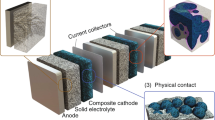
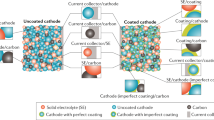
Abstract
Solid-state electrolytes (SSEs) have emerged as high-priority materials for safe, energy-dense and reversible storage of electrochemical energy in batteries. In this Review, we assess recent progress in the design, synthesis and analysis of SSEs, and identify key failure modes, performance limitations and design concepts for creating SSEs to meet requirements for practical applications. We provide an overview of the development and characteristics of SSEs, followed by analysis of ion transport in the bulk and at interfaces based on different single-valent (Li+, Na+, K+) and multivalent (Mg2+, Zn2+, Ca2+, Al3+) cation carriers of contemporary interest. We analyse the progress in overcoming issues associated with the poor ionic conductivity and high interfacial resistance of inorganic SSEs and the poor oxidative stability and cation transference numbers of polymer SSEs. Perspectives are provided on the design requirements for future generations of SSEs, with a focus on the chemical, geometric, mechanical, electrochemical and interfacial transport features required to accelerate progress towards practical solid-state batteries in which metals are paired with energetic cathode chemistries, including Ni-rich and Li-rich intercalating materials, sustainable organic materials, S8, O2 and CO2.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Tikekar, M. D., Choudhury, S., Tu, Z. & Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016).
Manthiram, A., Yu, X. W. & Wang, S. F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Bachman, J. C. et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116, 140–162 (2016).
Hu, Y.-S. Batteries: getting solid. Nat. Energy 1, 16042 (2016).
Li, J., Ma, C., Chi, M., Liang, C. & Dudney, N. J. Solid electrolyte: the key for high-voltage lithium batteries. Adv. Energy Mater. 5, 1401408 (2015).
Bruce, P. G. (ed.) Solid State Electrochemistry Ch. 1 1–6 (Cambridge Univ. Press, 1995).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016). This paper reports the highest ionic conductivity for an SIE, achieved using multiple strategies to increase ion conduction through the material.
He, X., Zhu, Y. & Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017). This paper explains why only a few materials can deliver exceptionally high ionic conductivity and how to design fast ion conductors following simple principles.
Bockris, J. O. M. & Reddy, A. K. N. Modern Electrochemistry Vol. 1 (Springer, 1998).
Fenton, D. E., Parker, J. M. & Wright, P. V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 14, 589 (1973).
Weston, J. & Steele, B. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion. 7, 75–79 (1982).
He, Y., Chen, Z., Zhang, Z., Wang, C. & Chen, L. Effect of dispersed phase particles on electrical conduction of PEO-NaSCN. Chem. Res. Chin. Univ. 2, 97–101 (1986).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Zhou, W. et al. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 138, 9385–9388 (2016). One of the first reports of a multilayer electrolyte that combines the benefits of its organic and inorganic components.
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications 2nd edn (Wiley, 2001).
Jost, W. Diffusion and electrolytic conduction in crystals (ionic semiconductors). J. Chem. Phys. 1, 466–475 (1933).
Corish, J. in Handbook of Materials Modeling (ed. Yip, S.) 1889–1899 (Springer, 2005).
Knauth, P. & Tuller, H. L. Solid-state ionics: roots, status, and future prospects. J. Am. Ceram. Soc. 85, 1654–1680 (2002).
Minami, T. Fast ion conducting glasses. J. Non-Cryst. Solids 73, 273–284 (1985).
Angell, C. A. Mobile ions in amorphous solids. Annu. Rev. Phys. Chem. 43, 693–717 (1992).
Souquet, J. L. Ionic transport in amorphous solid electrolytes. Annu. Rev. Mater. Sci. 11, 211–231 (1981).
Wang, Y. et al. Design principles for solid-state lithium superionic conductors. Nat. Mater. 14, 1026–1031 (2015). This paper reveals a fundamental relationship between anion packing and ionic transport in fast Li-ion-conducting materials and exposes the desirable structural attributes of good Li-ion conductors.
Noda, Y. et al. Computational and experimental investigation of the electrochemical stability and Li-ion conduction mechanism of LiZr2(PO4)3. Chem. Mater. 29, 8983–8991 (2017).
Weber, D. A. et al. Structural insights and 3D diffusion pathways within the lithium superionic conductor Li10GeP2S12. Chem. Mater. 28, 5905–5915 (2016).
Kwon, O. et al. Synthesis, structure, and conduction mechanism of lithium superionic conductor Li10+δGe1+δP2−δS12. J. Mater. Chem. A 3, 438–446 (2015).
Iwasaki, R. et al. Weak anisotropic lithium-ion conductivity in single crystals of Li10GeP2S12. Chem. Mater. 31, 3694–3699 (2019).
Zhao, C. Z. et al. An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 4, eaat3446 (2018).
Nitzan, A. & Ratner, M. A. Conduction in polymers - dynamic disorder transport. J. Phys. Chem. 98, 1765–1775 (1994).
Borodin, O. & Smith, G. D. Mechanism of ion transport in amorphous poly(ethylene oxide)/LiTFSI from molecular dynamics simulations. Macromolecules 39, 1620–1629 (2006).
Teran, A. A., Tang, M. H., Mullin, S. A. & Balsara, N. P. Effect of molecular weight on conductivity of polymer electrolytes. Solid State Ion. 203, 18–21 (2011).
Gadjourova, Z., Andreev, Y. G., Tunstall, D. P. & Bruce, P. G. Ionic conductivity in crystalline polymer electrolytes. Nature 412, 520–523 (2001). This paper provides the first evidence of ion conduction in the crystalline phase of polymers.
Staunton, E., Andreev, Y. G. & Bruce, P. G. Structure and conductivity of the crystalline polymer electrolyte β-PEO6:LiAsF6. J. Am. Chem. Soc. 127, 12176–12177 (2005).
Henderson, W. A. & Passerini, S. Ionic conductivity in crystalline-amorphous polymer electrolytes – P(EO)6: LiX phases. Electrochem. Commun. 5, 575–578 (2003).
Xue, S. et al. Diffusion of lithium ions in amorphous and crystalline poly(ethylene oxide)3:LiCF3SO3 polymer electrolytes. Electrochim. Acta 235, 122–128 (2017).
Gregori, G., Merkle, R. & Maier, J. Ion conduction and redistribution at grain boundaries in oxide systems. Prog. Mater. Sci. 89, 252–305 (2017).
Dawson, J. A., Canepa, P., Famprikis, T., Masquelier, C. & Islam, M. S. Atomic-scale influence of grain boundaries on Li-ion conduction in solid electrolytes for all-solid-state batteries. J. Am. Chem. Soc. 140, 362–368 (2018).
