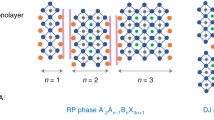
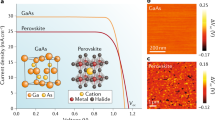
Abstract
The surface of a semiconductor often has a key role in determining its properties. For metal halide perovskites, understanding the surface features and their impact on the materials and devices is becoming increasingly important. At length scales down to the nanoscale regime, surface features become dominant in regulating the properties of perovskite materials, owing to the high surface-to-volume ratio. For perovskite bulk films in the micrometre range, defects and structural disorder readily form at the surface and affect device performance. Through concerted efforts to optimize processing techniques, high-quality perovskite thin films can now be fabricated with monolayer-like polycrystalline grains or even single crystals. Surface defects therefore remain the major obstacle to progress, pushing surface studies to the forefront of perovskite research. In this Review, we summarize and assess recent advances in the understanding of perovskite surfaces and surface strategies towards improving perovskite materials and the efficiency and stability of perovskite devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Davison, S. G., Steshcka, M. in Basic Theory of Surface States (eds Davison, S. G. & Stęślicka, M.) 46–54 (Clarendon Press, 1992).
Shockley, W. On the surface states associated with a periodic potential. Phys. Rev. 56, 317–323 (1939).
Kim, H. S. et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012).
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Yuan, M. et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 11, 872–877 (2016).
National Renewable Energy Laboratory. Best research-cell efficiencies chart. NREL https://www.nrel.gov/pv/cell-efficiency.html (2019).
Xu, W. et al. Rational molecular passivation for high-performance perovskite light-emitting diodes. Nat. Photonics 13, 418–424 (2019).
Cao, Y. et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 562, 249–253 (2018).
Lin, K. et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 562, 245–248 (2018).
Meggiolaro, D., Mosconi, E. & De Angelis, F. Formation of surface defects dominates ion migration in lead-halide perovskites. ACS Energy Lett. 4, 779–785 (2019).
Wang, R. et al. A review of perovskites solar cell stability. Adv. Funct. Mater. 29, 1808843 (2019).
Boles, M. A., Ling, D., Hyeon, T. & Talapin, D. V. The surface science of nanocrystals. Nat. Mater. 15, 141–153 (2016).
Levchuk, I. et al. Brightly luminescent and color-tunable formamidinium lead halide perovskite FAPbX3 (X = Cl, Br, I) colloidal nanocrystals. Nano Lett. 17, 2765–2770 (2017).
Kagan, C. R. & Murray, C. B. Charge transport in strongly coupled quantum dot solids. Nat. Nanotechnol. 10, 1013–1026 (2015).
Wang, R. et al. Constructive molecular configurations for surface-defect passivation of perovskite photovoltaics. Science 366, 1509–1513 (2019).
Chen, Z. et al. Thin single crystal perovskite solar cells to harvest below-bandgap light absorption. Nat. Commun. 8, 1890 (2017).
Min, H. et al. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 366, 749–753 (2019).
Jung, E. H. et al. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 567, 511–515 (2019).
Jiang, Q. et al. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 13, 460–466 (2019).
Callen, H. B. Thermodynamics and an Introduction to Thermostatistics 27–35 (Wiley, 1985)
Uratani, H. & Yamashita, K. Charge carrier trapping at surface defects of perovskite solar cell absorbers: a first-principles study. J. Phys. Chem. Lett. 8, 742–746 (2017).
Mosconi, E., Ronca, E. & De Angelis, F. First-principles investigation of the TiO2/organohalide perovskites interface: the role of interfacial chlorine. J. Phys. Chem. Lett. 5, 2619–2625 (2014).
Quarti, C., De Angelis, F. & Beljonne, D. Influence of surface termination on the energy level alignment at the CH3NH3PbI3 perovskite/C60 interface. Chem. Mater. 29, 958–968 (2017).
Haruyama, J., Sodeyama, K., Han, L. & Tateyama, Y. Termination dependence of tetragonal CH3NH3PbI3 surfaces for perovskite solar cells. J. Phys. Chem. Lett. 5, 2903–2909 (2014).
Haruyama, J., Sodeyama, K., Han, L. & Tateyama, Y. Surface properties of CH3NH3PbI3 for perovskite solar cells. Acc. Chem. Res. 49, 554–561 (2016).
Huang, X., Paudel, T. R., Dowben, P. A., Dong, S. & Tsymbal, E. Y. Electronic structure and stability of the CH3NH3PbBr3 (001) surface. Phys. Rev. B 94, 195309 (2016).
Komesu, T. et al. Surface electronic structure of hybrid organo lead bromide perovskite single crystals. J. Phys. Chem. C 120, 21710–21715 (2016).
Torres, A. & Rego, L. G. C. Surface effects and adsorption of methoxy anchors on hybrid lead iodide perovskites: insights for spiro-MeOTAD attachment. J. Phys. Chem. C 118, 26947–26954 (2014).
Ran, C., Xu, J., Gao, W., Huang, C. & Dou, S. Defects in metal triiodide perovskite materials towards high-performance solar cells: origin, impact, characterization, and engineering. Chem. Soc. Rev. 47, 4581–4610 (2018).
Yin, W.-J., Shi, T. & Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 104, 063903 (2014).
Liu, Y. et al. Atomistic origins of surface defects in CH3NH3PbBr3 perovskite and their electronic structures. ACS Nano 11, 2060–2065 (2017).
