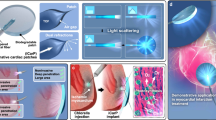
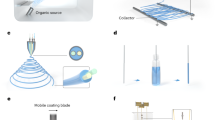
Abstract
Numerous light-based diagnostic and therapeutic devices are routinely used in the clinic. These devices have a familiar look as items plugged in the wall or placed at patients’ bedsides, but recently, many new ideas have been proposed for the realization of implantable or wearable functional devices. Many advances are being fuelled by the development of multifunctional materials for photonic healthcare devices. However, the finite depth of light penetration in the body is still a serious constraint for their clinical applications. In this Review, we discuss the basic concepts and some examples of state-of-the-art implantable and wearable photonic healthcare devices for diagnostic and therapeutic applications. First, we describe emerging multifunctional materials critical to the advent of next-generation implantable and wearable photonic healthcare devices and discuss the path for their clinical translation. Then, we examine implantable photonic healthcare devices in terms of their properties and diagnostic and therapeutic functions. We next describe exemplary cases of noninvasive, wearable photonic healthcare devices across different anatomical applications. Finally, we discuss the future research directions for the field, in particular regarding mobile healthcare and personalized medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yun, S. H. & Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 1, 0008 (2017). This review paper describes the fundamental concept of light, its interactions with biological matters and its applications to diagnosis, therapy and surgery.
Van Soest, G., Regar, E. & van der Steen, A. F. W. Photonics in cardiovascular medicine. Nat. Photonics 9, 626–629 (2015).
Kim, H. et al. Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv. Mater. 30, 1701460 (2018). This review paper describes the fundamental science, synthetic concepts, characteristics and biomedical applications of multifunctional photonic nanomaterials that can interact through light with biological systems.
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Tuchin, V. V. Polarized light interaction with tissues. J. Biomed. Opt. 21, 071114 (2016).
Weisleder, R. Molecular imaging in cancer. Science 312, 1168–1171 (2006).
Lee, M.-Y. et al. Biodegradable photonic melanoidin for theranostic applications. ACS Nano 10, 822–831 (2016).
Tak, S., Uga, M., Flandin, G., Dan, I. & Penny, W. D. Sensor space group analysis for fNIRS data. J. Neurosci. Methods 264, 103–112 (2016).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Yokota, T. et al. Ultraflexible organic photonic skin. Sci. Adv. 2, e1501856 (2016).
Lee, D.-E. et al. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 41, 2656–2672 (2012).
Shao, J. et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 7, 12967 (2016).
Liu, K. et al. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew. Chem. Int. Ed. 55, 3036–3039 (2016).
Hamblin, M. R., Huang, Y.-Y. & Heiskanen, V. Non-mammalian hosts and photobiomodulation: do all life-forms respond to light? Photochem. Photobiol. 95, 126–139 (2019).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Kuffler, D. P. Photobiomodulation in promoting wound healing: a review. Regen. Med. 11, 107–122 (2016).
Wang, Y., Huang, Y.-Y., Wang, Y., Lyu, P. & Hamblin, M. R. Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim. Biophys. Acta 1861, 441–449 (2017).
Hamblin, M. R. Shining light on the head: photobiomodulation for brain disorders. Biochim. Biophys. Acta Clin. 6, 113–124 (2016).
Choi, M. H. et al. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photonics 7, 987–994 (2013). This paper describes light-guiding hydrogel implants for in vivo optogenetic nanotoxicity sensing and optogenetic therapy in diabetic mice.
Mickle, A. D. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019). This paper describes a wireless, miniaturized bio-optoelectronic implant for the optogenetic modulation of the peripheral nervous system.
Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Shin, G. et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521 (2017).
Shirasaki, Y., Supran, G. J., Bawendi, M. G. & Bulovic, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 7, 13–23 (2013).
Biju, V., Itoh, T., Anas, A., Sujith, A. & Ishikawa, M. Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 391, 2469–2495 (2008).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Medintz, I. L., Uyeda, H. T., Goldman, E. R. & Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 4, 435–446 (2005).
Hutter, E. & Fendler, J. H. Exploitation of localized surface plasmon resonance. Adv. Mater. 16, 1685–1706 (2004).
Yang, X., Yang, M., Pang, B., Vara, M. & Xia, Y. Gold nanomaterials at work in biomedicine. Chem. Rev. 115, 10410–10488 (2015).