Inaguma, Y. et al. High ionic conductivity in lithium lanthanum titanate. Solid. State Commun. 86, 689–693 (1993).
Bruce, P. G. The A-C conductivity of polycrystalline LISICON, Li2+2xZn1−xGeO4, and a model for intergranular constriction resistances. J. Electrochem. Soc. 130, 662–6691 (1983).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46, 7778–7781 (2007).
Yu, S. & Siegel, D. J. Grain boundary contributions to Li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 29, 9639–9647 (2017).
Kotobuki, M., Kanamura, K., Sato, Y., Yamamoto, K. & Yoshida, T. Electrochemical properties of Li7La3Zr2O12 solid electrolyte prepared in argon atmosphere. J. Power Sources 199, 346–349 (2012).
Chen, C. C., Fu, L. & Maier, J. Synergistic, ultrafast mass storage and removal in artificial mixed conductors. Nature 536, 159–164 (2016).
Chen, C.-C. & Maier, J. Decoupling electron and ion storage and the path from interfacial storage to artificial electrodes. Nat. Energy 3, 102–108 (2018).
Swift, M. W. & Qi, Y. First-principles prediction of potentials and space-charge layers in all-solid-state batteries. Phys. Rev. Lett. 122, 167701 (2019).
Nomura, Y. et al. Direct observation of a Li-ionic space-charge layer formed at an electrode/solid-electrolyte interface. Angew. Chem. Int. Ed. 58, 5292–5296 (2019). This paper reports the ionic and potential profiles in the charge-redistribution layer formed at an SSE–electrode interface using phase-shifting electron holography and spatially resolved electron energy-loss spectroscopy.
Thokchom, J. S. & Kumar, B. The effects of crystallization parameters on the ionic conductivity of a lithium aluminum germanium phosphate glass–ceramic. J. Power Sources 195, 2870–2876 (2010).
Haruyama, J., Sodeyama, K., Han, L., Takada, K. & Tateyama, Y. Space–charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 26, 4248–4255 (2014).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Lu, Y., Li, L., Zhang, Q., Niu, Z. & Chen, J. Electrolyte and interface engineering for solid-state sodium batteries. Joule 2, 1747–1770 (2018).
Hong, H. Y. P. Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors. Mater. Res. Bull. 13, 117–124 (1978).
Wang, Y., Richards, W. D., Bo, S. H., Miara, L. J. & Ceder, G. Computational prediction and evaluation of solid-state sodium superionic conductors Na7P3X11 (X = O, S, Se). Chem. Mater. 29, 7475–7482 (2017).
Sagane, F., Abe, T., Iriyama, Y. & Ogumi, Z. Li+ and Na+ transfer through interfaces between inorganic solid electrolytes and polymer or liquid electrolytes. J. Power Sources 146, 749–752 (2005).
Okoshi, M., Yamada, Y., Yamada, A. & Nakai, H. Theoretical analysis on de-solvation of lithium, sodium, and magnesium cations to organic electrolyte solvents. J. Electrochem. Soc. 160, A2160–A2165 (2013).
Chusid, O. et al. Solid-state rechargeable magnesium batteries. Adv. Mater. 15, 627–630 (2003).
Ikeda, S., Takahashi, M., Ishikawa, J. & Ito, K. Solid electrolytes with multivalent cation conduction. 1. Conducting species in Mg-Zr-PO4 system. Solid State Ion. 23, 125–129 (1987).
Higashi, S., Miwa, K., Aoki, M. & Takechi, K. A novel inorganic solid state ion conductor for rechargeable Mg batteries. Chem. Commun. 50, 1320–1322 (2014).
Yamanaka, T., Hayashi, A., Yamauchi, A. & Tatsumisago, M. Preparation of magnesium ion conducting MgS–P2S5–MgI2 glasses by a mechanochemical technique. Solid State Ion. 262, 601–603 (2014).
Nomura, K., Ikeda, S., Ito, K. & Einaga, H. Framework structure, phase transition and ionic conductivity of MgZr4(PO4)6 and ZnZr4(PO4)6. J. Electroanal. Chem. 326, 351–356 (1992).
Imanaka, N., Okazaki, Y. & Adachi, G. Divalent magnesium ionic conduction in Mg1−2x(Zr1−xNbx)4P6O24 (x = 0–0.4) solid solutions. Electrochem. Solid-State Lett. 3, 327–329 (1999).
Imanaka, N., Okazaki, Y. & Adachi, G.-y Divalent magnesium ion conducting characteristics in phosphate based solid electrolyte composites. J. Mater. Chem. 10, 1431–1435 (2000).
Matsuo, M. et al. Complex hydrides with (BH4)− and (NH2)− anions as new lithium fast-ion conductors. J. Am. Chem. Soc. 131, 16389–16391 (2009).
Canepa, P. et al. High magnesium mobility in ternary spinel chalcogenides. Nat. Commun. 8, 1759 (2017).
Yang, L., Huq, R., Farrington, G. & Chiodelli, G. Preparation and properties of PEO complexes of divalent cation salts. Solid State Ion. 18–19, 291–294 (1986).
Shao, Y. et al. Nanocomposite polymer electrolyte for rechargeable magnesium batteries. Nano Energy 12, 750–759 (2015).
Zhao, Q. et al. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 4, eaao1761 (2018).
Farrington, G. C. & Dunn, B. Divalent beta″-aluminas: high conductivity solid electrolytes for divalent cations. Solid State Ion. 7, 267–281 (1982).
Ikeda, S. Solid electrolytes with multivalent cation conduction (2) zinc ion conduction in Zn-Zr-PO4 system. Solid State Ion. 40–41, 79–82 (1990).
Martinolich, A. J. et al. Solid-state divalent ion conduction in ZnPS3. Chem. Mater. 31, 3652–3661 (2019).
Abrantes, T., Alcacer, L. & Sequeira, C. Thin film solid state polymer electrolytes containing silver, copper and zinc ions as charge carriers. Solid State Ion. 18–19, 315–320 (1986).
Lee, B. S. et al. Dendrite suppression membranes for rechargeable zinc batteries. ACS Appl. Mater. Interfaces 10, 38928–38935 (2018).
Lin, C. et al. Solid-state rechargeable zinc–air battery with long shelf life based on nanoengineered polymer electrolyte. ChemSusChem 11, 3215–3224 (2018).
Nomura, K., Ikeda, S., Ito, K. & Einaga, H. Framework structure, phase transition, and transport properties in MIIZr4(PO4)6 compounds (MII = Mg, Ca, Sr, Ba, Mn, Co, Ni, Zn, Cd, and Pb). Bull. Chem. Soc. Jpn. 65, 3221–3227 (1992).