Kim, J., Lee, S. H., Lee, J. H. & Hong, K. H. The role of intrinsic defects in methylammonium lead iodide perovskite. J. Phys. Chem. Lett. 5, 1312–1317 (2014).
Liu, N. & Yam, C. Y. First-principles study of intrinsic defects in formamidinium lead triiodide perovskite solar cell absorbers. Phys. Chem. Chem. Phys. 20, 6800–6804 (2018).
Kabakova, I. V. et al. The effect of ionic composition on acoustic phonon speeds in hybrid perovskites from Brillouin spectroscopy and density functional theory. J. Mater. Chem. C 6, 3861–3868 (2018).
Ohmann, R. et al. Real-space imaging of the atomic structure of organic-inorganic perovskite. J. Am. Chem. Soc. 137, 16049–16054 (2015).
She, L., Liu, M. & Zhong, D. Atomic structures of CH3NH3PbI3 (001) surfaces. ACS Nano 10, 1126–1131 (2016).
Stecker, C. et al. Surface defect dynamics in organic –inorganic hybrid perovskites: from mechanism to interfacial properties. ACS Nano 13, 12127–12136 (2019).
Hieulle, J. et al. Imaging of the atomic structure of all-inorganic halide perovskites. J. Phys. Chem. Lett. 11, 818–823 (2020).
Akkerman, Q. A. et al. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 137, 10276–10281 (2015).
Meggiolaro, D., Mosconi, E. & De Angelis, F. Modeling the interaction of molecular iodine with MAPbI3: a probe of lead-halide perovskites defect chemistry. ACS Energy Lett. 3, 447–451 (2018).
Thind, A. S. et al. Atomic structure and electrical activity of grain boundaries and Ruddlesden–Popper faults in cesium lead bromide perovskite. Adv. Mater. 31, 1805047 (2019).
Rothmann, M. U. et al. Direct observation of intrinsic twin domains in tetragonal CH3NH3PbI3. Nat. Commun. 8, 14547 (2017).
Kim, T. W. et al. Self-organized superlattice and phase coexistence inside thin film organometal halide perovskite. Adv. Mater. 30, 1705230 (2018).
Aristidou, N. et al. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 8, 15218 (2017).
Zhang, L., Ju, M. G. & Liang, W. The effect of moisture on the structures and properties of lead halide perovskites: a first-principles theoretical investigation. Phys. Chem. Chem. Phys. 18, 23174–23183 (2016).
Mosconi, E., Azpiroz, J. M. & De Angelis, F. Ab initio molecular dynamics simulations of methylammonium lead iodide perovskite degradation by water. Chem. Mater. 27, 4885–4892 (2015).
Koocher, N. Z., Saldana-Greco, D., Wang, F., Liu, S. & Rappe, A. M. Polarization dependence of water adsorption to CH3NH3PbI3 (001) surfaces. J. Phys. Chem. Lett. 6, 4371–4378 (2015).
Yin, W. J., Yang, J. H., Kang, J., Yan, Y. & Wei, S. H. Halide perovskite materials for solar cells: a theoretical review. J. Mater. Chem. A 3, 8926–8942 (2015).
Schulz, P., Cahen, D. & Kahn, A. Halide perovskites: is it all about the interfaces? Chem. Rev. 119, 3349–3417 (2019).
Xiao, Z., Song, Z. & Yan, Y. From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 31, 1803792 (2019).
Brandt, R. E. et al. Searching for “defect-tolerant” photovoltaic materials: combined theoretical and experimental screening. Chem. Mater. 29, 4667–4674 (2017).
Cheng, X., Yang, S., Cao, B., Tao, X. & Chen, Z. Single crystal perovskite solar cells: development and perspectives. Adv. Funct. Mater. 30, 1905021 (2019).
Geng, W. et al. Effect of surface composition on electronic properties of methylammonium lead iodide perovskite. J. Mater. 1, 213–220 (2015).
Stranks, S. D. & Plochocka, P. The influence of the Rashba effect. Nat. Mater. 17, 381–382 (2018).
Etienne, T., Mosconi, E. & De Angelis, F. Dynamical origin of the Rashba effect in organohalide lead perovskites: a key to suppressed carrier recombination in perovskite solar cells? J. Phys. Chem. Lett. 7, 1638–1645 (2016).
Mosconi, E., Etienne, T. & De Angelis, F. Rashba band splitting in organohalide lead perovskites: bulk and surface effects. J. Phys. Chem. Lett. 8, 2247–2252 (2017).
Niesner, D. et al. Giant Rashba splitting in CH3NH3PbBr3 organic-inorganic perovskite. Phys. Rev. Lett. 117, 126401 (2016).
Li, Z. et al. Optoelectronic dichotomy of mixed halide CH3NH3Pb(Br1−xClx)3 single crystals: surface versus bulk photoluminescence. J. Am. Chem. Soc. 140, 11811–11819 (2018).
Yang, Y. et al. Top and bottom surfaces limit carrier lifetime in lead iodide perovskite films. Nat. Energy 2, 16207 (2017).
Yu, Z. L., Ma, Q. R., Zhao, Y. Q., Liu, B. & Cai, M. Q. Surface termination - a key factor to influence electronic and optical properties of CsSnI3. J. Phys. Chem. C 122, 9275–9282 (2018).
Chen, B., Rudd, P. N., Yang, S., Yuan, Y. & Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 48, 3842–3867 (2019).