Giljohann, D. A. et al. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 49, 3280–3294 (2010).
Manzeli, S., Ovchinnikov, D., Pasquier, D., Yazyev, O. V. & Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2, 17033 (2017).
Xu, M., Liang, T., Shi, M. & Chen, H. Graphene-like two-dimensional material. Chem. Rev. 113, 3766–3798 (2013).
Haase, M. & Schäfer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 50, 5808–5829 (2011).
Zhou, B., Shi, B., Jin, D. & Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 10, 924–936 (2015).
Tian, G. et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 24, 1226–1231 (2012).
Friend, R. H. et al. Electroluminescence in conjugated polymers. Nature 397, 121–128 (1999). This review paper describes the background science, materials fabrication and semiconductor physics of electroluminescent conjugated polymers.
Bernholc, J., Brenner, D., Nardelli, M. B., Meunier, V. & Roland, C. Mechanical and electrical properties of nanotubes. Annu. Rev. Mater. Res. 32, 347–375 (2002).
Mochalin, V. N., Shenderova, O., Ho, D. & Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 7, 11–23 (2012).
Dreyer, D. R., Park, S., Bielawski, C. W. & Ruoff, R. S. The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228–240 (2010).
Park, Y., Yoo, J., Lim, B., Kwon, W. & Rhee, S.-W. Improving the functionality of carbon nanodots: doping and surface functionalization. J. Mater. Chem. A 4, 11582–11603 (2016).
Kim, H. et al. Dual-color-emitting carbon nanodots for multicolor bioimaging and optogenetic control of ion channels. Adv. Sci. 4, 1700325 (2017).
Del Alamo, J. A. Nanometre-scale electronics with III–V compound semiconductors. Nature 479, 317–323 (2011).
Chen, K. et al. Direct growth of single-crystalline III–V semiconductors on amorphous substrates. Nat. Commun. 7, 10502 (2016).
Okamoto, K. Fundamentals of Optical Waveguides 2nd edn Ch. 1 (Academic, 2006).
Parker, G. J. in Encyclopedia of Materials: Science and Technology 2nd edn (eds Buschow, K. H. J. et al.) 3703–3707 (Pergamon, 2001).
Sparta, D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2012).
Humar, M. et al. Toward biomaterial-based implantable photonic devices. Nanophotonics 6, 414–434 (2017).
Dupuis, A. et al. Prospective for biodegradable microstructured optical fibers. Opt. Lett. 32, 109–111 (2007).
Parker, S. T. et al. Biocompatible silk printed optical waveguides. Adv. Mater. 21, 2411–2415 (2009).
Shan, D. et al. Flexible biodegradable citrate-based polymeric step-index optical fiber. Biomaterials 143, 142–148 (2017).
Nizamoglu, S. et al. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun. 7, 10374 (2016).
Guo, J., Zhou, M. & Yang, C. Fluorescent hydrogel waveguide for on-site detection of heavy metal ions. Sci. Rep. 7, 7902 (2017).
Jain, A., Yang, A. H. J. & Erickson, D. Gel-based optical waveguides with live cell encapsulation and integrated microfluidics. Opt. Lett. 37, 1472–1474 (2012).
Manocchi, A. K., Domachuk, P., Omenetto, F. G. & Yi, H. Facile fabrication of gelatin-based biopolymeric optical waveguides. Biotechnol. Bioeng. 103, 725–732 (2009).
Wang, S., Oh, J. Y., Xu, J., Tran, H. & Bao, Z. Skin-inspired electronics: an emerging paradigm. Acc. Chem. Res. 51, 1033–1045 (2018). This review paper describes intrinsically stretchable materials for conductors, semiconductors and insulators, and futuristic self-healable or biodegradable electronic materials.
Oh, J. Y. & Bao, Z. Second skin enabled by advanced electronics. Adv. Sci. 6, 1900186 (2019).
Irimia-Vladu, M. “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 43, 588–610 (2014).
Kong, D. et al. Capacitance characterization of elastomeric dielectrics for applications in intrinsically stretchable thin film transistors. Adv. Funct. Mater. 26, 4680–4686 (2016).
Kim, D.-H. et al. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl Acad. Sci. USA 105, 18675–18680 (2008).
Matsuhisa, N., Chen, X., Bao, Z. & Someya, T. Materials and structure designs of stretchable conductors. Chem. Soc. Rev. 48, 2946–2966 (2019).