Zhao, Q. et al. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 4, eaau8131 (2018).
Wu, G. M., Lin, S. J. & Yang, C. C. Alkaline Zn-air and Al-air cells based on novel solid PVA/PAA polymer electrolyte membranes. J. Membr. Sci. 280, 802–808 (2006).
Sun, X. G. et al. Polymer gel electrolytes for application in aluminum deposition and rechargeable aluminum ion batteries. Chem. Commun. 52, 292–295 (2016).
Yu, Z. et al. Flexible stable solid-state Al-ion batteries. Adv. Funct. Mater. 29, 1806799 (2019).
Han, F., Zhu, Y., He, X., Mo, Y. & Wang, C. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6, 1501590 (2016).
Wenzel, S., Leichtweiss, T., Krüger, D., Sann, J. & Janek, J. Interphase formation on lithium solid electrolytes—an in situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ion. 278, 98–105 (2015).
Wenzel, S. et al. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode. Chem. Mater. 28, 2400–2407 (2016).
Lewis, J. A. et al. Interphase morphology between a solid-state electrolyte and lithium controls cell failure. ACS Energy Lett. 4, 591–599 (2019).
Leung, K. et al. Kinetics-controlled degradation reactions at crystalline LiPON/LixCoO2 and crystalline LiPON/Li-metal interfaces. ChemSusChem. 11, 1956–1969 (2018).
Zhang, W. et al. The detrimental effects of carbon additives in Li10GeP2S12-based solid-state batteries. ACS Appl. Mater. Interfaces 9, 35888–35896 (2017).
Han, F. D., Gao, T., Zhu, Y. J., Gaskell, K. J. & Wang, C. S. A battery made from a single material. Adv. Mater. 27, 3473–3483 (2015).
Sahu, G. et al. Air-stable, high-conduction solid electrolytes of arsenic-substituted Li4SnS4. Energy Environ. Sci. 7, 1053–1058 (2014).
Hallinan, D. T., Rausch, A. & McGill, B. An electrochemical approach to measuring oxidative stability of solid polymer electrolytes for lithium batteries. Chem. Eng. Sci. 154, 34–41 (2016).
Xia, Y. Y., Fujieda, T., Tatsumi, K., Prosini, P. P. & Sakai, T. Thermal and electrochemical stability of cathode materials in solid polymer electrolyte. J. Power Sources 92, 234–243 (2001).
Zhao, Q., Liu, X., Stalin, S., Khan, K. & Archer, L. A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 4, 365–373 (2019). This study reports that SPEs formed in situ exhibit fast interfacial transport.
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396–A404 (2005).
Han, F. D. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Xu, C., Ahmad, Z., Aryanfar, A., Viswanathan, V. & Greer, J. R. Enhanced strength and temperature dependence of mechanical properties of Li at small scales and its implications for Li metal anodes. Proc. Natl Acad. Sci. USA 114, 57–61 (2017).
Swamy, T. et al. Lithium metal penetration induced by electrodeposition through solid electrolytes: example in single-crystal Li6La3ZrTaO12 garnet. J. Electrochem. Soc. 165, A3648–A3655 (2018).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7, 1701003 (2017).
Aguesse, F. et al. Investigating the dendritic growth during full cell cycling of garnet electrolyte in direct contact with Li metal. ACS Appl. Mater. Interfaces 9, 3808–3816 (2017).
Song, Y. et al. Revealing the short-circuiting mechanism of garnet-based solid-state electrolyte. Adv. Energy Mater. 9, 1900671 (2019).
Wu, B. B. et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 11, 1803–1810 (2018). This paper reveals the origin and evolution of Li dendrite growth along the cracks and holes of ceramics.
Sharafi, A., Meyer, H. M., Nanda, J., Wolfenstine, J. & Sakamoto, J. Characterizing the Li–Li7La3Zr2O12 interface stability and kinetics as a function of temperature and current density. J. Power Sources 302, 135–139 (2016).
Raj, R. & Wolfenstine, J. Current limit diagrams for dendrite formation in solid-state electrolytes for Li-ion batteries. J. Power Sources 343, 119–126 (2017).
Dejonghe, L. C., Feldman, L. & Beuchele, A. Slow degradation and electron conduction in sodium-beta-aluminas. J. Mater. Sci. 16, 780–786 (1981).
Richards, W. D., Wang, Y., Miara, L. J., Kim, J. C. & Ceder, G. Design of Li1+2xZn1−xPS4, a new lithium ion conductor. Energy Environ. Sci. 9, 3272–3278 (2016).
Garcia, A. New lithium ion conductors based on the γ-LiAlO2 structure. Solid State Ion. 40–41, 13–17 (1990).
Kawai, H. Lithium ion conductivity of A-site deficient perovskite solid solution La0.67−xLi3xTiO3. J. Electrochem. Soc. 141, L78–L79 (1994).
Park, K. H. et al. Design strategies, practical considerations, and new solution processes of sulfide solid electrolytes for all-solid-state batteries. Adv. Energy Mater. 8, 1800035 (2018).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Adeli, P. et al. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution. Angew. Chem. Int. Ed. 58, 8681–8686 (2019).
Zhang, L. et al. Na3PSe4: A novel chalcogenide solid electrolyte with high ionic conductivity. Adv. Energy Mater. 5, 1501294 (2015).
Banerjee, A. et al. Na3SbS4: A solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. Int. Ed. 55, 9634–9638 (2016).
Kim, K. & Siegel, D. J. Correlating lattice distortions, ion migration barriers, and stability in solid electrolytes. J. Mater. Chem. A 7, 3216–3227 (2019).
Lacivita, V. et al. Resolving the amorphous structure of lithium phosphorus oxynitride (Lipon). J. Am. Chem. Soc. 140, 11029–11038 (2018).
Zheng, Z. F., Fang, H. Z., Yang, F., Liu, Z. K. & Wang, Y. Amorphous LiLaTiO3 as solid electrolyte material. J. Electrochem. Soc. 161, A473–A479 (2014).
Sendek, A. D. et al. Holistic computational structure screening of more than 12000 candidates for solid lithium-ion conductor materials. Energy Environ. Sci. 10, 306–320 (2017).
Nolan, A. M., Zhu, Y. Z., He, X. F., Bai, Q. & Mo, Y. F. Computation-accelerated design of materials and interfaces for all-solid-state lithium-ion batteries. Joule 2, 2016–2046 (2018).
