Abstract
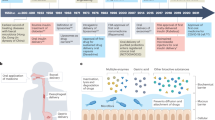
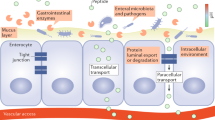
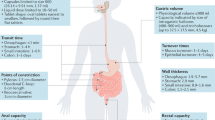
Throughout history, oral administration has been regarded as the most convenient mode of drug delivery, as it requires minimal expertise and invasiveness. Although oral delivery works well for small-molecule drugs, oral delivery of macromolecules (particularly proteins and peptides) has been limited by acidic conditions in the stomach and low permeability across the intestinal epithelium. Accordingly, the large numbers of biologic drugs that have become available in the past 10 years typically require administration by injection or infusion. As such, a renewed emphasis has been placed on the development of novel materials that overcome the physiological challenges of oral delivery for macromolecular agents. This Review provides an overview of physiological barriers to the oral delivery of biologics and highlights the advances made in materials across various length scales, from small molecules to macroscopic devices. This Review also describes the current status of materials for oral delivery of protein and peptide drugs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
08 January 2020
The originally published article contained a typographical error in the final text box of Figure 1, which has been corrected in the HTML and PDF versions of the manuscript.
References
US Food and Drug Administration. Novel drug approvals for 2018. FDA https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2018 (2018).
Toutain, P. L. & Bousquet-Mélou, A. Bioavailability and its assessment. J. Vet. Pharmacol. Ther. 27, 455–466 (2004).
Bardal, S. K., Waechter, J. E. & Martin, D. S. Applied Pharmacology 17–34 (Elsevier Saunders, 2011).
Shone, A., Burnside, J., Chipchase, S., Game, F. & Jeffcoate, W. Probing the validity of the probe-to-bone test in the diagnosis of osteomyelitis of the foot in diabetes. Diabetes Care 29, 945 (2006).
Thomaidou, E. & Ramot, Y. Injection site reactions with the use of biological agents. Dermatol. Ther. 32, e12817 (2019).
Hilhorst, N., Spanoudi-Kitrimi, I., Goemans, N. & Morren, M. A. Injection site reactions after long-term subcutaneous delivery of drisapersen: a retrospective study. Eur. J. Pediatr. 178, 253–258 (2018).
Messer, L. H., Berget, C., Beatson, C., Polsky, S. & Forlenza, G. P. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol. Ther. 20, S254–S264 (2018).
Richardson, T. & Kerr, D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am. J. Clin. Dermatol. 4, 661–667 (2003).
Kerbleski, J. F. & Gottlieb, A. B. Dermatological complications and safety of anti-TNF treatments. Gut 58, 1033–1039 (2009).
Liu, N. F. et al. Stigma in people with type 1 or type 2 diabetes. Clin. Diabetes 35, 27–34 (2017).
Spain, C. V., Wright, J. J., Hahn, R. M., Wivel, A. & Martin, A. A. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin. Ther. 38, 1653–1664.e1 (2016).
Crawford, A., Jewell, S., Mara, H., McCatty, L. & Pelfrey, R. Managing treatment fatigue in patients with multiple sclerosis on long-term therapy: the role of multiple sclerosis nurses. Patient Prefer. Adherence 8, 1093–1099 (2014).
Zhong, W. et al. Age and sex patterns of drug prescribing in a defined American population. Mayo Clin. Proc. 88, 697–707 (2013).
Zelikin, A. N., Ehrhardt, C. & Healy, A. M. Materials and methods for delivery of biological drugs. Nat. Chem. 8, 997–1007 (2016).
Antosova, Z., Mackova, M., Kral, V. & Macek, T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 27, 628–635 (2009).
Roger, E., Lagarce, F., Garcion, E. & Benoit, J. P. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine 5, 287–306 (2010).
Zhou, X. H. & Po, A. L. W. Peptide and protein drugs: II. Non-parenteral routes of delivery. Int. J. Pharm. 75, 117–130 (1991).