Agiorgousis, M. L., Sun, Y. Y., Zeng, H. & Zhang, S. Strong covalency-induced recombination centers in perovskite solar cell material CH3NH3PbI3. J. Am. Chem. Soc. 136, 14570–14575 (2014).
Sarmah, S. P. et al. Double charged surface layers in lead halide perovskite crystals. Nano Lett. 17, 2021–2027 (2017).
Andaji-Garmaroudi, Z. et al. A highly emissive surface layer in mixed-halide multication perovskites. Adv. Mater. 31, 1902374 (2019).
Anderson, N. C., Hendricks, M. P., Choi, J. J. & Owen, J. S. Ligand exchange and the stoichiometry of metal chalcogenide nanocrystals: spectroscopic observation of facile metal-carboxylate displacement and binding. J. Am. Chem. Soc. 135, 18536–18548 (2013).
Akkerman, Q. A., Rainò, G., Kovalenko, M. V. & Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 17, 394–405 (2018).
De Roo, J. et al. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano 10, 2071–2081 (2016).
Ravi, V. K. et al. Origin of the substitution mechanism for the binding of organic ligands on the surface of CsPbBr3 perovskite nanocubes. J. Phys. Chem. Lett. 8, 4988–4994 (2017).
Almeida, G. et al. Role of acid-base equilibria in the size, shape, and phase control of cesium lead bromide nanocrystals. ACS Nano 12, 1704–1711 (2018).
Nedelcu, G. et al. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 15, 5635–5640 (2015).
Li, J. et al. 50-Fold EQE improvement up to 6.27% of solution-processed all-inorganic perovskite CsPbBr3 QLEDs via surface ligand density control. Adv. Mater. 29, 1603885 (2017).
Pan, A. et al. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: the role of organic acid, base, and cesium precursors. ACS Nano 10, 7943–7954 (2016).
Ten Brinck, S. & Infante, I. Surface termination, morphology, and bright photoluminescence of cesium lead halide perovskite nanocrystals. ACS Energy Lett. 1, 1266–1272 (2016).
Forde, A., Inerbaev, T., Hobbie, E. K. & Kilin, D. S. Excited-state dynamics of a CsPbBr3 nanocrystal terminated with binary ligands: sparse density of states with giant spin-orbit coupling suppresses carrier cooling. J. Am. Chem. Soc. 141, 4388–4397 (2019).
Ten Brinck, S., Zaccaria, F. & Infante, I. Defects in lead halide perovskite nanocrystals: analogies and (many) differences with the bulk. ACS Energy Lett. 4, 2739–2747 (2019).
Bodnarchuk, M. I. et al. Rationalizing and controlling the surface structure and electronic passivation of cesium lead halide nanocrystals. ACS Energy Lett. 4, 63–74 (2019).
Saidaminov, M. I. et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 3, 648–654 (2018).
Giridharagopal, R., Cox, P. A. & Ginger, D. S. Functional scanning probe imaging of nanostructured solar energy materials. Acc. Chem. Res. 49, 1769–1776 (2016).
Hou, Y. et al. Overcoming the interface losses in planar heterojunction perovskite-based solar cells. Adv. Mater. 28, 5112–5120 (2016).
Tan, H. et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 355, 722–726 (2017).
Li, X. et al. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells. Science 353, 58–62 (2016).
Lee, J. W. et al. A bifunctional Lewis base additive for microscopic homogeneity in perovskite solar cells. Chem 3, 290–302 (2017).
Tennyson, E. M., Doherty, T. A. S. & Stranks, S. D. Heterogeneity at multiple length scales in halide perovskite semiconductors. Nat. Rev. Mater. 4, 573–587 (2019).
Nie, W. et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 347, 522–525 (2015).
Lin, L. et al. Bulk recrystallization for efficient mixed-cation mixed-halide perovskite solar cells. J. Mater. Chem. A 7, 25511–25520 (2019).
Yang, W. S. et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 356, 1376–1379 (2017).
Hieulle, J. et al. Unraveling the impact of halide mixing on perovskite stability. J. Am. Chem. Soc. 141, 3515–3523 (2019).
Hsu, H. C. et al. Photodriven dipole reordering: key to carrier separation in metalorganic halide perovskites. ACS Nano 13, 4402–4409 (2019).
Kim, T. W. et al. Real-time in situ observation of microstructural change in organometal halide perovskite induced by thermal degradation. Adv. Funct. Mater. 28, 1804039 (2018).
Fan, Z. et al. Layer-by-layer degradation of methylammonium lead tri-iodide perovskite microplates. Joule 1, 548–562 (2017).
Klein-Kedem, N., Cahen, D. & Hodes, G. Effects of light and electron beam irradiation on halide perovskites and their solar cells. Acc. Chem. Res. 49, 347–354 (2016).
Zhou, Y., Sternlicht, H. & Padture, N. P. Transmission electron microscopy of halide perovskite materials and devices. Joule 3, 641–661 (2019).
Rothmann, M. U. et al. Structural and chemical changes to CH3NH3PbI3 induced by electron and gallium ion beams. Adv. Mater. 30, 1800629 (2018).
Li, Y. et al. Unravelling degradation mechanisms and atomic structure of organic-inorganic halide perovskites by cryo-EM. Joule 3, 2854–2866 (2019).
Ji, F. et al. Simultaneous evolution of uniaxially oriented grains and ultralow-density grain-boundary network in CH3NH3PbI3 perovskite thin films mediated by precursor phase metastability. ACS Energy Lett. 2, 2727–2733 (2017).