Muy, S. et al. Tuning mobility and stability of lithium ion conductors based on lattice dynamics. Energy Environ. Sci. 11, 850–859 (2018).
Xiong, S. et al. Computation-guided design of LiTaSiO5, a new lithium ionic conductor with sphene structure. Adv. Energy Mater. 9, 1803821 (2019).
Barteau, K. P. Poly(glycidyl ether)-based Battery Electrolytes: Correlating Polymer Properties to Ion Transport. Thesis, Univ. California, Santa Barbara (2015).
Wheatle, B. K., Keith, J. R., Mogurampelly, S., Lynd, N. A. & Ganesan, V. Influence of dielectric constant on ionic transport in polyether-based electrolytes. ACS Macro Lett. 6, 1362–1367 (2017).
Mindemark, J., Imholt, L., Montero, J. & Brandell, D. Allyl ethers as combined plasticizing and crosslinkable side groups in polycarbonate-based polymer electrolytes for solid-state Li batteries. J. Polym. Sci. A 54, 2128–2135 (2016).
Abraham, K. M. & Alamgir, M. Li+-conductive solid polymer electrolytes with liquid-like conductivity. J. Electrochem. Soc. 137, 1657–1658 (1990).
Fu, G. & Kyu, T. Effect of side-chain branching on enhancement of ionic conductivity and capacity retention of a solid copolymer electrolyte membrane. Langmuir 33, 13973–13981 (2017).
Fan, L. Z., Hu, Y. S., Bhattacharyya, A. J. & Maier, J. Succinonitrile as a versatile additive for polymer electrolytes. Adv. Funct. Mater. 17, 2800–2807 (2007).
Guzmán, G., Nava, D. P., Vazquez-Arenas, J. & Cardoso, J. Design of a zwitterion polymer electrolyte based on poly[poly (ethylene glycol) methacrylate]: the effect of sulfobetaine group on thermal properties and ionic conduction. Macromol. Symp. 374, 1600136 (2017).
Pesko, D. M. et al. Universal relationship between conductivity and solvation-site connectivity in ether-based polymer electrolytes. Macromolecules 49, 5244–5255 (2016).
Lu, Y. et al. A compatible anode/succinonitrile-based electrolyte interface in all-solid-state Na–CO2 batteries. Chem. Sci. 10, 4306–4312 (2019).
Choudhury, S., Stalin, S., Deng, Y. & Archer, L. A. Soft colloidal glasses as solid-state electrolytes. Chem. Mater. 30, 5996–6004 (2018).
Lin, Y., Wang, X., Liu, J. & Miller, J. D. Natural halloysite nano-clay electrolyte for advanced all-solid-state lithium-sulfur batteries. Nano Energy 31, 478–485 (2017).
Liu, W. et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2, 17035 (2017). This paper reports a composite SPE with well-aligned, inorganic, Li +-conductive nanowires with an ionic conductivity an order of magnitude higher than that of previous SPEs.
Zaman, W., Hortance, N., Dixit, M. B., De Andrade, V. & Hatzell, K. B. Visualizing percolation and ion transport in hybrid solid electrolytes for Li–metal batteries. J. Mater. Chem. A 7, 23914–23921 (2019).
Chen, X. C. et al. Study of segmental dynamics and ion transport in polymer–ceramic composite electrolytes by quasi-elastic neutron scattering. Mol. Syst. Des. Eng. 4, 379–385 (2019).
Chen, X. C. et al. Determining and minimizing resistance for ion transport at the polymer/ceramic electrolyte interface. ACS Energy Lett. 4, 1080–1085 (2019).
Zhou, W. et al. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv. Mater. 31, e1805574 (2019). The first report of a multilayer SPE with continuous ion transport across interfaces for high-voltage operation.
Seki, S., Kobayashi, Y., Miyashiro, H., Mita, Y. & Iwahori, T. Fabrication of high-voltage, high-capacity all-solid-state lithium polymer secondary batteries by application of the polymer electrolyte/inorganic electrolyte composite concept. Chem. Mater. 17, 2041–2045 (2005).
Choudhury, S. et al. Stabilizing polymer electrolytes in high-voltage lithium batteries. Nat. Commun. 10, 3091 (2019).
Dong, T. T. et al. A multifunctional polymer electrolyte enables ultra-long cycle-life in a high-voltage lithium metal battery. Energy Environ. Sci. 11, 1197–1203 (2018).
Zhao, Q., Chen, P. Y., Li, S. K., Liu, X. T. & Archer, L. A. Solid-state polymer electrolytes stabilized by task-specific salt additives. J. Mater. Chem. A 7, 7823–7830 (2019).
Xu, K., Zhang, S. S., Jow, T. R., Xu, W. & Angell, C. A. LiBOB as salt for lithium-ion batteries: a possible solution for high temperature operation. Electrochem. Solid-State Lett. 5, A26–A29 (2002).
Li, S. et al. A superionic conductive, electrochemically stable dual-salt polymer electrolyte. Joule 2, 1838–1856 (2018). One of the first reports of the use of crosslinking polymers to wet the porous cathode material before crosslinking to form continuous transport pathways, along with additives to enable operation at high voltages.
Liang, W. F., Shao, Y. F., Chen, Y. M. & Zhu, Y. A 4 V cathode compatible, superionic conductive solid polymer electrolyte for solid lithium metal batteries with long cycle life. ACS Appl. Energy Mater. 1, 6064–6071 (2018).
Chai, J. et al. In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv. Sci. 4, 1600377 (2017).
Auvergniot, J. et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 29, 3883–3890 (2017).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Interfacial observation between LiCoO2 electrode and Li2S−P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy. Chem. Mater. 22, 949–956 (2010).
Ohta, N. et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 18, 2226–2229 (2006).
Ito, Y. et al. Application of LiCoO2 particles coated with lithium ortho-oxosalt thin films to sulfide-type all-solid-state lithium batteries. J. Electrochem. Soc. 162, A1610–A1616 (2015).
Woo, J. H., Travis, J. J., George, S. M. & Lee, S.-H. Utilization of Al2O3 atomic layer deposition for Li ion pathways in solid state Li batteries. J. Electrochem. Soc. 162, A344–A349 (2014).
Hallinan, D. T., Mullin, S. A., Stone, G. M. & Balsara, N. P. Lithium metal stability in batteries with block copolymer electrolytes. J. Electrochem. Soc. 160, A464–A470 (2013).
Capuano, F. Composite polymer electrolytes. J. Electrochem. Soc. 138, 1918–1922 (1991).
Harry, K. J., Liao, X. X., Parkinson, D. Y., Minor, A. M. & Balsara, N. P. Electrochemical deposition and stripping behavior of lithium metal across a rigid block copolymer electrolyte membrane. J. Electrochem. Soc. 162, A2699–A2706 (2015).