Fjellestad-Paulsen, A., Hoglund, P., Lundin, S. & Paulsen, O. Pharmacokinetics of 1-deamino-8-D-arginine vasopressin after various routes of administration in healthy volunteers. Clin. Endocrinol. 38, 177–182 (1993). Reports the oral bioavailability (0.1%) of one of the first oral formulations of the synthetic hormone desmopressin.
Fábián, T. K., Hermann, P., Beck, A., Fejérdy, P. & Fábián, G. Salivary defense proteins: their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 13, 4295–4320 (2012).
Allen, A. & Carroll, N. J. Adherent and soluble mucus in the stomach and duodenum. Dig. Dis. Sci. 30, 55S–62S (1985).
Allen, A., Flemstrom, G., Garner, A. & Kivilaakso, E. Gastroduodenal mucosal protection. Physiol. Rev. 73, 823–857 (1993).
Whitcomb, D. C. & Lowe, M. E. Human pancreatic digestive enzymes. Dig. Dis. Sci. 52, 1–17 (2007).
Masaoka, Y., Tanaka, Y., Kataoka, M., Sakuma, S. & Yamashita, S. Site of drug absorption after oral administration: assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur. J. Pharm. Sci. 29, 240–250 (2006).
Kararli, T. T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16, 351–380 (1995).
Daugherty, A. L. & Mrsny, R. J. Transcellular uptake mechanisms of the intestinal epithelial barrier Part one. Pharm. Sci. Technol. Today 2, 144–151 (1999).
Golub, A. L., Frost, R. W., Betlach, C. J. & Gonzalez, M. A. Physiologic considerations in drug absorption from the gastrointestinal tract. J. Allergy Clin. Immunol. 78, 689–694 (1986).
Perez-Vilar, J. & Hill, R. L. The structure and assembly of secreted mucins. J. Biol. Chem. 274, 31751–31754 (1999).
Murgia, X., Loretz, B., Hartwig, O., Hittinger, M. & Lehr, C. M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 124, 82–97 (2018).
Ensign, L. M., Cone, R. & Hanes, J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 64, 557–570 (2012). Outlines key features of the mucus barriers that can impede oral delivery.
Lai, S. K., Wang, Y. Y. & Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61, 158–171 (2009).
Yildiz, H. M., McKelvey, C. A., Marsac, P. J. & Carrier, R. L. Size selectivity of intestinal mucus to diffusing particulates is dependent on surface chemistry and exposure to lipids. J. Drug Target. 23, 768–774 (2015).
Maisel, K., Ensign, L., Reddy, M., Cone, R. & Hanes, J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J. Control. Release 197, 48–57 (2015).
Carlson, T. L., Lock, J. Y. & Carrier, R. L. Engineering the mucus barrier. Annu. Rev. Biomed. Eng. 20, 197–220 (2018).
Ducheˇne, D., Touchard, F. & Peppas, N. A. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug Dev. Ind. Pharm. 14, 283–318 (1988).
Ch’Ng, H. S., Park, H., Kelly, P. & Robinson, J. R. Bioadhesive polymers as platforms for oral controlled drug delivery II: synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers. J. Pharm. Sci. 74, 399–405 (1985).
Pullan, R. D. et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35, 353–359 (1994).
Elderman, M. et al. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLOS One 12, e0184274 (2017).
Bahari, H. M., Ross, I. N. & Turnberg, L. A. Demonstration of a pH gradient across the mucus layer on the surface of human gastric mucosa in vitro. Gut 23, 513–516 (1982).
Mayhew, T. M., Myklebust, R., Whybrow, A. & Jenkins, R. Epithelial integrity, cell death and cell loss in mammalian small intestine. Histol. Histopathol. 14, 257–267 (1999).
Middleton, C. Crypts, villi and microvilli in the small intestine of the rat. A stereological study of their variability within and between animals. J. Anat. 141, 1–17 (1985).
Salim, S. Y. & Söderholm, J. D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 362–381 (2011).
Allaire, J. M. et al. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 39, 677–696 (2018). Provides a thorough overview of the intestinal epithelium and surrounding environment.