Park, M. et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 7, 803–809 (2012).
Matsuhisa, N. et al. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 16, 834–840 (2017).
Park, M., Park, J. & Jeong, U. Design of conductive composite elastomers for stretchable electronics. Nano Today 9, 244–260 (2014).
Lipomi, D. J. et al. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 6, 788–792 (2011).
Vosgueritchian, M., Lipomi, D. J. & Bao, Z. Highly conductive and transparent PEDOT:PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes. Adv. Funct. Mater. 22, 421–428 (2012).
Oh, J. Y., Kim, S., Baik, H. K. & Jeong, U. Conducting polymer dough for deformable electronics. Adv. Mater. 28, 4455–4461 (2016).
Wang, Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017).
Dickey, M. D. Stretchable and soft electronics using liquid metals. Adv. Mater. 29, 1606425 (2017).
Yan, J., Lu, Y., Chen, G., Yang, M. & Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 47, 2518–2533 (2018).
Xu, F. et al. Highly stretchable carbon nanotube transistors with ion gel gate dielectrics. Nano Lett. 14, 682–686 (2014).
Müller, C. et al. Tough, semiconducting polyethylene-poly(3-hexylthiophene) diblock copolymers. Adv. Funct. Mater. 17, 2674–2679 (2007).
Oh, J. Y. et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 539, 411–415 (2016).
Wang, G. J. N. et al. Inducing elasticity through oligosiloxane crosslinks for intrinsically stretchable semiconducting polymers. Adv. Funct. Mater. 26, 7254–7262 (2016).
Xu, J. et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017).
Kang, J., Tok, J. B.-H. & Bao, Z. Self-healing soft electronics. Nat. Electron. 2, 144–150 (2019).
Rao, Y.-L. et al. Stretchable self-healing polymeric dielectrics cross-linked through metal-ligand coordination. J. Am. Chem. Soc. 138, 6020–6027 (2016).
Kang, J. et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater. 30, 1706846 (2018).
Hart, L. R. et al. Perylene as an electron-rich moiety in healable, complementary π–π stacked, supramolecular polymer systems. Polymer 69, 293–300 (2015).
Hohlbein, N., Shaaban, A. & Schmidt, A. Remote-controlled activation of self-healing behavior in magneto-responsive ionomeric composites. Polymer 69, 301–309 (2015).
Tee, B. C., Wang, C., Allen, R. & Bao, Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 7, 825–832 (2012).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Lei, T. et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl Acad. Sci. USA 114, 5107–5112 (2017).
Irimia-Vladu, M. et al. Biocompatible and biodegradable materials for organic field-effect transistors. Adv. Funct. Mater. 20, 4069–4076 (2010).
Bettinger, C. J. & Bao, Z. Organic thin-film transistors fabricated on resorbable biomaterial substrates. Adv. Mater. 22, 651–655 (2010).
Hwang, S.-W. et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 15, 2801–2808 (2015).
Boutry, C. M. et al. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 27, 6954–6961 (2015).
Hastings, J. W. Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J. Mol. Evol. 19, 309–321 (1983).
Jones, K. A. et al. Orthogonal luciferase–luciferin pairs for bioluminescence imaging. J. Am. Chem. Soc. 139, 2351–2358 (2017).
Yeh, H.-W. et al. Red-shifted luciferase–luciferin pairs for enhanced bioluminescence imaging. Nat. Methods 14, 971–974 (2017).
Maguire, C. A. et al. Triple bioluminescence imaging for in vivo monitoring of cellular processes. Mol. Ther. Nucl. Acids 2, e99 (2013).
Bhaumik, S. & Gambhir, S. S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl Acad. Sci. USA 99, 377–382 (2002).
Pfleger, K. D. G. & Eidne, K. A. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat. Methods 3, 165–174 (2006).
James, J. R., Oliveira, M. I., Carmo, A. M., Iaboni, A. & Davis, S. J. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat. Methods 3, 1001–1006 (2006).
Salahpour, A. et al. BRET biosensors to study GPCR biology, pharmacology, and signal transduction. Front. Endocrinol. 3, 105 (2012).
Kobayashi, H., Picard, L. P., Schönegge, A. M. & Bouvier, M. Bioluminescence resonance energy transfer–based imaging of protein–protein interactions in living cells. Nat. Protoc. 14, 1084–1107 (2019).