Liu, L. et al. Grain-boundary “patches” by in situ conversion to enhance perovskite solar cells stability. Adv. Mater. 30, 1800544 (2018).
Kim, M., Motti, S. G., Sorrentino, R. & Petrozza, A. Enhanced solar cell stability by hygroscopic polymer passivation of metal halide perovskite thin film. Energy Environ. Sci. 11, 2609–2619 (2018).
Wang, S. et al. Targeted therapy for interfacial engineering toward stable and efficient perovskite solar cells. Adv. Mater. 31, 1903691 (2019).
Steirer, K. X. et al. Defect tolerance in methylammonium lead triiodide perovskite. ACS Energy Lett. 1, 360–366 (2016).
Xie, H. et al. Effects of precursor ratios and annealing on electronic structure and surface composition of CH3NH3PbI3 perovskite films. J. Phys. Chem. C 120, 215–220 (2016).
Kim, T. G., Seo, S. W., Kwon, H., Hahn, J. & Kim, J. W. Influence of halide precursor type and its composition on the electronic properties of vacuum deposited perovskite films. Phys. Chem. Chem. Phys. 17, 24342–24348 (2015).
Olthof, S. & Meerholz, K. Substrate-dependent electronic structure and film formation of MAPbI3 perovskites. Sci. Rep. 7, 40267 (2017).
Hawash, Z. et al. Interfacial modification of perovskite solar cells using an ultrathin MAI layer leads to enhanced energy level alignment, efficiencies, and reproducibility. J. Phys. Chem. Lett. 8, 3947–3953 (2017).
Xue, J. et al. Crystalline liquid-like behavior: surface-induced secondary grain growth of photovoltaic perovskite thin film. J. Am. Chem. Soc. 141, 13948–13953 (2019).
Li, N. et al. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat. Energy 4, 408–415 (2019).
Wu, Y. et al. Perovskite solar cells with 18.21% efficiency and area over 1 cm2 fabricated by heterojunction engineering. Nat. Energy 1, 16148 (2016).
Christians, J. A. et al. Tailored interfaces of unencapsulated perovskite solar cells for >1,000 hour operational stability. Nat. Energy 3, 68–74 (2018).
Hou, Y. et al. A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 358, 1192–1197 (2017).
Zhao, Q. et al. High efficiency perovskite quantum dot solar cells with charge separating heterostructure. Nat. Commun. 10, 2842 (2019).
Li, Z. et al. Extrinsic ion migration in perovskite solar cells. Energy Environ. Sci. 10, 1234–1242 (2017).
Alharbi, E. A. et al. Atomic-level passivation mechanism of ammonium salts enabling highly efficient perovskite solar cells. Nat. Commun. 10, 3008 (2019).
Szostak, R. et al. Nanoscale mapping of chemical composition in organic-inorganic hybrid perovskite films. Sci. Adv. 5, eaaw6619 (2019).
Zhang, M. et al. Reconfiguration of interfacial energy band structure for high-performance inverted structure perovskite solar cells. Nat. Commun. 10, 4593 (2019).
Xiao, M. et al. Effect of interfacial molecular orientation on power conversion efficiency of perovskite solar cells. J. Am. Chem. Soc. 139, 3378–3386 (2017).
Xiao, M., Lu, T., Lin, T., Andre, J. S. & Chen, Z. Understanding molecular structures of buried interfaces in halide perovskite photovoltaic devices nondestructively with sub‐monolayer sensitivity using sum frequency generation vibrational spectroscopy. Adv. Energy Mater. 10, 1903053 (2019).
Yang, J.-P. et al. Band dispersion and hole effective mass of methylammonium lead iodide perovskite. Sol. RRL 2, 1800132 (2018).
Zu, F. et al. Constructing the electronic structure of CH3NH3PbI3 and CH3NH3PbBr3 perovskite thin films from single-crystal band structure measurements. J. Phys. Chem. Lett. 10, 601–609 (2019).
Lee, M. I. et al. First determination of the valence band dispersion of CH3NH3PbI3 hybrid organic–inorganic perovskite. J. Phys. D Appl. Phys. 50, 26LT02 (2017).
Wang, C. et al. Valence band dispersion measurements of perovskite single crystals using angle-resolved photoemission spectroscopy. Phys. Chem. Chem. Phys. 19, 5361–5365 (2017).
Tao, S. et al. Absolute energy level positions in tin- and lead-based halide perovskites. Nat. Commun. 10, 2560 (2019).
Noel, N. K. et al. Enhanced photoluminescence and solar cell performance via Lewis base passivation of organic–inorganic lead halide perovskites. ACS Nano 8, 9815–9821 (2014).
Que, M. et al. Quantum-dot-induced cesium-rich surface imparts enhanced stability to formamidinium lead iodide perovskite solar cells. ACS Energy Lett. 4, 1970–1975 (2019).
Cho, K. T. et al. Selective growth of layered perovskites for stable and efficient photovoltaics. Energy Environ. Sci. 11, 952–959 (2018).
Lu, J. et al. Interfacial benzenethiol modification facilitates charge transfer and improves stability of cm-sized metal halide perovskite solar cells with up to 20% efficiency. Energy Environ. Sci. 11, 1880–1889 (2018).
Kutes, Y. et al. Mapping the photoresponse of CH3NH3PbI3 hybrid perovskite thin films at the nanoscale. Nano Lett. 16, 3434–3441 (2016).