Mace, O. J., Tehan, B. & Marshall, F. Pharmacology and physiology of gastrointestinal enteroendocrine cells. Pharmacol. Res. Perspect. 3, e00155 (2015).
Denker, B. M. & Nigam, S. K. Molecular structure and assembly of the tight junction. Am. J. Physiol. 274, F1–F9 (1998).
Fine, K. D., Santa Ana, C. A., Porter, J. L. & Fordtran, J. S. Effect of changing intestinal flow rate on a measurement of intestinal permeability. Gastroenterology 108, 983–989 (1995).
Linnankoski, J. et al. Paracellular porosity and pore size of the human intestinal epithelium in tissue and cell culture models. J. Pharm. Sci. 99, 2166–2175 (2010).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Amin, M. L. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 7, 27–34 (2013).
Sjöstedt, N., Holvikari, K., Tammela, P. & Kidron, H. Inhibition of breast cancer resistance protein and multidrug resistance associated protein 2 by natural compounds and their derivatives. Mol. Pharm. 14, 135–146 (2017).
Lea, T. in The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models (eds Verhoeckx, K. et al.) 95–102 (Springer International, 2015).
Karasov, W. H. Integrative physiology of transcellular and paracellular intestinal absorption. J. Exp. Biol. 220, 2495–2501(2017).
Pereira de Sousa, I. & Bernkop-Schnürch, A. Pre-systemic metabolism of orally administered drugs and strategies to overcome it. J. Control. Release 192, 301–309 (2014).
Goldberg, M. & Gomez-Orellana, I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov. 2, 289–295 (2003).
Bittner, B. et al. Erratum: development of a subcutaneous formulation for trastuzumab — nonclinical and clinical bridging approach to the approved intravenous dosing regimen. Drug Res 63, 602 (2013).
Pivot, X. et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 14, 962–970 (2013).
Hourcade-Potelleret, F. et al. Use of a population pharmacokinetic approach for the clinical development of a fixed-dose subcutaneous formulation of trastuzumab. CPT Pharmacometrics Syst. Pharmacol. 3, e87 (2014).
Shah, R. B., Patel, M., Maahs, D. M. & Shah, V. N. Insulin delivery methods: Past, present and future. Int. J. Pharm. Investig. 6, 1–9 (2016). Outlines key ways in which insulin has been attempted to be delivered throughout history.
US Food and Drug Administration. FDA approves first oral GLP-1 treatment for type 2 diabetes. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-glp-1-treatment-type-2-diabetes (2019). This is the first FDA-approved oral biologic for type 2 diabetes mellitus.
Winstanley, P. A. & Orme, M. L. The effects of food on drug bioavailability. Br. J. Clin. Pharmacol. 28, 621–628 (1989).
Melander, A. Influence of food on the bioavailability of drugs. Clin. Pharmacokinet. 3, 337–351 (1978).
Karsdal, M. A. et al. Influence of food intake on the bioavailability and efficacy of oral calcitonin. Br. J. Clin. Pharmacol. 67, 413–420 (2009).
Yasuji, T., Kondo, H. & Sako, K. The effect of food on the oral bioavailability of drugs: a review of current developments and pharmaceutical technologies for pharmacokinetic control. Ther. Deliv. 3, 81–90 (2012).
Hoppu, K. Prehepatic metabolism of drugs — a mechanism for low and variable oral bioavailability. Pediatr. Nephrol. 13, 85–89 (1999).
Agrawal, S. & Panchagnula, R. Implication of biopharmaceutics and pharmacokinetics of rifampicin in variable bioavailability from solid oral dosage form. Biopharm. Drug Dispos. 26, 321–334 (2005).
El-Kattan, A. & Varma, M. in Topics on Drug Metabolism Ch. 1 (ed Paxton, J.) (IntechOpen, 2012).
Lamson, N. L., Berger, A., Fein, K. C., & Whitehead, K. A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-019-0465-5 (2019).
Bernkop-Schnürch, A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J. Control. Release 52, 1–16 (1998).