Harry, K. J., Higa, K., Srinivasan, V. & Balsara, N. P. Influence of electrolyte modulus on the local current density at a dendrite tip on a lithium metal electrode. J. Electrochem. Soc. 163, A2216–A2224 (2016).
Young, R. & Lovell, P. Introduction to Polymers 3rd edn Ch. 21 (CRC, 2011).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014). The first report of the use of polymer crosslinking to incorporate a high-modulus component in the electrolyte for the suppression of dendrite growth.
Zeng, X. X. et al. Reshaping lithium plating/stripping behavior via bifunctional polymer electrolyte for room-temperature solid Li metal batteries. J. Am. Chem. Soc. 138, 15825–15828 (2016).
Choudhury, S. et al. Confining electrodeposition of metals in structured electrolytes. Proc. Natl Acad. Sci. USA 115, 6620–6625 (2018). This paper reports the visual elucidation of the mechanism of dendrite suppression by crosslinked polymer electrolytes and the role of mesh size of the network in stabilizing electrodeposition.
Liu, K. et al. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 139, 4815–4820 (2017).
Zheng, G. Y. et al. High-performance lithium metal negative electrode with a soft and flowable polymer coating. ACS Energy Lett. 1, 1247–1255 (2016).
Lopez, J. et al. Effects of polymer coatings on electrodeposited lithium metal. J. Am. Chem. Soc. 140, 11735–11744 (2018).
Zhao, C. Z. et al. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl Acad. Sci. USA 114, 11069–11074 (2017).
Tung, S. O., Ho, S., Yang, M., Zhang, R. & Kotov, N. A. A dendrite-suppressing composite ion conductor from aramid nanofibres. Nat. Commun. 6, 6152 (2015).
Wang, C. et al. Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 9, 13694–13702 (2017).
Gao, Y. et al. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013). This work reports a multifunctional, single-ion SPE based on polyanionic block copolymers.
Ma, Q. et al. Single lithium-ion conducting polymer electrolytes based on a super-delocalized polyanion. Angew. Chem. Int. Ed. 55, 2521–2525 (2016).
Bannister, D. J., Davies, G. R., Ward, I. M. & McIntyre, J. E. Ionic conductivities for poly(ethylene oxide) complexes with lithium salts of monobasic and dibasic acids and blends of poly(ethylene oxide) with lithium salts of anionic polymers. Polymer 25, 1291–1296 (1984).
Matsumoto, K. & Endo, T. Synthesis of networked polymers with lithium counter cations from a difunctional epoxide containing poly(ethylene glycol) and an epoxide monomer carrying a lithium sulfonate salt moiety. J. Polym. Sci. A 48, 3113–3118 (2010).
Zhu, Y. S. et al. A single-ion polymer electrolyte based on boronate for lithium ion batteries. Electrochem. Commun. 22, 29–32 (2012).
Porcarelli, L. et al. Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett. 1, 678–682 (2016).
Snyder, J. F., Ratner, M. A. & Shriver, D. F. Ion conductivity of comb polysiloxane polyelectrolytes containing oligoether and perfluoroether sidechains. J. Electrochem. Soc. 150, A1090–A1094 (2003).
Rojas, A. A. et al. Effect of lithium-ion concentration on morphology and ion transport in single-ion-conducting block copolymer electrolytes. Macromolecules 48, 6589–6595 (2015).
Porcarelli, L. et al. Single ion conducting polymer electrolytes based on versatile polyurethanes. Electrochim. Acta 241, 526–534 (2017).
Savoie, B. M., Webb, M. A. & Miller, T. F. III Enhancing cation diffusion and suppressing anion diffusion via Lewis-acidic polymer electrolytes. J. Phys. Chem. Lett. 8, 641–646 (2017).
Forsyth, M., Porcarelli, L., Wang, X., Goujon, N. & Mecerreyes, D. Innovative electrolytes based on ionic liquids and polymers for next-generation solid-state batteries. Acc. Chem. Res. 52, 686–694 (2019).
Porcarelli, L. et al. Single-ion block copoly(ionic liquid)s as electrolytes for all-solid state lithium batteries. ACS Appl. Mater. Interfaces 8, 10350–10359 (2016).
Park, S. S., Tulchinsky, Y. & Dinca˘, M. Single-ion Li+, Na+, and Mg2+ solid electrolytes supported by a mesoporous anionic Cu–azolate metal–organic framework. J. Am. Chem. Soc. 139, 13260–13263 (2017).
Fischer, S. et al. A metal–organic framework with tetrahedral aluminate sites as a single-ion Li+ solid electrolyte. Angew. Chem. Int. Ed. 57, 16683–16687 (2018).
Jeong, K. et al. Solvent-free, single lithium-ion conducting covalent organic frameworks. J. Am. Chem. Soc. 141, 5880–5885 (2019).
Luo, W. et al. Transition from superlithiophobicity to superlithiophilicity of garnet solid-state electrolyte. J. Am. Chem. Soc. 138, 12258–12262 (2016).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017). The first paper to propose strategies to decrease the interfacial resistance of garnet electrolytes and Li-metal electrodes using ultrathin Al 2O 3.
Wang, C. et al. Conformal, nanoscale ZnO surface modification of garnet-based solid-state electrolyte for lithium metal anodes. Nano Lett. 17, 565–571 (2017).
Luo, W. et al. Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer. Adv. Mater. 29, 1606042 (2017).
Hao, F. et al. Taming active material-solid electrolyte interfaces with organic cathode for all-solid-state batteries. Joule 3, 1349–1359 (2019).
Jin, Y. et al. An intermediate temperature garnet-type solid electrolyte-based molten lithium battery for grid energy storage. Nat. Energy 3, 732–738 (2018).
Cheng, Q. et al. Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 3, 1510–1522 (2019).
Yu, Q. et al. Constructing effective interfaces for Li1.5Al0.5Ge1.5(PO4)3 pellets to achieve room-temperature hybrid solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 11, 9911–9918 (2019).
Tian, Y. et al. Reactivity-guided interface design in Na metal solid-state batteries. Joule 3, 1037–1050 (2019).
Gao, Y. et al. Salt-based organic–inorganic nanocomposites: towards a stable lithium metal/Li10GeP2S12 solid electrolyte interface. Angew. Chem. Int. Ed. 57, 13608–13612 (2018).
Zhang, Z. et al. Interface re-engineering of Li10GeP2S12 electrolyte and lithium anode for all-solid-state lithium batteries with ultralong cycle life. ACS Appl. Mater. Interfaces 10, 2556–2565 (2018).