Yun, J. S. et al. Benefit of grain boundaries in organic–inorganic halide planar perovskite solar cells. J. Phys. Chem. Lett. 6, 875–880 (2015).
Shao, Y. et al. Grain boundary dominated ion migration in polycrystalline organic-inorganic halide perovskite films. Energy Environ. Sci. 9, 1752–1759 (2016).
Xue, J. et al. A small-molecule “charge driver” enables perovskite quantum dot solar cells with efficiency approaching 13%. Adv. Mater. 31, 1900111 (2019).
Leblebici, S. Y. et al. Facet-dependent photovoltaic efficiency variations in single grains of hybrid halide perovskite. Nat. Energy 1, 16093 (2016).
Eperon, G. E., Moerman, D. & Ginger, D. S. Anticorrelation between local photoluminescence and photocurrent suggests variability in contact to active layer in perovskite solar cells. ACS Nano 10, 10258–10266 (2016).
Hermes, I. M. et al. Ferroelastic fingerprints in methylammonium lead iodide perovskite. J. Phys. Chem. C 120, 5724–5731 (2016).
Strelcov, E. et al. CH3NH3PbI3 perovskites: ferroelasticity revealed. Sci. Adv. 3, e1602165 (2017).
Zhao, T., Chueh, C. C., Chen, Q., Rajagopal, A. & Jen, A. K. Y. Defect passivation of organic–inorganic hybrid perovskites by diammonium iodide toward high-performance photovoltaic devices. ACS Energy Lett. 1, 757–763 (2016).
Chen, C. et al. Achieving a high open-circuit voltage in inverted wide-bandgap perovskite solar cells with a graded perovskite homojunction. Nano Energy 61, 141–147 (2019).
Chen, P. et al. In situ growth of 2D perovskite capping layer for stable and efficient perovskite solar cells. Adv. Funct. Mater. 28, 1706923 (2018).
Tennyson, E. M. et al. Cesium-incorporated triple cation perovskites deliver fully reversible and stable nanoscale voltage response. ACS Nano 13, 1538–1546 (2019).
Garrett, J. L. et al. Real-time nanoscale open-circuit voltage dynamics of perovskite solar cells. Nano Lett. 17, 2554–2560 (2017).
Zhang, F. et al. Complexities of contact potential difference measurements on metal halide perovskite surfaces. J. Phys. Chem. Lett. 10, 890–896 (2019).
Chatterjee, S. & Pal, A. J. Influence of metal substitution on hybrid halide perovskites: towards lead-free perovskite solar cells. J. Mater. Chem. A 6, 3793–3823 (2018).
Dasgupta, U., Bera, A. & Pal, A. J. Band diagram of heterojunction solar cells through scanning tunneling spectroscopy. ACS Energy Lett. 2, 582–591 (2017).
Paul, G., Chatterjee, S., Bhunia, H. & Pal, A. J. Self-doping in hybrid halide perovskites via precursor stoichiometry: to probe the type of conductivity through scanning tunneling spectroscopy. J. Phys. Chem. C 122, 20194–20199 (2018).
Yost, A. J. et al. Coexistence of two electronic nano-phases on a CH3NH3PbI3–xClx surface observed in STM measurements. ACS Appl. Mater. Interfaces 8, 29110–29116 (2016).
Yang, Y. et al. Low surface recombination velocity in solution-grown CH3NH3PbBr3 perovskite single crystal. Nat. Commun. 6, 7961 (2015).
Chen, X., Wang, K. & Beard, M. C. Ultrafast probes at the interfaces of solar energy conversion materials. Phys. Chem. Chem. Phys. 21, 16399–16407 (2019).
DeQuilettes, D. W. et al. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 348, 683–686 (2015).
Koushik, D. et al. On the effect of atomic layer deposited Al2O3 on the environmental degradation of hybrid perovskite probed by positron annihilation spectroscopy. J. Mater. Chem. C 7, 5275–5284 (2019).
Sundar, C. S. & Viswanathan, B. Positron annihilation spectroscopy. Met. Mater. Process. 8, 1–8 (1996).
Lin, Y. et al. π-Conjugated Lewis base: efficient trap-passivation and charge-extraction for hybrid perovskite solar cells. Adv. Mater. 29, 1604545 (2017).
Bai, Y. et al. Oligomeric silica-wrapped perovskites enable synchronous defect passivation and grain stabilization for efficient and stable perovskite photovoltaics. ACS Energy Lett. 4, 1231–1240 (2019).
Zou, M. et al. Strengthened perovskite/fullerene interface enhances efficiency and stability of inverted planar perovskite solar cells via a tetrafluoroterephthalic acid interlayer. ACS Appl. Mater. Interfaces 11, 33515–33524 (2019).
Wu, T. et al. Efficient and stable CsPbI3 solar cells via regulating lattice distortion with surface organic terminal groups. Adv. Mater. 31, 1900605 (2019).
Sun, C. et al. Amino-functionalized conjugated polymer as an efficient electron transport layer for high-performance planar-heterojunction perovskite solar cells. Adv. Energy Mater. 6, 1501534 (2016).
Chaudhary, B. et al. Poly(4-vinylpyridine)-based interfacial passivation to enhance voltage and moisture stability of lead halide perovskite solar cells. ChemSusChem 10, 2473–2479 (2017).
Li, H. et al. Enhancing efficiency of perovskite solar cells via surface passivation with graphene oxide interlayer. ACS Appl. Mater. Interfaces 9, 38967–38976 (2017).
