synthesis of monodispersed CdSe quantum dots nanocrystals by using mixed fatty amines as ligands. Chem. Eng. J. 285, 20–26 (2016).
Shu, Y., Jiang, P., Pang, D.-W. & Zhang, Z.-L. Droplet-based microreactor for synthesis of water-soluble Ag2S quantum dots. Nanotechnology 26, 275701 (2015).
Lignos, I. et al. Millisecond-timescale monitoring of PbS nanoparticle nucleation and growth using droplet-based microfluidics. Small 11, 4009–4017 (2015).
Cheng, Y., Ling, S. D., Geng, Y., Wang, Y. & Xu, J. Microfluidic synthesis of quantum dots and their applications in bio-sensing and bio-imaging. Nanoscale Adv. 3, 2180–2195 (2021).
Jambovane, S. R. et al. Continuous, one-pot synthesis and post-synthetic modification of nanoMOFs using droplet nanoreactors. Sci. Rep. 6, 36657 (2016).
González-Estefan, J. H., Gonidec, M., Daro, N., Marchivie, M. & Chastanet, G. Extreme downsizing in the surfactant-free synthesis of spin-crossover nanoparticles in a microfluidic flow-focusing junction. Chem. Commun. 54, 8040–8043 (2018).
Zhu, P. & Wang, L. Passive and active droplet generation with microfluidics: a review. Lab Chip 17, 34–75 (2017).
Liu, J. et al. Yolk/shell nanoparticles: new platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 47, 12578–12591 (2011).
Wang, X., Feng, J., Bai, Y., Zhang, Q. & Yin, Y. Synthesis, properties, and applications of hollow micro-/nanostructures. Chem. Rev. 116, 10983–11060 (2016).
Kumar, A., Jeon, K.-W., Kumari, N. & Lee, I. S. Spatially confined formation and transformation of nanocrystals within nanometer-sized reaction media. Acc. Chem. Res. 51, 2867–2879 (2018).
Lou, X. W., Archer, L. A. & Yang, Z. Hollow micro-/nanostructures: synthesis and applications. Adv. Mater. 20, 3987–4019 (2008).
Shaik, F. Ligand-free yolk-shell nanoparticles: synthesis and catalytic applications. ChemNanoMat 6, 1449–1473 (2020).
Hah, H. J., Um, J. I., Han, S. H. & Koo, S. M. New synthetic route for preparing rattle-type silica particles with metal cores. Chem. Commun. 2004, 1012–1013 (2004).
Cheng, D., Zhou, X., Xia, H. & Chan, H. S. O. Novel method for the preparation of polymeric hollow nanospheres containing silver cores with different sizes. Chem. Mater. 17, 3578–3581 (2005).
Gao, C., Zhang, Q., Lu, Z. & Yin, Y. Templated synthesis of metal nanorods in silica nanotubes. J. Am. Chem. Soc. 133, 19706–19709 (2011).
Shaik, F., Zhang, W. & Niu, W. A novel photochemical method for the synthesis of Au triangular nanoplates inside nanocavity of mesoporous silica shells. J. Phys. Chem. C 121, 9572–9578 (2017).
Shaik, F., Zhang, W., Niu, W. & Lu, X. Volume-confined synthesis of ligand-free gold nanoparticles with tailored sizes for enhanced catalytic activity. Chem. Phys. Lett. 613, 95–99 (2014).
Shaik, F., Zhang, W. & Niu, W. A generalized method for the synthesis of ligand-free M@SiO2 (M = Ag, Au, Pd, Pt) Yolk–shell nanoparticles. Langmuir 33, 3281–3286 (2017).
Zhang, L. et al. Spatially controlled reduction and growth of silver in hollow gold nanoshell particles. J. Phys. Chem. C 123, 10614–10621 (2019).
Yeo, K. M., Choi, S., Anisur, R. M., Kim, J. & Lee, I. S. Surfactant-free platinum-on-gold nanodendrites with enhanced catalytic performance for oxygen reduction. Angew. Chem. Int. Ed. 50, 745–748 (2011).
Kim, D. et al. Confined nucleation and growth of PdO nanocrystals in a seed-free solution inside hollow nanoreactor. ACS Appl. Mater. Interfaces 9, 29992–30001 (2017).
Jeong, K., Kim, S. M. & Lee, I. S. A seed-engineering approach toward a hollow nanoreactor suitable for the confined synthesis of less-noble Ni-based nanocrystals. Chem. Commun. 51, 499–502 (2015).
Wu, S.-H. et al. Catalytic nano-rattle of Au@hollow silica: towards a poison-resistant nanocatalyst. J. Mater. Chem. 21, 789–794 (2011).
Uchida, M. et al. Biological containers: protein cages as multifunctional nanoplatforms. Adv. Mater. 19, 1025–1042 (2007).