Bernkop-Schnürch, A. & Marschütz, M. K. Development and in vitro evaluation of systems to protect peptide drugs from aminopeptidase N. Pharm. Res. 14, 181–185 (1997).
Hastewell, J., Antonin, K. H., Fox, R. & Mackay, M. The colonic absorption of human calcitonin: the effects of increasing local concentration and co-administration with a protease inhibitor. Int. J. Pharm. 126, 245–251 (1995).
Yamamoto, A. et al. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm. Res. 11, 1496–1500 (1994).
Otsuki, M., Ohki, A., Okabayashi, Y., Suehiro, I. & Baba, S. Effect of synthetic protease inhibitor camostate on pancreatic exocrine function in rats. Pancreas 2, 164–169 (1987).
Melmed, R. N., El-Aaser, A. A. A. & Holt, S. J. Hypertrophy and hyperplasia of the neonatal rat exocrine pancreas induced by orally administered soybean trypsin inhibitor. Biochim. Biophys. Acta 421, 280–288 (1976).
Kunin, C. M. Nephrotoxicity of antibiotics. JAMA 202, 204–208 (1967).
Friess, H., Kleeff, J., Isenmann, R., Malfertheiner, P. & Büchler, M. W. Adaptation of the human pancreas to inhibition of luminal proteolytic activity. Gastroenterology 115, 388–396 (1998).
Binkley, N. et al. A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: the Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial. J. Bone Miner. Res. 27, 1821–1829 (2012).
Tomita, M., Hayashi, M. & Awazu, S. Absorption-enhancing mechanism of EDTA, caprate, and decanoylcarnitine in Caco-2 cells. J. Pharm. Sci. 85, 608–611 (1996).
Welling, S. H. et al. The role of citric acid in oral peptide and protein formulations: relationship between calcium chelation and proteolysis inhibition. Eur. J. Pharm. Biopharm. 86, 544–551 (2014).
Bolourchian, N. & Dadashzadeh, S. pH-independent release of propranolol hydrochloride from HPMC-based matrices using organic acids. Daru 16, 136–142 (2008).
Dvorˇácˇková, K. et al. The effect of acid pH modifiers on the release characteristics of weakly basic drug from hydrophlilic–lipophilic matrices. AAPS PharmSciTech 14, 1341–1348 (2013).
Noach, A. B. J., Kurosaki, Y., Blom-Roosemalen, M. C. M., de Boer, A. G. & Breimer, D. D. Cell-polarity dependent effect of chelation on the paracellular permeability of confluent Caco-2 cell monolayers. Int. J. Pharm. 90, 229–237 (1993).
Shen, L., Zhao, H. Y., Du, J. & Wang, F. Anti-tumor activities of four chelating agents against human neuroblastoma cells. In Vivo 19, 233–236 (2005).
Collares-Buzato, C. B., McEwan, G. T. A., Jepson, M. A., Simmons, N. L. & Hirst, B. H. Paracellular barrier and junctional protein distribution depend on basolateral extracellular Ca2+ in cultured epithelia. Biochim. Biophys. Acta 1222, 147–158 (1994).
Lueßen, H. L. et al. Mucoadhesive polymers in peroral peptide drug delivery. I. Influence of mucoadhesive excipients on the proteolytic activity of intestinal enzymes. Eur. J. Pharm. Sci. 4, 117–128 (1996).
Lindahl, A., Ungell, A.-L., Knutson, L. & Lennernäs, H. Characterization of fluids from the stomach and proximal jejunum in men and women. Pharm. Res. 14, 497–502 (1997).
Bernkop-Schnürch, A. & Krajicek, M. E. Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: synthesis and evaluation of different chitosan-EDTA conjugates. J. Control. Release 50, 215–223 (1998).
Lannigan, R. S. & Yamarik, T. A. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA and trisodium HEDTA. Int. J. Toxicol. 21, 95–142 (2002).
Ilbäck, N. G., Stålhandske, T. & Lindh, U. Effects of EDTA on trace elements and cardiovascular function in the anesthetised rabbit. Biol. Trace Elem. Res. 76, 133–148 (2000).