Yang, G., Qin, P., Fang, G. & Li, G. A Lewis base-assisted passivation strategy towards highly efficient and stable perovskite solar cells. Sol. RRL 2, 1800055 (2018).
Dequilettes, D. W. et al. Photoluminescence lifetimes exceeding 8 μs and quantum yields exceeding 30% in hybrid perovskite thin films by ligand passivation. ACS Energy Lett. 1, 438–444 (2016).
Yang, X. et al. Efficient green light-emitting diodes based on quasi-two-dimensional composition and phase engineered perovskite with surface passivation. Nat. Commun. 9, 570 (2018).
Wang, Y. et al. Stabilizing heterostructures of soft perovskite semiconductors. Science 365, 687–691 (2019).
Godding, J. S. W. et al. Oxidative passivation of metal halide perovskites. Joule 3, 2716–2731 (2019).
Shao, Y., Xiao, Z., Bi, C., Yuan, Y. & Huang, J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 5, 5784 (2014).
Yang, S. et al. Stabilizing halide perovskite surfaces for solar cell operation with wide-bandgap lead oxysalts. Science 365, 473–478 (2019).
Zhao, Y. et al. Correlations between immobilizing ions and suppressing hysteresis in perovskite solar cells. ACS Energy Lett. 1, 266–272 (2016).
Abate, A. et al. Supramolecular halogen bond passivation of organic–inorganic halide perovskite solar cells. Nano Lett. 14, 3247–3254 (2014).
Xu, J. et al. Perovskite–fullerene hybrid materials suppress hysteresis in planar diodes. Nat. Commun. 6, 7081 (2015).
Tu, Y. et al. Diboron-assisted interfacial defect control strategy for highly efficient planar perovskite solar cells. Adv. Mater. 30, 1805085 (2018).
Song, Z. et al. Impact of processing temperature and composition on the formation of methylammonium lead iodide perovskites. Chem. Mater. 27, 4612–4619 (2015).
Yang, M. et al. Facile fabrication of large-grain CH3NH3PbI3−xBrx films for high-efficiency solar cells via CH3NH3Br-selective Ostwald ripening. Nat. Commun. 7, 12305 (2016).
Han, G. et al. Facile method to reduce surface defects and trap densities in perovskite photovoltaics. ACS Appl. Mater. Interfaces 9, 21292–21297 (2017).
Dong, Q. et al. Abnormal crystal growth in CH3NH3PbI3–xClx using a multi-cycle solution coating process. Energy Environ. Sci. 8, 2464–2470 (2015).
Lin, Y. et al. Enhanced thermal stability in perovskite solar cells by assembling 2D/3D stacking structures. J. Phys. Chem. Lett. 9, 654–658 (2018).
Tai, M. et al. In situ formation of a 2D/3D heterostructure for efficient and stable CsPbI2Br solar cells. J. Mater. Chem. A 7, 22675–22682 (2019).
Gharibzadeh, S. et al. Record open-circuit voltage wide-bandgap perovskite solar cells utilizing 2D/3D perovskite heterostructure. Adv. Energy Mater. 9, 1803699 (2019).
Xu, Z. et al. Br-containing alkyl ammonium salt-enabled scalable fabrication of high-quality perovskite films for efficient and stable perovskite modules. J. Mater. Chem. A 7, 26849–26857 (2019).
Yoo, J. J. et al. An interface stabilized perovskite solar cell with high stabilized efficiency and low voltage loss. Energy Environ. Sci. 12, 2192–2199 (2019).
Wang, Y., Zhang, T., Kan, M. & Zhao, Y. Bifunctional stabilization of all-Inorganic α-CsPbI3 perovskite for 17% efficiency photovoltaics. J. Am. Chem. Soc. 140, 12345–12348 (2018).
Niu, T. et al. Interfacial engineering at the 2D/3D heterojunction for high-performance perovskite solar cells. Nano Lett. 19, 7181–7190 (2019).
Li, N. et al. Enhanced moisture stability of cesium-containing compositional perovskites by a feasible interfacial engineering. Adv. Mater. Interfaces 4, 1700598 (2017).
Wang, Y. et al. Efficient α-CsPbI3 photovoltaics with surface terminated organic cations. Joule 2, 2065–2075 (2018).
Zhou, L. et al. Highly efficient and stable planar perovskite solar cells with modulated diffusion passivation toward high power conversion efficiency and ultrahigh fill factor. Sol. RRL 3, 1900293 (2019).
Zhou, Q. et al. High-performance perovskite solar cells with enhanced environmental stability based on a (p-FC6H4C2H4NH3)2[PbI4] capping layer. Adv. Energy Mater. 9, 1802595 (2019).
Dong, H. et al. A modulated double‐passivation strategy toward highly efficient perovskite solar cells with efficiency over 21%. Sol. RRL 3, 1900291 (2019).
Liu, Y. et al. Ultrahydrophobic 3D/2D fluoroarene bilayer-based water-resistant perovskite solar cells with efficiencies exceeding 22%. Sci. Adv. 5, eaaw2543 (2019).
Zhang, T. et al. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 3, e1700841 (2017).
Jokar, E. et al. Slow surface passivation and crystal relaxation with additives to improve device performance and durability for tin-based perovskite solar cells. Energy Environ. Sci. 11, 2353–2362 (2018).
Wang, Y. et al. Thermodynamically stabilized β-CsPbI3–based perovskite solar cells with efficiencies >18%. Science 365, 591–595 (2019).