Jutz, G., van Rijn, P., Santos Miranda, B. & Böker, A. Ferritin: a versatile building block for bionanotechnology. Chem. Rev. 115, 1653–1701 (2015).
Yamashita, I. in Biological Magnetic Materials and Applications 1st edn Ch. 6 (eds Matsunaga T., Tanaka T. & Kisailus D.) 135–153 (Springer, 2018).
Abe, S., Maity, B. & Ueno, T. Design of a confined environment using protein cages and crystals for the development of biohybrid materials. Chem. Commun. 52, 6496–6512 (2016).
Huang, J. et al. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 44, 6330–6374 (2015).
Dickerson, M. B., Sandhage, K. H. & Naik, R. R. Protein- and peptide-directed syntheses of inorganic materials. Chem. Rev. 108, 4935–4978 (2008).
Pan, Y., Paschoalino, W. J., Szuchmacher Blum, A. & Mauzeroll, J. Recent advances in bio-templated metallic nanomaterial synthesis and electrocatalytic applications. ChemSusChem 14, 758–791 (2021).
Douglas, T. & Young, M. Host–guest encapsulation of materials by assembled virus protein cages. Nature 393, 152–155 (1998).
Zhou, Z., Bedwell, G. J., Li, R., Prevelige, P. E. & Gupta, A. Formation mechanism of chalcogenide nanocrystals confined inside genetically engineered virus-like particles. Sci. Rep. 4, 3832 (2014).
Peng, T., Paramelle, D., Sana, B., Lee, C. F. & Lim, S. Designing non-native iron-binding site on a protein cage for biological synthesis of nanoparticles. Small 10, 3131–3138 (2014).
Douglas, T. et al. Protein engineering of a viral cage for constrained nanomaterials synthesis. Adv. Mater. 14, 415–418 (2002).
Melman, A. in Fine Particles in Medicine and Pharmacy (ed. Matijević, E.) 195–221 (Springer, 2012).
Jin, Y., He, J., Fan, K. & Yan, X. Ferritin variants: inspirations for rationally designing protein nanocarriers. Nanoscale 11, 12449–12459 (2019).
Meldrum, F. C., Wade, V. J., Nimmo, D. L., Heywood, B. R. & Mann, S. Synthesis of inorganic nanophase materials in supramolecular protein cages. Nature 349, 684–687 (1991).
Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry (Oxford Univ. Press, 2001).
Wang, Z. et al. Biomineralization-inspired synthesis of copper sulfide–ferritin nanocages as cancer theranostics. ACS Nano 10, 3453–3460 (2016).
Li, T. et al. Synthesis and characterization of Au-core Ag-shell nanoparticles from unmodified apoferritin. J. Mater. Chem. 22, 14458–14464 (2012).
Wu, H., Engelhard, M. H., Wang, J., Fisher, D. R. & Lin, Y. Synthesis of lutetium phosphate–apoferritin core–shell nanoparticles for potential applications in radioimmunoimaging and radioimmunotherapy of cancers. J. Mater. Chem. 18, 1779–1783 (2008).
Suzuki, M. et al. Preparation and catalytic reaction of Au/Pd bimetallic nanoparticles in Apo-ferritin. Chem. Commun. 2009, 4871–4873 (2009).
Shin, Y., Dohnalkova, A. & Lin, Y. Preparation of homogeneous gold–silver alloy nanoparticles using the apoferritin cavity as a nanoreactor. J. Phys. Chem. C 114, 5985–5989 (2010).
Allen, M., Willits, D., Mosolf, J., Young, M. & Douglas, T. Protein cage constrained synthesis of ferrimagnetic iron oxide nanoparticles. Adv. Mater. 14, 1562–1565 (2002).
Allen, M., Willits, D., Young, M. & Douglas, T. Constrained synthesis of cobalt oxide nanomaterials in the 12-subunit protein cage from Listeria innocua. Inorg. Chem. 42, 6300–6305 (2003).
Iwahori, K. et al. Cadmium sulfide nanoparticle synthesis in Dps protein from Listeria innocua. Chem. Mater. 19, 3105–3111 (2007).
Matsuura, K. Synthetic approaches to construct viral capsid-like spherical nanomaterials. Chem. Commun. 54, 8944–8959 (2018).
Klem, M. T., Young, M. & Douglas, T. Biomimetic synthesis of β-TiO2 inside a viral capsid. J. Mater. Chem. 18, 3821–3823 (2008).
Bedwell, G. J. et al. Selective biotemplated synthesis of TiO2 inside a protein cage. Biomacromolecules 16, 214–218 (2015).
Zhang, W., Zhang, Z.-P., Zhang, X.-E. & Li, F. Reaction inside a viral protein nanocage: mineralization on a nanoparticle seed after encapsulation via self-assembly. Nano Res. 10, 3285–3294 (2017).
Liu, A., Yang, L., Verwegen, M., Reardon, D. & Cornelissen, J. J. L. M. Construction of core-shell hybrid nanoparticles templated by virus-like particles. RSC Adv. 7, 56328–56334 (2017).