Wang, C. H. et al. Boosting the performance of lithium batteries with solid-liquid hybrid electrolytes: Interfacial properties and effects of liquid electrolytes. Nano Energy 48, 35–43 (2018).
Xu, B. Y., Duan, H. A., Liu, H. Z., Wang, C. A. & Zhong, S. W. Stabilization of garnet/liquid electrolyte interface using superbase additives for hybrid Li batteries. ACS Appl. Mater. Interfaces 9, 21077–21082 (2017).
Zhang, Z. Z. et al. A self-forming composite electrolyte for solid-state sodium battery with ultralong cycle life. Adv. Energy Mater. 7, 1601196 (2017). Study in which the charge-transfer rate at the electrode–electrolyte interface is increased using a small amount of non-flammable and non-volatile ionic liquid at the cathode in solid-state sodium batteries.
Oh, D. Y. et al. Excellent compatibility of solvate ionic liquids with sulfide solid electrolytes: toward favorable ionic contacts in bulk-type all-solid-state lithium-ion batteries. Adv. Energy Mater. 5, 1500865 (2015).
Duan, H. et al. Dendrite-free Li-metal battery enabled by a thin asymmetric solid electrolyte with engineered layers. J. Am. Chem. Soc. 140, 82–85 (2018).
Chen, X. Z., He, W. J., Ding, L. X., Wang, S. Q. & Wang, H. H. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 12, 938–944 (2019).
Pan, Q. et al. Correlating electrode–electrolyte interface and battery performance in hybrid solid polymer electrolyte-based lithium metal batteries. Adv. Energy Mater. 7, 1701231 (2017).
Liu, Y. et al. Transforming from planar to three-dimensional lithium with flowable interphase for solid lithium metal batteries. Sci. Adv. 3, eaao0713 (2017).
Aldalur, I. et al. Jeffamine based polymers as highly conductive polymer electrolytes and cathode binder materials for battery application. J. Power Sources 347, 37–46 (2017).
Porcarelli, L., Gerbaldi, C., Bella, F. & Nair, J. R. Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci. Rep. 6, 19892 (2016). One of the first papers to report an all-ethylene-oxide-based crosslinked SSE with improved and continuous ion transport through electrode–electrolyte interfaces.
Dong, D. et al. Polymer electrolyte glue: a universal interfacial modification strategy for all-solid-state Li batteries. Nano Lett. 19, 2343–2349 (2019).
Du, A. et al. A crosslinked polytetrahydrofuran-borate-based polymer electrolyte enabling wide-working-temperature-range rechargeable magnesium batteries. Adv. Mater. 31, e1805930 (2019).
Wang, Y.-X. et al. Room-temperature sodium–sulfur batteries: a comprehensive review on research progress and cell chemistry. Adv. Energy Mater. 7, 1602829 (2017).
Jacoby, M. Batteries get flexible. Chem. Eng. News 91, 13–18 (2013).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Sun, C., Liu, J., Gong, Y., Wilkinson, D. P. & Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33, 363–386 (2017).
Wan, J. et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 14, 705–711 (2019).
Ates, T., Keller, M., Kulisch, J., Adermann, T. & Passerini, S. Development of an all-solid-state lithium battery by slurry-coating procedures using a sulfidic electrolyte. Energy Storage Mater. 17, 204–210 (2019).
Xiao, N., Ren, X., McCulloch, W. D., Gourdin, G. & Wu, Y. Potassium superoxide: a unique alternative for metal–air batteries. Acc. Chem. Res. 51, 2335–2343 (2018).
Al Sadat, W. I. & Archer, L. A. The O2-assisted Al/CO2 electrochemical cell: A system for CO2 capture/conversion and electric power generation. Sci. Adv. 2, e1600968 (2016).
Lei, X. et al. Flexible lithium–air battery in ambient air with an in situ formed gel electrolyte. Angew. Chem. Int. Ed. 57, 16131–16135 (2018).
Wu, H., Zhuo, D., Kong, D. & Cui, Y. Improving battery safety by early detection of internal shorting with a bifunctional separator. Nat. Commun. 5, 5193 (2014).
Koerver, R. et al. Capacity fade in solid-state batteries: interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 29, 5574–5582 (2017).
Wang, S., Xu, H., Li, W., Dolocan, A. & Manthiram, A. Interfacial chemistry in solid-state batteries: formation of interphase and its consequences. J. Am. Chem. Soc. 140, 250–257 (2018).
Park, K. et al. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem. Mater. 28, 8051–8059 (2016).
Sun, F. et al. Revealing hidden facts of Li anode in cycled lithium oxygen batteries through X-ray and neutron tomography. ACS Energy Lett. 4, 306–316 (2019).
Lv, S. et al. Operando monitoring the lithium spatial distribution of lithium metal anodes. Nat. Commun. 9, 2152 (2018).
Wang, C. W. et al. In situ neutron depth profiling of lithium metal–garnet interfaces for solid state batteries. J. Am. Chem. Soc. 139, 14257–14264 (2017).
Chandrashekar, S. et al. 7Li MRI of Li batteries reveals location of microstructural lithium. Nat. Mater. 11, 311–315 (2012).
Harry, K. J., Hallinan, D. T., Parkinson, D. Y., MacDowell, A. A. & Balsara, N. P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2014).
Li, Y. Z. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Matsuda, Y. et al. In situ Raman spectroscopy of LiCoO2 cathode in Li/Li3PO4/LiCoO2 all-solid-state thin-film lithium battery. Solid State Ion. 335, 7–14 (2019).
Wang, Z. et al. In situ STEM-EELS observation of nanoscale interfacial phenomena in all-solid-state batteries. Nano Lett. 16, 3760–3767 (2016).
Yao, Y.-F. Y. & Kummer, J. T. Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 29, 2453–2475 (1967).
Goodenough, J. B., Hong, H. Y. P. & Kafalas, J. A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976).
Hong, H. Y. P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 11, 173–182 (1976).
Aono, H., Imanaka, N. & Adachi, G.-y High Li+ conducting ceramics. Acc. Chem. Res. 27, 265–270 (1994).
Boukamp, B. A. & Huggins, R. A. Ionic conductivity in lithium imide. Phys. Lett. A 72, 464–466 (1979).
Matsuo, M. et al. Sodium and magnesium ionic conduction in complex hydrides. J. Alloys Compd. 580, S98–S101 (2013).
Bates, J. B. et al. Electrical properties of amorphous lithium electrolyte thin films. Solid State Ion. 53–56, 647–654 (1992).