Zheng, X. et al. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2, 17102 (2017).
Dong, H. et al. Conjugated molecules “bridge”: functional ligand toward highly efficient and long-term stable perovskite solar cell. Adv. Funct. Mater. 29, 1808119 (2019).
Weng, Y. et al. Electric dipole moment-assisted charge extraction and effective defect passivation in perovskite solar cells by depositing a PCBM:TIPD blend film on a CH3NH3PbI3 layer. J. Mater. Chem. C 7, 11559–11568 (2019).
Zhao, S. et al. General nondestructive passivation by 4-fluoroaniline for perovskite solar cells with improved performance and stability. Small 14, 1803350 (2018).
Noel, N. K. et al. Interfacial charge-transfer doping of metal halide perovskites for high performance photovoltaics. Energy Environ. Sci. 12, 3063–3073 (2019).
Wang, Q., Dong, Q., Li, T., Gruverman, A. & Huang, J. Thin insulating tunneling contacts for efficient and water-resistant perovskite solar cells. Adv. Mater. 28, 6734–6739 (2016).
Bai, S. et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571, 245–250 (2019).
Zhang, Y. et al. High efficiency (16.37%) of cesium bromide-passivated all‐inorganic CsPbI2Br perovskite solar cells. Sol. RRL 3, 1900254 (2019).
Zhang, Y. et al. Fusing nanowires into thin films: fabrication of graded-heterojunction perovskite solar cells with enhanced performance. Adv. Energy Mater. 9, 1900243 (2019).
Tan, F. et al. In situ back-contact passivation improves photovoltage and fill factor in perovskite solar cells. Adv. Mater. 31, 1807435 (2019).
Luo, D. et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 360, 1442–1446 (2018).
Kim, H. et al. Optimal interfacial engineering with different length of alkylammonium halide for efficient and stable perovskite solar cells. Adv. Energy Mater. 9, 1902740 (2019).
Wu, Z. et al. Highly efficient and stable perovskite solar cells via modification of energy levels at the perovskite/carbon electrode interface. Adv. Mater. 31, 1804284 (2019).
Koushik, D. et al. High-efficiency humidity-stable planar perovskite solar cells based on atomic layer architecture. Energy Environ. Sci. 10, 91–100 (2017).
Li, B. et al. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 9, 1076 (2018).
Tavakoli, M. M. et al. Controllable perovskite crystallization via antisolvent technique using chloride additives for highly efficient planar perovskite solar cells. Adv. Energy Mater. 9, 1803587 (2019).
Poli, I., Liang, X., Baker, R., Eslava, S. & Cameron, P. J. Enhancing the hydrophobicity of perovskite solar cells using C18 capped CH3NH3PbI3 nanocrystals. J. Mater. Chem. C 6, 7149–7156 (2018).
Qian, F. et al. Novel surface passivation for stable FA0.85MA0.15PbI3 perovskite solar cells with 21.6% efficiency. Sol. RRL 3, 1900072 (2019).
Wang, F. et al. Phenylalkylamine passivation of organolead halide perovskites enabling high-efficiency and air-stable photovoltaic cells. Adv. Mater. 28, 9986–9992 (2016).
Zheng, X. et al. Quantum dots supply bulk- and surface-passivation agents for efficient and stable perovskite solar cells. Joule 3, 1963–1976 (2019).
Bi, C., Kershaw, S. V., Rogach, A. L. & Tian, J. Improved stability and photodetector performance of CsPbI3 perovskite quantum dots by ligand exchange with aminoethanethiol. Adv. Funct. Mater. 29, 1902446 (2019).
Cao, W. et al. Halide-rich synthesized cesium lead bromide perovskite nanocrystals for light-emitting diodes with improved performance. Chem. Mater. 29, 5168–5173 (2017).
Li, X. et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 26, 2435–2445 (2016).
Zhang, B. et al. Alkyl phosphonic acids deliver CsPbBr3 nanocrystals with high photoluminescence quantum yield and truncated octahedron shape. Chem. Mater. 31, 9140–9147 (2019).
Koscher, B. A., Swabeck, J. K., Bronstein, N. D. & Alivisatos, A. P. Essentially trap-free CsPbBr3 colloidal nanocrystals by postsynthetic thiocyanate surface treatment. J. Am. Chem. Soc. 139, 6566–6569 (2017).
Liu, F. et al. Highly luminescent phase-stable CsPbI3 perovskite quantum dots achieving near 100% absolute photoluminescence quantum yield. ACS Nano 11, 10373–10383 (2017).
Lu, D. et al. Giant light-emission enhancement in lead halide perovskites by surface oxygen passivation. Nano Lett. 18, 6967–6973 (2018).
Nenon, D. P. et al. Design principles for trap-free CsPbX3 nanocrystals: enumerating and eliminating surface halide vacancies with softer Lewis bases. J. Am. Chem. Soc. 140, 17760–17772 (2018).
Almeida, G., Infante, I. & Manna, L. Resurfacing halide perovskite nanocrystals. Science 364, 833–834 (2019).
Sanehira, E. M. et al. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells. Sci. Adv. 3, eaao4204 (2017).
Li, G. et al. Surface ligand engineering for near-unity quantum yield inorganic halide perovskite QDs and high-performance QLEDs. Chem. Mater. 30, 6099–6107 (2018).
Song, J. et al. Organic–inorganic hybrid passivation enables perovskite QLEDs with an EQE of 16.48%. Adv. Mater. 30, 1805409 (2018).