Fendler, J. H. Atomic and molecular clusters in membrane mimetic chemistry. Chem. Rev. 87, 877–899 (1987).
Vargas, K. M. & Shon, Y.-S. Hybrid lipid–nanoparticle complexes for biomedical applications. J. Mater. Chem. B 7, 695–708 (2019).
Dong, R., Liu, W. & Hao, J. Soft vesicles in the synthesis of hard materials. Acc. Chem. Res. 45, 504–513 (2012).
Mann, S., Hannington, J. P. & Williams, R. J. P. Phospholipid vesicles as a model system for biomineralization. Nature 324, 565–567 (1986).
Korgel, B. A. & Monbouquette, H. G. Synthesis of size-monodisperse CdS nanocrystals using phosphatidylcholine vesicles as true reaction compartments. J. Phys. Chem. 100, 346–351 (1996).
Kennedy, M. T., Korgel, B. A., Monbouquette, H. G. & Zasadzinski, J. A. Cryo-transmission electron microscopy confirms controlled synthesis of cadmium sulfide nanocrystals within lecithin vesicles. Chem. Mater. 10, 2116–2119 (1998).
Korgel, B. A. & Monbouquette, H. G. Controlled synthesis of mixed core and layered (Zn,Cd)S and (Hg,Cd)S nanocrystals within phosphatidylcholine vesicles. Langmuir 16, 3588–3594 (2000).
Genç, R., Clergeaud, G., Ortiz, M. & O’Sullivan, C. K. Green synthesis of gold nanoparticles using glycerol-incorporated nanosized liposomes. Langmuir 27, 10894–10900 (2011).
Clergeaud, G., Genç, R., Ortiz, M. & O’Sullivan, C. K. Liposomal nanoreactors for the synthesis of monodisperse palladium nanoparticles using glycerol. Langmuir 29, 15405–15413 (2013).
Genç, R., Ortiz, M. & O’Sullivan, C. K. Diffusion-controlled synthesis of gold nanoparticles: nano-liposomes as mass transfer barrier. J. Nanopart. Res. 16, 2329 (2014).
Genc, R., Clergeaud, G., Ortiz, M. & O’Sullivan, C. Shape directed biomineralization of gold nanoparticles using self-assembled lipid structures. Biomater. Sci. 2, 1128–1134 (2014).
Witzigmann, D. et al. Formation of lipid and polymer based gold nanohybrids using a nanoreactor approach. RSC Adv. 5, 74320–74328 (2015).
Song, W., Liu, X., Yang, Y., Han, X. & Deng, Q. Synthesis of magnetic core–shell structure Fe3O4@MCM-41 nanoparticle by vesicles in aqueous solutions. Chin. J. Chem. Eng. 23, 1398–1402 (2015).
Lee, J.-H. et al. General and programmable synthesis of hybrid liposome/metal nanoparticles. Sci. Adv. 2, e1601838 (2016).
Hawker, C. J. & Russell, T. P. Block copolymer lithography: merging “bottom-up” with “top-down” processes. MRS Bull. 30, 952–966 (2011).
Dai, Y., Lu, P., Cao, Z., Campbell, C. T. & Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 47, 4314–4331 (2018).
Glass, R., Möller, M. & Spatz, J. P. Block copolymer micelle nanolithography. Nanotechnology 14, 1153–1160 (2003).
Möller, M., Spatz, J. P. & Roescher, A. Gold nanoparticles in micellar poly(styrene)-b-poly(ethylene oxide) films — size and interparticle distance control in monoparticulate films. Adv. Mater. 8, 337–340 (1996).
Spatz, J. P. et al. Ordered deposition of inorganic clusters from micellar block copolymer films. Langmuir 16, 407–415 (2000).
Arnold, M. et al. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 5, 383–388 (2004).
Glass, R. et al. Micro-nanostructured interfaces fabricated by the use of inorganic block copolymer micellar monolayers as negative resist for electron-beam lithography. Adv. Funct. Mater. 13, 569–575 (2003).
Liu, P. & Ding, J. Fabrication of micro–nano hybrid patterns on a solid surface. Langmuir 26, 492–497 (2010).
Lohmueller, T., Bock, E. & Spatz, J. P. Synthesis of quasi-hexagonal ordered arrays of metallic nanoparticles with tuneable particle size. Adv. Mater. 20, 2297–2302 (2008).
Gorzolnik, B., Mela, P. & Moeller, M. Nano-structured micropatterns by combination of block copolymer self-assembly and UV photolithography. Nanotechnology 17, 5027–5032 (2006).
Cummins, C., Ghoshal, T., Holmes, J. D. & Morris, M. A. Strategies for inorganic incorporation using neat block copolymer thin films for etch mask function and nanotechnological application. Adv. Mater. 28, 5586–5618 (2016).