Yao, H., Zhang, Y., Xiao, F., Xia, Z. & Rao, J. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew. Chem. Int. Ed. 46, 4346–4349 (2007).
Sarkar, K., Xue, Y. & Sant, S. in The Immune Response to Implanted Materials and Devices (ed. Corradetti, B.) 81–105 (Springer, 2017).
Singh, A. et al. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 13, 988–995 (2014).
Merrill, E. W. in Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications (ed. Harris, J. M.) 199–220 (Plenum, 1992).
Zhang, L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013).
Kastellorizios, M., Papadimitrakopoulos, F. & Burgess, D. J. Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. J. Control. Rel. 214, 103–111 (2015).
Laissue, P. P., Alghamdi, R. A., Tomancak, P., Reynaud, E. G. & Shroff, H. Assessing phototoxicity in live fluorescence imaging. Nat. Methods 14, 657–661 (2017).
Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–79 (2016).
Salles, G. N. et al. A novel bioresorbable device as a controlled release system for protecting cells from oxidative stress from Alzheimer’s disease. Mol. Neurobiol. 54, 6827–6838 (2017).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Zhang, H. et al. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 5, eaaw0873 (2019).
Lee, J. O. et al. A microscale optical implant for continuous in vivo monitoring of intraocular pressure. Microsyst. Nanoeng. 3, 17057 (2017).
Bansal, A., Yang, F., Xi, T., Zhang, Y. & Ho, J. S. In vivo wireless photonic photodynamic therapy. Proc. Natl Acad. Sci. USA 115, 1469–1474 (2018).
Yamagishi, K. et al. Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng. 3, 27–36 (2019). This paper describes an implantable optoelectronic device to overcome the light-penetration issue in the application of photodynamic therapy.
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Samineni, V. K. et al. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Sci. Rep. 7, 15865 (2017).
Park, S.-I. et al. Printed assemblies of inorganic light-emitting diodes for deformable and semitransparent displays. Science 325, 977–981 (2009).
Kim, H.-S. et al. Unusual strategies for using indium gallium nitride grown on silicon (111) for solid-state lighting. Proc. Natl Acad. Sci. USA 108, 10072–10077 (2011).
Kim, T.-I. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Kim, S. et al. Microstructured elastomeric surfaces with reversible adhesion and examples of their use in deterministic assembly by transfer printing. Proc. Natl Acad. Sci. USA 107, 17095–17100 (2010).
Agarwal, K., Jegadeesan, R., Guo, Y. X. & Thakor, N. V. Wireless power transfer strategies for implantable bioelectronics. IEEE Rev. Biomed. Eng. 10, 136–161 (2017).
Hinchet, R. et al. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 365, 491–494 (2019).
Djourno, A. & Eyries, C. Auditory prosthesis by means of a distant electrical stimulation of the sensory nerve with the use of an indwelt coiling. Presse Med. 65, 1417 (1957).
Brindley, G. S. & Lewin, W. S. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 196, 479–493 (1968).
Kelly, S. K. & Rizzo, J. in Artificial Vision (ed. Gabel, V. P.) 85–97 (Springer, 2017).
Gather, M. C. & Yun, S. H. Single-cell biological lasers. Nat. Photonics 5, 406–410 (2011).
Humar, M. & Yun, S. H. Intracellular microlasers. Nat. Photonics 9, 572–576 (2015).
Koo, J. et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 24, 1830–1836 (2018).
Reiner, R. & Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 3, 17076 (2017).
Hong, Y. J., Jeong, H., Cho, K. W., Lu, N. & Kim, D.-H. Wearable and implantable devices for cardiovascular healthcare: from monitoring to therapy based on flexible and stretchable electronics. Adv. Funct. Mater. 29, 1808247 (2019).
Xu, H., Yin, L., Lui, C., Sheng, X. & Zhao, N. Recent advances in biointegrated optoelectronic devices. Adv. Mater. 30, 1800156 (2018).
Samineni, V. K. et al. Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain 158, 2108–2116 (2017).
Park, S. I. et al. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proc. Natl Acad. Sci. USA 113, E8169–E8177 (2016).
Kim, R.-H. et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater. 9, 929–937 (2010).
Lee, W. et al. Transparent, conformable, active multielectrode array using organic electrochemical transistors. Proc. Natl Acad. Sci. USA 114, 10554–10559 (2017).
Park, S. et al. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature 561, 516–521 (2018).