Xue, J. et al. Surface ligand management for stable FAPbI3 perovskite quantum dot solar cells. Joule 2, 1866–1878 (2018).
Chiba, T. et al. High-efficiency perovskite quantum-dot light-emitting devices by effective washing process and interfacial energy level alignment. ACS Appl. Mater. Interfaces 9, 18054–18060 (2017).
Wang, Q. et al. μ-Graphene crosslinked CsPbI3 quantum dots for high efficiency solar cells with much improved stability. Adv. Energy Mater. 8, 1800007 (2018).
Pan, J. et al. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 28, 8718–8725 (2016).
Vickers, E. T. et al. Improving charge carrier delocalization in perovskite quantum dots by surface passivation with conductive aromatic ligands. ACS Energy Lett. 3, 2931–2939 (2018).
Dai, J. et al. Charge transport between coupling colloidal perovskite quantum dots assisted by functional conjugated ligands. Angew. Chem. Int. Ed. 57, 5754–5758 (2018).
Pan, J. et al. Bidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable near-unity photoluminescence quantum yield and efficient red light-emitting diodes. J. Am. Chem. Soc. 140, 562–565 (2018).
Krieg, F. et al. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 3, 641–646 (2018).
Swarnkar, A. et al. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 354, 92–95 (2016).
Wheeler, L. M. et al. Targeted ligand exchange chemistry on cesium lead halide perovskite quantum dots for high-efficiency photovoltaics. J. Am. Chem. Soc. 140, 10504–10513 (2018).
Luo, B. et al. Organolead halide perovskite nanocrystals: branched capping ligands control crystal size and stability. Angew. Chem. Int. Ed. 55, 8864–8868 (2016).
Sun, C. et al. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 28, 10088–10094 (2016).
Yoon, Y. J. et al. Enabling tailorable optical properties and markedly enhanced stability of perovskite quantum dots by permanently ligating with polymer hairs. Adv. Mater. 31, 1901602 (2019).
Zhang, H. et al. Embedding perovskite nanocrystals into a polymer matrix for tunable luminescence probes in cell imaging. Adv. Funct. Mater. 27, 1604382 (2017).
Huang, S. et al. Enhancing the stability of CH3NH3PbBr3 quantum dots by embedding in silica spheres derived from tetramethyl orthosilicate in ‘waterless’ toluene. J. Am. Chem. Soc. 138, 5749–5752 (2016).
Huang, H. et al. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 7, 5699–5703 (2016).
Meyns, M. et al. Polymer-enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Appl. Mater. Interfaces 8, 19579–19586 (2016).
Zhong, Q. et al. One-pot synthesis of highly stable CsPbBr3@SiO2 core–shell nanoparticles. ACS Nano 12, 8579–8587 (2018).
He, Y. et al. Unconventional route to dual-shelled organolead halide perovskite nanocrystals with controlled dimensions, surface chemistry, and stabilities. Sci. Adv. 5, eaax4424 (2019).
Bhaumik, S. et al. Highly stable, luminescent core–shell type methylammonium–octylammonium lead bromide layered perovskite nanoparticles. Chem. Commun. 52, 7118–7121 (2016).
Quan, L. N. et al. Highly emissive green perovskite nanocrystals in a solid state crystalline matrix. Adv. Mater. 29, 1605945 (2017).
Ravi, V. K., Scheidt, R. A., Dubose, J. & Kamat, P. V. Hierarchical arrays of cesium lead halide perovskite nanocrystals through electrophoretic deposition. J. Am. Chem. Soc. 140, 8887–8894 (2018).
Yang, D. et al. CsPbBr3 quantum dots 2.0: benzenesulfonic acid equivalent ligand awakens complete purification. Adv. Mater. 31, 1900767 (2019).
Yassitepe, E. et al. Amine-free synthesis of cesium lead halide perovskite quantum dots for efficient light-emitting diodes. Adv. Funct. Mater. 26, 8757–8763 (2016).
Wu, W.-Q. et al. Reducing surface halide deficiency for efficient and stable iodide-based perovskite solar cells. J. Am. Chem. Soc. 142, 3989–3996 (2020).
Acknowledgements
This article is based on work supported by the US Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under the Solar Energy Technologies Office award number DE-EE0008751. The authors thank A. Z. Stieg and S. Gilbert Corder for helpful discussions regarding AFM and nano-FTIR techniques, and S. Nuryyeva, M. Mujahid and S. Tan for help with language editing prior to submission.
Author information
Authors and Affiliations
Contributions
J.X. and R.W. researched most of the data and prepared the first draft. Y.Y. revised the manuscript before submission and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, J., Wang, R. & Yang, Y. The surface of halide perovskites from nano to bulk. Nat Rev Mater 5, 809–827 (2020). https://doi.org/10.1038/s41578-020-0221-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-020-0221-1
This article is cited by
-
The dynamic adsorption affinity of ligands is a surrogate for the passivation of surface defects
Nature Communications (2024)
-
Strain regulates the photovoltaic performance of thick-film perovskites
Nature Communications (2024)
-
Perovskite single-crystal thin films: preparation, surface engineering, and application
Nano Convergence (2023)
-
Fabrication of perovskite solar cells in ambient air by blocking perovskite hydration with guanabenz acetate salt
Nature Energy (2023)
-
Long-term operating stability in perovskite photovoltaics
Nature Reviews Materials (2023)