Lohmüller, T. et al. Nanopatterning by block copolymer micelle nanolithography and bioinspired applications. Biointerphases 6, MR1–MR12 (2011).
Yap, F. L. & Krishnamoorthy, S. Fabricating 2D arrays of chemical templates for in situ synthesis of inorganic nanostructures using self-assembly based nanolithography. J. Mater. Chem. 20, 10211–10216 (2010).
Aizawa, M. & Buriak, J. M. Block copolymer templated chemistry for the formation of metallic nanoparticle arrays on semiconductor. Surf. Chem. Mater. 19, 5090–5101 (2007).
Pan, D., Fu, Q. & Lu, J. Nanolithography through mixture of block copolymer micelles. Nanotechnology 23, 305302 (2012).
Bosworth, J. K. et al. Control of self-assembly of lithographically patternable block copolymer films. ACS Nano 2, 1396–1402 (2008).
Zu, X., Tu, W. & Deng, Y. General approach for fabricating nanoparticle arrays via patterned block copolymer nanoreactors. J. Nanopart. Res. 13, 1–13 (2011).
Shin, I., Han, K. H., Cha, S. K., Kim, S. O. & Seo, M. Synthesis of carboxylic acid-functionalized polymethacrylate-b-polystyrene as an Ag ion-loadable block copolymer thin film template. Polymer 217, 123462 (2021).
Roulet, M., Vayer, M. & Sinturel, C. A simple route to ordered metal oxide nanoparticle arrays using block copolymer thin films. Eur. Polym. J. 49, 3897–3903 (2013).
Mendoza, C. et al. In situ synthesis and alignment of Au nanoparticles within hexagonally packed cylindrical domains of diblock copolymers in bulk. Langmuir 25, 9571–9578 (2009).
Kruss, S., Srot, V., van Aken, P. A. & Spatz, J. P. Au–Ag hybrid nanoparticle patterns of tunable size and density on glass and polymeric supports. Langmuir 28, 1562–1568 (2012).
Cha, S. K. et al. Au–Ag core–shell nanoparticle array by block copolymer lithography for synergistic broadband plasmonic properties. ACS Nano 9, 5536–5543 (2015).
Tang, Z. et al. Fabrication of Au nanoparticle arrays on flexible substrate for tunable localized surface plasmon resonance. ACS Appl. Mater. Interfaces 13, 9281–9288 (2021).
Daripa, S. et al. Metal-immobilized micellar aggregates of a block copolymer from a mixed solvent for a SERS-active sensing substrate and versatile dip catalysis. Langmuir 37, 2445–2456 (2021).
Darhuber, A. A., Troian, S. M., Miller, S. M. & Wagner, S. Morphology of liquid microstructures on chemically patterned surfaces. J. Appl. Phys. 87, 7768–7775 (2000).
Tebbe, M., Galati, E., Walker, G. C. & Kumacheva, E. Homopolymer nanolithography. Small 13, 1702043 (2017).
Yan, N., Liu, X., Zhu, J., Zhu, Y. & Jiang, W. Well-ordered inorganic nanoparticle arrays directed by block copolymer nanosheets. ACS Nano 13, 6638–6646 (2019).
Chang, T., Du, B., Huang, H. & He, T. Highly tunable complementary micro/submicro-nanopatterned surfaces combining block copolymer self-assembly and colloidal lithography. ACS Appl. Mater. Interfaces 8, 22705–22713 (2016).
Kim, S.-S. & Sohn, B.-H. Template-assisted self-assembly of diblock copolymer micelles for non-hexagonal arrays of Au nanoparticles. RSC Adv. 6, 41331–41339 (2016).
Han, S. T. et al. Microcontact printing of ultrahigh density gold nanoparticle monolayer for flexible flash memories. Adv. Mater. 24, 3556–3561 (2012).
Liu, P. & Ding, J. Fabrication of micro–nano hybrid patterns on a solid surface. Langmuir 26, 492–497 (2010).
Kästle, G. et al. Micellar nanoreactors — preparation and characterization of hexagonally ordered arrays of metallic nanodots. Adv. Funct. Mater. 13, 853–861 (2003).
Möller, M. & Spatz, J. P. Mineralization of nanoparticles in block copolymer micelles. Curr. Opin. Colloid Interface Sci. 2, 177–187 (1997).
Bera, A., Bhattacharya, A., Tiwari, N., Jha, S. N. & Bhattacharyya, D. Morphology, stability, and X-ray absorption spectroscopic study of iron oxide (hematite) nanoparticles prepared by micelle nanolithography. Surf. Sci. 669, 145–153 (2018).
Li, X., Lau, K. H., Kim, D. H. & Knoll, W. High-density arrays of titania nanoparticles using monolayer micellar films of diblock copolymers as templates. Langmuir 21, 5212–5217 (2005).