Jansen, M. & Henseler, U. Synthesis, structure determination, and ionic conductivity of sodium tetrathiophosphate. J. Solid State Chem. 99, 110–119 (1992).
Kanno, R. & Murayama, M. Lithium ionic conductor thio-LISICON: the Li2S-GeS2-P2S5 system. J. Electrochem. Soc. 148, A742–A746 (2001).
Zhang, Z. et al. Na11Sn2PS12: a new solid state sodium superionic conductor. Energy Environ. Sci. 11, 87–93 (2018). Report of a Na SSE with an unprecedented 3D structure type that exhibits an ionic conductivity of 1.4 mS cm −1.
Schwering, G., Honnerscheid, A., van Wullen, L. & Jansen, M. High lithium ionic conductivity in the lithium halide hydrates Li3−n(OHn)Cl (0.83 ≤ n ≤ 2) and Li3−n(OH)nBr (1 ≤ n ≤ 2) at ambient temperatures. ChemPhysChem 4, 343–348 (2003).
Zhao, Y. & Daemen, L. L. Superionic conductivity in lithium-rich anti-perovskites. J. Am. Chem. Soc. 134, 15042–15047 (2012).
Thangadurai, V., Kaack, H. & Weppner, W. J. F. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 86, 437–440 (2003).
Hooper, A. & North, J. M. The fabrication and performance of all solid state polymer-based rechargeable lithium cells. Solid State Ion. 9–10, 1161–1166 (1983).
Watanabe, M. et al. High lithium ionic conductivity of polymeric solid electrolytes. Makromol. Chem. Rapid Commun. 2, 741–744 (1981).
Alarco, P. J., Abu-Lebdeh, Y., Abouimrane, A. & Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 3, 476–481 (2004).
Fan, L.-Z. & Maier, J. Composite effects in poly(ethylene oxide)–succinonitrile based all-solid electrolytes. Electrochem. Commun. 8, 1753–1756 (2006).
Meziane, R., Bonnet, J.-P., Courty, M., Djellab, K. & Armand, M. Single-ion polymer electrolytes based on a delocalized polyanion for lithium batteries. Electrochim. Acta 57, 14–19 (2011).
Maccallum, J., Smith, M. & Vincent, C. The effects of radiation-induced crosslinking on the conductance of LiClO4·PEO electrolytes. Solid State Ion. 11, 307–312 (1984).
Xia, D., Soltz, D. & Smid, J. Conductivities of solid polymer electrolyte complexes of alkali salts with polymers of methoxypolyethyleneglycol methacrylates. Solid State Ion. 14, 221–224 (1984).
Giles, J. R. M., Gray, F. M., MacCallum, J. R. & Vincent, C. A. Synthesis and characterization of ABA block copolymer-based polymer electrolytes. Polymer 28, 1977–1981 (1987).
Yamaguchi, G. & Suzuki, K. On the structures of alkali polyaluminates. Bull. Chem. Soc. Jpn. 41, 93–99 (1968).
Francisco, B. E., Stoldt, C. R. & M’Peko, J.-C. Lithium-ion trapping from local structural distortions in sodium super ionic conductor (NASICON) electrolytes. Chem. Mater. 26, 4741–4749 (2014).
Stramare, S., Thangadurai, V. & Weppner, W. Lithium lanthanum titanates: a review. Chem. Mater. 15, 3974–3990 (2003).
Deng, Z., Radhakrishnan, B. & Ong, S. P. Rational composition optimization of the lithium-rich Li3OCl1−xBrx anti-perovskite superionic conductors. Chem. Mater. 27, 3749–3755 (2015).
Jalem, R. et al. Concerted migration mechanism in the Li ion dynamics of garnet-type Li7La3Zr2O12. Chem. Mater. 25, 425–430 (2013).
Cheung, I. W. et al. Electrochemical and solid state NMR characterization of composite PEO-based polymer electrolytes. Electrochim. Acta 48, 2149–2156 (2003).
Shin, J. H. & Passerini, S. Effect of fillers on the electrochemical and interfacial properties of PEO–LiN(SO2CF2CF3)2 polymer electrolytes. Electrochim. Acta 49, 1605–1612 (2004).
Croce, F., Settimi, L., Scrosati, B. & Zane, D. Nanocomposite, PEO-LiBOB polymer electrolytes for low temperature, lithium rechargeable batteries. J. New Mater. Electrochem. Syst. 9, 3–9 (2006).
Wong, D. H. et al. Nonflammable perfluoropolyether-based electrolytes for lithium batteries. Proc. Natl Acad. Sci. USA 111, 3327–3331 (2014).
Mindemark, J., Lacey, M. J., Bowden, T. & Brandell, D. Beyond PEO—alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 81, 114–143 (2018).
Zoppi, R. A., Fonseca, C. M. N. P., DePaoli, M. A. & Nunes, S. P. Solid electrolytes based on poly(amide 6-b-ethylene oxide). Solid State Ion. 91, 123–130 (1996).
Schaefer, J. L., Yanga, D. A. & Archer, L. A. High lithium transference number electrolytes via creation of 3-dimensional, charged, nanoporous networks from dense functionalized nanoparticle composites. Chem. Mater. 25, 834–839 (2013).
Zhang, Y. et al. Toward ambient temperature operation with all-solid-state lithium metal batteries with a sp 3 boron-based solid single ion conducting polymer electrolyte. J. Power Sources 306, 152–161 (2016).
Choudhury, S., Mangal, R., Agrawal, A. & Archer, L. A. A highly reversible room-temperature lithium metal battery based on cross-linked hairy nanoparticles. Nat. Commun. 6, 10101 (2015).
Ben Youcef, H., Garcia-Calvo, O., Lago, N., Devaraj, S. & Armand, M. Cross-linked solid polymer electrolyte for all-solid-state rechargeable lithium batteries. Electrochim. Acta 220, 587–594 (2016).
Tärneberg, R. & Lunde˙n, A. Ion diffusion in the high-temperature phases Li2SO4, LiNaSO4, LiAgSO4 and Li4Zn(SO4)3. Solid State Ion. 90, 209–220 (1996).
Kummer, J. T. β-alumina electrolytes. Prog. Solid State Chem. 7, 141–175 (1972).
Liu, Z. et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 135, 975–978 (2013).
Kimura, T. et al. Preparation and characterization of lithium ion conductive Li3SbS4 glass and glass-ceramic electrolytes. Solid State Ion. 333, 45–49 (2019).
Yu, C. et al. Accessing the bottleneck in all-solid state batteries, lithium-ion transport over the solid-electrolyte-electrode interface. Nat. Commun. 8, 1086 (2017).