Nihongaki, Y., Kawano, F., Nakajima, T. & Sato, M. Photoactivatable CRISPR-Cas9 for optogenetic genome editing. Nat. Biotechnol. 33, 755–760 (2015).
Nussinovitch, U. & Gepstein, L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol. 13, 750–754 (2015).
Tan, P., He, L., Han, G. & Zhou, Y. Optogenetic immunomodulation: shedding light on antitumor immunity. Trends Biotechnol. 35, 215–226 (2017).
Park, J. H. et al. Optogenetic modulation of urinary bladder contraction for lower urinary tract dysfunction. Sci. Rep. 7, 40872 (2017).
Häusser, M. Optogenetics: the age of light. Nat. Methods 11, 1012–1014 (2014).
Iaccarino, H. F. et al. Gamma frequency entertainment attenuates amyloid load and modifies microglia. Nature 540, 230–235 (2016). This paper demonstrates the feasibility of optogenetic therapy to control brain signals with a gamma frequency of 40 Hz for the treatment of Alzheimer disease.
Aron, L. & Yankner, B. A. Neural synchronization in Alzheimer’s disease. Nature 540, 207–208 (2016).
Kim, C. K., Adhikari, A. & Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 18, 222–235 (2017).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Famm, K., Litt, B., Tracey, K. J., Boyden, E. S. & Slaoui, M. Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161 (2013).
Kim, J. et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2, e1600418 (2016).
Kim, J. et al. Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv. Funct. Mater. 27, 1604373 (2017).
White, M. S. et al. Ultrathin, highly flexible and stretchable PLEDs. Nat. Photonics 7, 811–816 (2013).
Kim, T. et al. Fully stretchable optoelectronic sensors based on colloidal quantum dots for sensing photoplethysmographic signals. ACS Nano 11, 5992–6003 (2017).
Kaltenbrunner, M. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 3, 770 (2012).
Yin, D. et al. Efficient and mechanically robust stretchable organic light-emitting devices by a laser-programmable buckling process. Nat. Commun. 7, 11573 (2016).
Han, T.-H. et al. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 6, 105–110 (2012).
Liang, J., Li, L., Niu, X., Yu, Z. & Pei, Q. Elastomeric polymer light-emitting devices and displays. Nat. Photonics 7, 817–824 (2013).
Liang, J. et al. Silver nanowire percolation network soldered with graphene oxide at room temperature and its application for fully stretchable polymer light-emitting diodes. ACS Nano 8, 1590–1600 (2014).
Bade, S. G. R. et al. Stretchable light-emitting diodes with organometal-halide-perovskite-polymer composite emitters. Adv. Mater. 29, 1607053 (2017).
Son, D. et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 13, 1057–1065 (2018).
Larson, C. et al. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 351, 1071–1074 (2016).
Takei, K., Honda, W., Harada, S., Arie, T. & Akita, S. Toward flexible and wearable human-interactive health-monitoring devices. Adv. Healthc. Mater. 4, 487–500 (2015).
Senior, M. Novartis signs up for Google smart lens. Nat. Biotechnol. 3, 856 (2014).
Park, J. et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 4, eaap9841 (2018). This paper describes smart contact lenses that can measure tear glucose concentration and display it using light-emitting diodes powered wirelessly.
Yao, H., Shum, A. J., Cowan, M., Lähdesmäki, I. & Parviz, B. A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 26, 3290–3296 (2011).
Elsherif, M., Hassan, M. U., Yetisen, A. K. & Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 12, 5452–5462 (2018).
Leonardi, M., Pitchon, E. M., Bertsch, A., Renaud, P. & Mermoud, A. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol. 87, 433–437 (2009).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Domschke, A., March, W. F., Kabilan, S. & Lowe, C. Initial clinical testing of a holographic non-invasive contact lens glucose sensor. Diabetes Technol. Ther. 8, 89–93 (2006).
Deng, J. et al. Self-reporting colorimetric analysis of drug release by molecular imprinted structural color contact lens. ACS Appl. Mater. Interfaces 10, 34611–34617 (2018).
March, W. F., Mueller, A. & Herbrechtsmeier, P. Clinical trial of a noninvasive contact lens glucose sensor. Diabetes Technol. Ther. 6, 782–789 (2004).
Badugu, R., Lakowicz, J. R. & Geddes, C. D. Noninvasive continuous monitoring of physiological glucose using a monosaccharide-sensing contact lens. Anal. Chem. 76, 610–618 (2004).