Zhao, H., Douglas, E. P., Harrison, B. S. & Schanze, K. S. Preparation of CdS nanoparticles in salt-induced block copolymer micelles. Langmuir 17, 8428–8433 (2001).
Cha, S. K. et al. Au–Ag core–shell nanoparticle array by block copolymer lithography for synergistic broadband plasmonic properties. ACS Nano 9, 5536–5543 (2015).
Mbenkum, B. N., Diaz-Ortiz, A., Gu, L., van Aken, P. A. & Schutz, G. Expanding micelle nanolithography to the self-assembly of multicomponent core–shell nanoparticles. J. Am. Chem. Soc. 132, 10671–10673 (2010).
Ethirajan, A. et al. A micellar approach to magnetic ultrahigh-density data-storage media: extending the limits of current colloidal methods. Adv. Mater. 19, 406–410 (2007).
Jahn, S., Lechner, S. J., Freichels, H., Moller, M. & Spatz, J. P. Precise AuxPt1−x alloy nanoparticle array of tunable composition for catalytic applications. Sci. Rep. 6, 20536 (2016).
Piner, R. D., Zhu, J., Xu, F., Hong, S. & Mirkin, C. A. “Dip-pen” nanolithography. Science 283, 661–663 (1999).
Liu, G., Petrosko, S. H., Zheng, Z. & Mirkin, C. A. Evolution of dip-pen nanolithography (DPN): from molecular patterning to materials discovery. Chem. Rev. 120, 6009–6047 (2020).
Maynor, B. W., Li, Y. & Liu, J. Au “ink” for AFM “dip-pen” nanolithography. Langmuir 17, 2575–2578 (2001).
Li, Y., Maynor, B. W. & Liu, J. Electrochemical AFM “dip-pen” nanolithography. J. Am. Chem. Soc. 123, 2105–2106 (2001).
Ding, L., Li, Y., Chu, H., Li, X. & Liu, J. Creation of cadmium sulfide nanostructures using AFM dip-pen nanolithography. J. Phys. Chem. B 109, 22337–22340 (2005).
Basnar, B. & Willner, I. Dip-pen-nanolithographic patterning of metallic, semiconductor, and metal oxide nanostructures on surfaces. Small 5, 28–44 (2009).
Chai, J. et al. Scanning probe block copolymer lithography. Proc. Natl Acad. Sci. USA 107, 20202–20206 (2010).
Huo, F. et al. Polymer pen lithography. Science 321, 1658–1660 (2008).
Liu, G. et al. Delineating the pathways for the site-directed synthesis of individual nanoparticles on surfaces. Proc. Natl Acad. Sci. USA 110, 887–891 (2013).
Chai, J., Liao, X., Giam, L. R. & Mirkin, C. A. Nanoreactors for studying single nanoparticle coarsening. J. Am. Chem. Soc. 134, 158–161 (2012).
Du, J. S., Chen, P.-C., Dravid, V. P. & Mirkin, C. A. Using STEM to probe the in-situ dynamics of multimetallic nanoparticles grown in polymer nanoreactors. Microsc. Microanal. 23, 872–873 (2017).
Chen, P. C. et al. Tip-directed synthesis of multimetallic nanoparticles. J. Am. Chem. Soc. 137, 9167–9173 (2015).
Behafarid, F. & Roldan Cuenya, B. Towards the understanding of sintering phenomena at the nanoscale: geometric and environmental effects. Top. Catal. 56, 1542–1559 (2013).
Chen, P.-C. et al. Polyelemental nanoparticle libraries. Science 352, 1565–1569 (2016).
Chen, P.-C. et al. Interface and heterostructure design in polyelemental nanoparticles. Science 363, 959–964 (2019).
Kluender, E. J., Bourgeois, M. R., Cherqui, C. R., Schatz, G. C. & Mirkin, C. A. Multimetallic nanoparticles on mirrors for SERS detection. J. Phys. Chem. C 125, 12784–12791 (2021).
Chen, P.-C. et al. Structural evolution of three-component nanoparticles in polymer nanoreactors. J. Am. Chem. Soc. 139, 9876–9884 (2017).
Du, J. S. et al. The structural fate of individual multicomponent metal-oxide nanoparticles in polymer nanoreactors. Angew. Chem. Int. Ed. 56, 7625–7629 (2017).
Kluender, E. J. et al. Catalyst discovery through megalibraries of nanomaterials. Proc. Natl Acad. Sci. USA 116, 40–45 (2019).
Huang, L. et al. Catalyst design by scanning probe block copolymer lithography. Proc. Natl Acad. Sci. USA 115, 3764–3769 (2018).
Shen, B. et al. Synthesis of metal-capped semiconductor nanowires from heterodimer nanoparticle catalysts. J. Am. Chem. Soc. 142, 18324–18329 (2020).
Yao, Y. et al. High-throughput, combinatorial synthesis of multimetallic nanoclusters. Proc. Natl Acad. Sci. USA 117, 6316–6322 (2020).