Hayashi, A., Noi, K., Tanibata, N., Nagao, M. & Tatsumisago, M. High sodium ion conductivity of glass–ceramic electrolytes with cubic Na3PS4. J. Power Sources 258, 420–423 (2014).
Wang, H. et al. An air-stable Na3SbS4 superionic conductor prepared by a rapid and economic synthetic procedure. Angew. Chem. Int. Ed. 55, 8551–8555 (2016).
Yu, Z. X. et al. Exceptionally high ionic conductivity in Na3P0.62As0.38S4 with improved moisture stability for solid-state sodium-ion batteries. Adv. Mater. 29, 1605561 (2017).
Chu, I. H. et al. Room-temperature all-solid-state rechargeable sodium-ion batteries with a Cl-doped Na3PS4 superionic conductor. Sci. Rep. 6, 33733 (2016).
Sudreau, F., Petit, D. & Boilot, J. P. Dimorphism, phase transitions, and transport properties in LiZr2(PO4)3. J. Solid State Chem. 83, 78–90 (1989).
Le Ruyet, R. et al. Investigation of Mg(BH4)(NH2)-based composite materials with enhanced Mg2+ ionic conductivity. J. Phys. Chem. C 123, 10756–10763 (2019).
Zhang, H. et al. Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte. Electrochim. Acta 133, 529–538 (2014).
Vasudevan, S. & Fullerton-Shirey, S. K. Effect of nanoparticle shape on the electrical and thermal properties of solid polymer electrolytes. J. Phys. Chem. C 123, 10720–10726 (2019).
Boschin, A. & Johansson, P. Characterization of NaX (X: TFSI, FSI) – PEO based solid polymer electrolytes for sodium batteries. Electrochim. Acta 175, 124–133 (2015).
Ni’mah, Y. L., Cheng, M.-Y., Cheng, J. H., Rick, J. & Hwang, B.-J. Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J. Power Sources 278, 375–381 (2015).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976).
Duan, H. et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries. Adv. Mater. 31, e1807789 (2019).
Li, X. et al. Constructing double buffer layers to boost electrochemical performances of NCA cathode for ASSLB. Energy Storage Mater. 18, 100–106 (2019).
Zheng, J. et al. Li- and Mn-rich cathode materials: challenges to commercialization. Adv. Energy Mater. 7, 1601284 (2017).
Xu, R. et al. Cathode-supported all-solid-state lithium–sulfur batteries with high cell-level energy density. ACS Energy Lett. 4, 1073–1079 (2019).
Fan, X. et al. High-performance all-solid-state Na–S battery enabled by casting–annealing technology. ACS Nano 12, 3360–3368 (2018).
Lu, X., Bowden, M. E., Sprenkle, V. L. & Liu, J. A low cost, high energy density, and long cycle life potassium-sulfur battery for grid-scale energy storage. Adv. Mater. 27, 5915–5922 (2015).
Zhu, Z. et al. All-solid-state lithium organic battery with composite polymer electrolyte and pillar[5]quinone cathode. J. Am. Chem. Soc. 136, 16461–16464 (2014).
Hong, X. et al. Nonlithium metal–sulfur batteries: steps toward a leap. Adv. Mater. 31, e1802822 (2019).
Zhao, Q., Zhu, Z. & Chen, J. Molecular engineering with organic carbonyl electrode materials for advanced stationary and redox flow rechargeable batteries. Adv. Mater. 25, 1607007 (2017).
Liu, Y. et al. Germanium thin film protected lithium aluminum germanium phosphate for solid-state Li batteries. Adv. Energy Mater. 8, 1702374 (2018).
Kang, Y. et al. Novel high-energy-density rechargeable hybrid sodium–air cell with acidic electrolyte. ACS Appl. Mater. Interfaces 10, 23748–23756 (2018).
Adelhelm, P. et al. From lithium to sodium: cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 6, 1016–1055 (2015).
Xu, Y., Zhao, Y., Ren, J., Zhang, Y. & Peng, H. An all-solid-state fiber-shaped aluminum–air battery with flexibility, stretchability, and high electrochemical performance. Angew. Chem. Int. Ed. 55, 7979–7982 (2016).
Fu, J. et al. Electrically rechargeable zinc–air batteries: progress, challenges, and perspectives. Adv. Mater. 29, 1604685 (2017).
Mokhtar, M. et al. Recent developments in materials for aluminum–air batteries: a review. J. Ind. Eng. Chem. 32, 1–20 (2015).
Hu, X., Li, Z. & Chen, J. Flexible Li–CO2 batteries with liquid-free electrolyte. Angew. Chem. Int. Ed. 56, 5785–5789 (2017).
Hu, X. et al. Quasi-solid state rechargeable Na–CO2 batteries with reduced graphene oxide Na anodes. Sci. Adv. 3, e1602396 (2017).
Acknowledgements
The authors acknowledge support from the US Department of Energy Basic Energy Sciences program (award no. DE-SC0016082), the US National Science Foundation Division of Materials Research (award no. DMR-1609125) and the Beijing Institute of Collaborative Innovation through the Cornell Joint Energy Materials and Systems Laboratory.
Author information
Authors and Affiliations
Contributions
Q.Z., S.S., C.-Z.Z. and L.A.A. researched data for the article. All authors contributed to the discussion of content, and writing and editing of the article prior to submission.
Corresponding author
Ethics declarations
Competing interests
L.A.A. is a founder and member of the board of directors of NOHMs Technologies, which develops and commercializes electrolytes for lithium-ion and lithium–sulfur battery technologies. Q.Z., S.S. and C.-Z.Z. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Bolloré Bluecar: https://www.bluecar.fr/sites/bluecar/files/medias/PDF/2_bluecar_20_p.pdf
Excellatron Thin Film Battery Technology: http://www.excellatron.com/thin-film-battery-technology/
Ionic Materials: https://ionicmaterials.com/the-solution/
NGK Insulators Ltd: https://www.ngk.co.jp/nas/why/comparison.html
Rights and permissions
About this article
Cite this article
Zhao, Q., Stalin, S., Zhao, CZ. et al. Designing solid-state electrolytes for safe, energy-dense batteries. Nat Rev Mater 5, 229–252 (2020). https://doi.org/10.1038/s41578-019-0165-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-019-0165-5
This article is cited by
-
Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries
Nature Communications (2024)
-
Recycling of solid-state batteries
Nature Energy (2024)
-
Nanoscale doping of polymeric semiconductors with confined electrochemical ion implantation
Nature Nanotechnology (2024)
-
Self-sufficient metal–air batteries for autonomous systems
Nature Chemical Engineering (2024)
-
Robotic synthesis decoded through phase diagram mastery
Nature Synthesis (2024)