Badugu, R., Lakowicz, J. R. & Geddes, C. D. A glucose-sensing contact lens: from bench top to patient. Curr. Opin. Biotechnol. 16, 100–107 (2005).
Zhang, J., Hodge, W., Hutnick, C. & Wang, X. Noninvasive diagnostic devices for diabetes through measuring tear glucose. J. Diabetes Sci. Technol. 5, 166–172 (2011).
Khalil, O. S. Spectroscopic and clinical aspects of noninvasive glucose measurements. Clin. Chem. 45, 165–177 (1999).
Maruo, K., Tsurugi, M., Tamura, M. & Ozaki, Y. In vivo noninvasive measurement of blood glucose by near-infrared diffuse-reflectance spectroscopy. Appl. Spectrosc. 57, 1236–1244 (2003).
Hahn, S. K., Lee, G.-H., Sim, J. Y., Koo, J. H. & Keum, D. H., Smart remotely controlled contact lens. Korean Patent 10-2017-0059225, PCT/KR2017/015243, Korean Patent 10-1956701 (2019).
Tang, J. et al. (2013). Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Investig. Ophthalmol. Vis. Sci. 54, 3681–3690 (2013).
Eells, J. T. et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl Acad. Sci. USA 100, 3439–3444 (2003).
Albarracin, R., Eells, J. T. & Valter, K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 52, 3582–3592 (2011).
Cashmore, A. R., Jarillo, J. A., Wu, Y. J. & Liu, D. Cryptochromes: blue light receptors for plants and animals. Science 284, 760–765 (1999).
Thresher, R. J. et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282, 1490–1494 (1998).
van der Horst, G. T. et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999).
Kim, W.-Y. et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449, 356–360 (2007).
Emery, P., So, W. V., Kaneko, M., Hall, J. C. & Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679 (1998).
Strong, R. E. et al. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress. Anxiety 26, 273–278 (2009).
Gordijn, M. C. & Meesters, Y. The effects of blue-enriched light treatment compared to standard light treatment in seasonal affective disorder. J. Affect. Disord. 136, 72–80 (2012).
Glickman, G., Byrne, B., Pineda, C., Hauck, W. W. & Brainard, G. C. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol. Psychiatry 59, 502–507 (2006).
Anderson, J. L., Glod, C. A., Dai, J., Cao, Y. & Lockley, S. W. Lux vs. wavelength in light treatment of seasonal affective disorder. Acta Psychiatr. Scand. 120, 203–212 (2009).
Bragard, I. & Coucke, P. A. Impact of the use of Luminette on well-being at work in a radiotherapy department. Cancer Radiother. 17, 731–735 (2013).
Langevin, R. H., Laurent, A. & Sauvéc, A. Preliminary assessment on the effectiveness of the Luminette in adolescents with a delayed sleep phase syndrome (DSPS): randomized single blind placebo-controlled study. Méd. Sommeil 11, 91–97 (2014).
Kirschbaum-Lesch, I., Gest, S., Legenbauer, T. & Holtmann, M. Feasibility and efficacy of bright light therapy in depressed adolescent inpatients. Z. Kinder Jugendpsychiatr. Psychother. 46, 423–429 (2018).
Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 8, 14–25 (2012).
Chua, C.-P. E., Redmond, S. J., McDarby, G. & Heneghan, C. Towards using photo-plethysmogram amplitude to measure blood pressure during sleep. Ann. Biomed. Eng. 38, 945–954 (2010).
Kim, M.-G., Kim, C., Alrowais, H. & Brand, O. Multiscale and uniform liquid metal thin-film patterning based on soft lithography for 3D heterogeneous integrated soft microsystems: additive stamping and subtractive reverse stamping. Adv. Mater. Technol. 3, 1800061 (2018).
Han, D. et al. Flexible blade-coated multicolor polymer light-emitting diodes for optoelectronic sensors. Adv. Mater. 29, 1606206 (2017).
Park, S. et al. Ultraflexible near-infrared organic photodetectors for conformal photoplethysmogram sensors. Adv. Mater. 30, 1802359 (2018).
Lee, H. et al. Toward all-day wearable health monitoring: an ultralow-power, reflective organic pulse oximetry sensing patch. Sci. Adv. 4, eaas9530 (2018). This paper describes thin-film photonic devices for pulse oximetry with significantly reduced power consumption, enabling all-day wearable healthcare.