Li, T. et al. Denary oxide nanoparticles as highly stable catalysts for methane combustion. Nat. Catal. 4, 62–70 (2021).
Yang, C. et al. Overcoming immiscibility toward bimetallic catalyst library. Sci. Adv. 6, eaaz6844 (2020).
Yao, Y. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 359, 1489–1494 (2018).
Yao, Y. et al. Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts. Sci. Adv. 6, eaaz0510 (2020).
Aslam, U., Chavez, S. & Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotechnol. 12, 1000–1005 (2017).
Wang, X. et al. Pd@Pt core–shell concave decahedra: a class of catalysts for the oxygen reduction reaction with enhanced activity and durability. J. Am. Chem. Soc. 137, 15036–15042 (2015).
Wang, X. et al. Palladium–platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 6, 7594 (2015).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Huang, L. et al. Multimetallic high-index faceted heterostructured nanoparticles. J. Am. Chem. Soc. 142, 4570–4575 (2020).
Chen, P.-C. et al. Chain-end functionalized polymers for the controlled synthesis of sub-2 nm particles. J. Am. Chem. Soc. 142, 7350–7355 (2020).
Oh, E., Golnabi, R., Walker, D. A. & Mirkin, C. A. Electrochemical polymer pen lithography. Small 17, 2100662 (2021).
Schneider, J. et al. Site-specific deposition of single gold nanoparticles by individual growth in electrohydrodynamically-printed attoliter droplet reactors. Nanoscale 7, 9510–9519 (2015).
Guardingo, M. et al. Synthesis of nanoscale coordination polymers in femtoliter reactors on surfaces. ACS Nano 10, 3206–3213 (2016).
Bellido, E., Cardona-Serra, S., Coronado, E. & Ruiz-Molina, D. Assisted-assembly of coordination materials into advanced nanoarchitectures by Dip Pen nanolithography. Chem. Commun. 47, 5175–5177 (2011).
Carbonell, C. et al. Femtolitre chemistry assisted by microfluidic pen lithography. Nat. Commun. 4, 2173 (2013).
Du, J. S. et al. Halide perovskite nanocrystal arrays: multiplexed synthesis and size-dependent emission. Sci. Adv. 6, eabc4959 (2020).
Ibanez, A., Maximov, S., Guiu, A., Chaillout, C. & Baldeck, P. L. Controlled nanocrystallization of organic molecules in sol-gel glasses. Adv. Mater. 10, 1540–1543 (1998).
Sawitowski, T., Miquel, Y., Heilmann, A. & Schmid, G. Optical properties of quasi one-dimensional chains of gold nanoparticles. Adv. Funct. Mater. 11, 435–440 (2001).
Wei, Q. et al. Rational design of novel nanostructured arrays based on porous AAO templates for electrochemical energy storage and conversion. Nano Energy 55, 234–259 (2019).
Kong, X., Zong, K. & Lee, S. S. Nanoconfining optoelectronic materials for enhanced performance and stability. Chem. Mater. 31, 4953–4970 (2019).
Cheng, S. et al. Facile synthesis of mesoporous gold–silica nanocomposite materials via sol–gel process with nonsurfactant templates. Chem. Mater. 15, 1560–1566 (2003).
Monnier, V., Sanz, N., Botzung-Appert, E., Bacia, M. & Ibanez, A. Confined nucleation and growth of organic nanocrystals in sol–gel matrices. J. Mater. Chem. 16, 1401–1409 (2006).
Sanz, N., Baldeck, P. L. & Ibanez, A. Organic nanocrystals embedded in sol–gel glasses for optical applications. Synth. Met. 115, 229–234 (2000).
Zhao, X.-G., Shi, J.-L., Hu, B., Zhang, L.-X. & Hua, Z.-L. In situ formation of silver nanoparticles inside pore channels of ordered mesoporous silica. Mater. Lett. 58, 2152–2156 (2004).
Chen, W., Cai, W., Zhang, L., Wang, G. & Zhang, L. Sonochemical processes and formation of gold nanoparticles within pores of mesoporous silica. J. Colloid Interface Sci. 238, 291–295 (2001).
Zhang, Y., Yuwono, A. H., Li, J. & Wang, J. Highly dispersed gold nanoparticles assembled in mesoporous titania films of cubic configuration. Microporous Mesoporous Mater. 110, 242–249 (2008).
Fan, W. et al. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity. Nat. Mater. 7, 984–991 (2008).
Kumai, Y. et al. Highly ordered platinum nanodot arrays with cubic symmetry in mesoporous thin films. Adv. Mater. 18, 760–762 (2006).
Fukuoka, A. et al. Template synthesis of nanoparticle arrays of gold and platinum in mesoporous silica films. Nano Lett. 2, 793–795 (2002).
Fukuoka, A. et al. Template synthesis of nanoparticle arrays of gold, platinum and palladium in mesoporous silica films and powders. J. Mater. Chem. 14, 752–756 (2004).
Besson, S., Gacoin, T., Ricolleau, C. & Boilot, J.-P. Silver nanoparticle growth in 3D-hexagonal mesoporous silica films. Chem. Commun. 2003, 360–361 (2003).
Wang, I., Baldeck, P. L., Botzung, E., Sanz, N. & Ibanez, A. Electric-field induced orientation of organic nanocrystals in sol–gel matrix. Opt. Mater. 21, 569–572 (2002).
Li, R., Zhu, X., Shou, D., Zhou, X. & Yan, X. The interparticle coupling effect of gold nanoparticles in confined ordered mesopores enhances high temperature catalytic oxidation. RSC Adv. 6, 88486–88489 (2016).
Wang, H. H., Han, C. Y., Willing, G. A. & Xiao, Z. Nanowire and nanotube syntheses through self-assembled nanoporous AAO templates. MRS Proc. 775, 4.8 (2003).
Zaraska, L., Sulka, G. D. & Jaskuła, M. Anodic alumina membranes with defined pore diameters and thicknesses obtained by adjusting the anodizing duration and pore opening/widening time. J. Solid State Electrochem. 15, 2427–2436 (2011).
Lv, X., Hu, G., Tang, J. & Wang, Y. New thoughts into the fabrication of ZnO nanoparticles: confined growth in the channels of AAO membrane and its formation mechanisms. J. Mater. Sci. Mater. Electron. 28, 14163–14169 (2017).
Ashley, M. J. et al. Templated synthesis of uniform perovskite nanowire arrays. J. Am. Chem. Soc. 138, 10096–10099 (2016).
Ozel, T., Bourret, G. R. & Mirkin, C. A. Coaxial lithography. Nat. Nanotechnol. 10, 319–324 (2015).
Fang, J. et al. A general soft-enveloping strategy in the templating synthesis of mesoporous metal nanostructures. Nat. Commun. 9, 521 (2018).
Kitahara, M. & Kuroda, K. Preparation of highly controlled nanostructured Au within mesopores using reductive deposition in non-polar environments. RSC Adv. 4, 27201–27206 (2014).
Anaya, M. et al. Strong quantum confinement and fast photoemission activation in CH3NH3PbI3 perovskite nanocrystals grown within periodically mesostructured films. Adv. Opt. Mater. 5, 1601087 (2017).
Demchyshyn, S. et al. Confining metal-halide perovskites in nanoporous thin films. Sci. Adv. 3, e1700738 (2017).
Malgras, V. et al. Observation of quantum confinement in monodisperse methylammonium lead halide perovskite nanocrystals embedded in mesoporous silica. J. Am. Chem. Soc. 138, 13874–13881 (2016).
Malgras, V., Henzie, J., Takei, T. & Yamauchi, Y. Stable blue luminescent CsPbBr3 perovskite nanocrystals confined in mesoporous thin films. Angew. Chem. Int. Ed. 130, 9019–9023 (2018).
Rubino, A. et al. Highly efficient and environmentally stable flexible color converters based on confined CH3NH3PbBr3 nanocrystals. ACS Appl. Mater. Interfaces 10, 38334–38340 (2018).
Rubino, A., Caliò, L., García-Bennett, A., Calvo, M. E. & Míguez, H. Mesoporous matrices as hosts for metal halide perovskite nanocrystals. Adv. Opt. Mater. 8, 1901868 (2020).
Qin, L., Park, S., Huang, L. & Mirkin, C. A. On-wire lithography. Science 309, 113–115 (2005).
Banholzer, M. J., Qin, L., Millstone, J. E., Osberg, K. D. & Mirkin, C. A. On-wire lithography: synthesis, encoding and biological applications. Nat. Protoc. 4, 838–848 (2009).
Aizenberg, J., Black, A. J. & Whitesides, G. M. Oriented growth of calcite controlled by self-assembled monolayers of functionalized alkanethiols supported on gold and silver. J. Am. Chem. Soc. 121, 4500–4509 (1999).
Jackman, R. J., Duffy, D. C., Ostuni, E., Willmore, N. D. & Whitesides, G. M. Fabricating large arrays of microwells with arbitrary dimensions and filling them using discontinuous dewetting. Anal. Chem. 70, 2280–2287 (1998).
Barton, J. E. & Odom, T. W. Mass-limited growth in zeptoliter beakers: a general approach for the synthesis of nanocrystals. Nano Lett. 4, 1525–1528 (2004).
Wang, L., Lee, M. H., Barton, J., Hughes, L. & Odom, T. W. Shape-control of protein crystals in patterned microwells. J. Am. Chem. Soc. 130, 2142–2143 (2008).
Briseno, A. L. et al. Patterning organic single-crystal transistor arrays. Nature 444, 913–917 (2006).