Khan, Y. et al. A flexible organic reflectance oximeter array. Proc. Natl Acad. Sci. USA 115, E11015–E11024 (2018).
Chu, B., Burnett, W., Chung, J. W. & Bao, Z. Bring on the bodynet. Nature 549, 328–330 (2017). This Comment article describes stretchable sensors, circuits and batteries to fabricate wearable devices on the bodynet, a network of sensors, screens and smart devices woven into our clothing, worn on our skin and implanted in our bodies.
Karu, T. I. Cellular mechanisms of low-power laser therapy. Proc. SPIE 5149, 60–66 (2003).
Jeon, Y. et al. A wearable photobiomodulation patch using a flexible red-wavelength OLED and its in vitro differential cell proliferation effects. Adv. Mater. Technol. 3, 1700391 (2018).
Lee, H. E. et al. Trichogenic photostimulation using monolithic flexible vertical AlGaInP light-emitting diodes. ACS Nano 12, 9587–9595 (2018).
Kalajian, T. A., Aldoukhi, A., Veronikis, A. J., Persons, K. & Holick, M. F. Ultraviolet B light emitting diodes (LEDs) are more efficient and effective in producing vitamin D3 in human skin compared to natural sunlight. Sci. Rep. 7, 11489 (2017).
Han, S. et al. Upconversion nanoparticles/hyaluronate–rose bengal conjugate complex for noninvasive photochemical tissue bonding. ACS Nano 11, 9979–9988 (2017).
Darlot, F. et al. Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann. Neurol. 79, 59–75 (2016).
Friedman, A. A., Letai, A., Fisher, D. E. & Flaherty, K. T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 15, 747–756 (2015).
Collins, F. S. & Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 372, 793–795 (2015).
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010).
Gong, Y. et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 350, 1361–1366 (2015).
Berlin, S. et al. Photoactivatable genetically encoded calcium indicators for targeted neuronal imaging. Nat. Methods 12, 852–858 (2015).
Kyung, T. et al. Optogenetic control of endogenous Ca2+ channels in vivo. Nat. Biotechnol. 33, 1092–1096 (2015).
Steinbeck, J. A. et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol. 33, 204–209 (2015).
Lapp, H. et al. Frequency-dependent drug screening using optogenetic stimulation of human iPSC-derived cardiomyocytes. Sci. Rep. 7, 9629 (2017).
PDB ID 1LCI. Conti, E., Franks, N. P. & Brick, P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 4, 287–298 (1996).
Acknowledgements
This research was supported by the Center for Advanced Soft Electronics (Global Frontier Project, CASE-2015M3A6A5072945), Engineering Research Center (ERC) Program (grant no. NRF-2017R1A5A1014708) and the Basic Science Research Program (2017R1E1A1A03070458) of the National Research Foundation (NRF) funded by the Ministry of Science and ICT, Korea. This work was also supported by the World Class 300 Project (S2482887) of the Small and Medium Business Administration (SMBA), Korea. The Stanford researchers acknowledge support from Stanford Catalyst for Collaborative Solutions Program and Stanford Bio-X seed funding. The Stanford researchers acknowledge support from Department of Defense Air Force Office of Scientific Research (FA9550-15-1-0106), Samsung Electronics and Stanford Catalyst for Collaborative Solutions Program.
Author information
Authors and Affiliations
Contributions
G.-H.L., H.M., H.K., G.H.L., W.K. and S.K.H. researched data and wrote the manuscript. S.Y., D.M., S.H.Y, Z.B. and S.K.H. reviewed and edited the manuscript. All authors discussed the contents and provided important contributions to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, GH., Moon, H., Kim, H. et al. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat Rev Mater 5, 149–165 (2020). https://doi.org/10.1038/s41578-019-0167-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-019-0167-3
This article is cited by
-
A detachable interface for stable low-voltage stretchable transistor arrays and high-resolution X-ray imaging
Nature Communications (2024)
-
Reconfiguring hydrogel assemblies using a photocontrolled metallopolymer adhesive for multiple customized functions
Nature Chemistry (2024)
-
Body-conformable light-emitting materials and devices
Nature Photonics (2024)
-
Clinical translation of wireless soft robotic medical devices
Nature Reviews Bioengineering (2024)
-
Nature-inspired interfacial engineering for energy harvesting
Nature Reviews Electrical Engineering (2024)