Yoo, W. C., Kumar, S., Penn, R. L., Tsapatsis, M. & Stein, A. Growth patterns and shape development of zeolite nanocrystals in confined syntheses. J. Am. Chem. Soc. 131, 12377–12383 (2009).
Lin, C. K. et al. Two-step patterning of scalable all-inorganic halide perovskite arrays. ACS Nano 14, 3500–3508 (2020).
Messina, G. M., Passiu, C., Rossi, A. & Marletta, G. Selective protein trapping within hybrid nanowells. Nanoscale 8, 16511–16519 (2016).
Wang, Y., Yu, Y., Liu, Y. & Yang, S. Template-confined site-specific electrodeposition of nanoparticle cluster-in-bowl arrays as surface enhanced Raman spectroscopy substrates. ACS Sens. 3, 2343–2350 (2018).
Zhu, C. et al. Silver nanoparticle-assembled micro-bowl arrays for sensitive SERS detection of pesticide residue. Nanotechnology 31, 205303 (2020).
Bi, K. et al. Direct electron-beam patterning of transferrable plasmonic gold nanoparticles using a HAuCl4/PVP composite resist. Nanoscale 11, 1245–1252 (2019).
Liao, X., Huang, Y. K., Mirkin, C. A. & Dravid, V. P. High throughput synthesis of multifunctional oxide nanostructures within nanoreactors defined by beam pen lithography. ACS Nano 11, 4439–4444 (2017).
Milliron, D. J., Caldwell, M. A. & Wong, H. S. P. Synthesis of metal chalcogenide nanodot arrays using block copolymer-derived nanoreactors. Nano Lett. 7, 3504–3507 (2007).
Jibril, L., Chen, P.-C., Hu, J., Odom, T. W. & Mirkin, C. A. Massively parallel nanoparticle synthesis in anisotropic nanoreactors. ACS Nano 13, 12408–12414 (2019).
Wang, D. & Schaaf, P. Solid-state dewetting for fabrication of metallic nanoparticles and influences of nanostructured substrates and dealloying. Phys. Status Solidi A 210, 1544–1551 (2013).
Thalladi, V. R. & Whitesides, G. M. Crystals of crystals: fabrication of encapsulated and ordered two-dimensional arrays of microcrystals. J. Am. Chem. Soc. 124, 3520–3521 (2002).
Han, D. et al. Nanopore-templated silver nanoparticle arrays photopolymerized in zero-mode waveguides. Front. Chem. 7, 216 (2019).
Laramy, C. R., O’Brien, M. N. & Mirkin, C. A. Crystal engineering with DNA. Nat. Rev. Mater. 4, 201–224 (2019).
McMillan, J. R., Hayes, O. G., Winegar, P. H. & Mirkin, C. A. Protein materials engineering with DNA. Acc. Chem. Res. 52, 1939–1948 (2019).
Zheng, B. et al. Sterically controlled docking of gold nanoparticles on ferritin surface by DNA hybridization. Nanotechnology 22, 275312 (2011).
Beyeh, N. K. et al. Crystalline cyclophane–protein cage frameworks. ACS Nano 12, 8029–8036 (2018).
Kostiainen, M. A. et al. Hierarchical self-assembly and optical disassembly for controlled switching of magnetoferritin nanoparticle magnetism. ACS Nano 5, 6394–6402 (2011).
Künzle, M., Eckert, T. & Beck, T. Binary protein crystals for the assembly of inorganic nanoparticle superlattices. J. Am. Chem. Soc. 138, 12731–12734 (2016).
Lach, M., Künzle, M. & Beck, T. Free-standing metal oxide nanoparticle superlattices constructed with engineered protein containers show in crystallo catalytic activity. Chem. Eur. J. 23, 17482–17486 (2017).
Acknowledgements
This work was supported by the Sherman Fairchild Foundation, the Air Force Office of Scientific Research award FA9550-16-1-0150, Kairos Ventures and the Air Force Research Laboratory agreement FA8650-15-2-5518. The US Government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force Research Laboratory or the US Government. L.J. was supported by the National Science Foundation through the National Science Foundation Graduate Research Fellowship Program under grant no. DGE-1842165. Any opinions, findings and conclusions or recommendations expressed in this work are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Contributions
C.A.M., J.H.S., L.J. and S.H.P. wrote and edited the manuscript. J.H.S. and L.J. prepared the first drafts of the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Materials thanks Jeroen Cornelissen, Yadong Yin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Swisher, J.H., Jibril, L., Petrosko, S.H. et al. Nanoreactors for particle synthesis. Nat Rev Mater 7, 428–448 (2022). https://doi.org/10.1038/s41578-021-00402-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00402-z
This article is cited by
-
Fibration of powdery materials
Nature Materials (2024)
-
Mesoporous carbon spheres with programmable interiors as efficient nanoreactors for H2O2 electrosynthesis
Nature Communications (2024)