Lee, H. J., McAuley, A., Schilke, K. F. & McGuire, J. Molecular origins of surfactant-mediated stabilization of protein drugs. Adv. Drug Deliv. Rev. 63, 1160–1171 (2011).
Jones, L. S., Bam, N. B. & Randolph, T. W. in Therapeutic Protein and Peptide Formulation and Delivery Ch. 12 (eds Shahrokh, Z., Sluzky, V., Cleland, J. L., Shire, S. J. & Randolph, T. W.) 206–222 (American Chemical Society, 1997).
Shao, Z., Li, Y., Krishnamoorthy, R., Chermak, T. & Mitra, A. K. Differential effects of anionic, cationic, nonionic, and physiologic surfactants on the dissociation, α-chymotryptic degradation, and enteral absorption of insulin hexamers. Pharm. Res. 10, 243–251 (1993).
Gupta, S., Kesarla, R. & Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 848043 (2013).
Dahlgren, D. et al. Effect of absorption-modifying excipients, hypotonicity, and enteric neural activity in an in vivo model for small intestinal transport. Int. J. Pharm. 549, 239–248 (2018).
Elsayed, A. et al. Chitosan–sodium lauryl sulfate nanoparticles as a carrier system for the in vivo delivery of oral insulin. AAPS PharmSciTech 12, 958–964 (2011).
Lo, Y. L. Relationships between the hydrophilic–lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J. Control. Release 90, 37–48 (2003).
Sugibayashi, K., Onuki, Y. & Takayama, K. Displacement of tight junction proteins from detergent-resistant membrane domains by treatment with sodium caprate. Eur. J. Pharm. Sci. 36, 246–253 (2009).
Kurasawa, M. et al. Regulation of tight junction permeability by sodium caprate in human keratinocytes and reconstructed epidermis. Biochem. Biophys. Res. Commun. 381, 171–175 (2009).
Maher, S., Leonard, T. W., Jacobsen, J. & Brayden, D. J. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv. Drug Deliv. Rev. 61, 1427–1449 (2009).
Heade, J., Maher, S., Bleiel, S. B. & Brayden, D. J. Labrasol and salts of medium-chain fatty acids can be combined in low concentrations to increase the permeability of a macromolecule marker across isolated rat intestinal mucosae. J. Pharm. Sci. 107, 1648–1655 (2018).
Keown, A. Merrion Pharma looks to wind up operations, announces liquidation plans. BioSpace https://www.biospace.com/article/merrion-pharma-looks-to-wind-up-operations-announces-liquidation-plans-/ (2016).
Leonard, T. W., Lynch, J., McKenna, M. J. & Brayden, D. J. Promoting absorption of drugs in humans using medium-chain fatty acid-based solid dosage forms: GIPET. Expert Opin. Drug Deliv. 3, 685–692 (2006).
Tucker, M. E. Oral basal insulin shows promise in type 2 diabetes. Medscape Medical News https://www.medscape.com/viewarticle/882211 (2017).
Halberg, I. B. et al. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabetes Endocrinol. 7, 179–188 (2019).
Muranushi, N., Mack, E. & Kim, S. W. The effects of fatty acids and their derivatives on the intestinal absorption of insulin in rat. Drug Dev. Ind. Pharm. 19, 929–941 (1993).
Chiasma. Chiasma Provides Update On Ongoing Mycapssa Phase 3 Clinical Trials. Chiasma http://ir.chiasmapharma.com/news-releases/news-release-details/chiasma-provides-update-ongoing-mycapssar-phase-3-clinical (2019).
Sharma, P., Varma, M. V. S., Chawla, H. P. S. & Panchagnula, R. Absorption enhancement, mechanistic and toxicity studies of medium chain fatty acids, cyclodextrins and bile salts as peroral absorption enhancers. Farmaco 60, 884–893 (2005).
Malkov, D. et al. Oral delivery of insulin with the Eligen technology: mechanistic studies. Curr. Drug Deliv. 2, 191–197 (2005).
Castelli, M. C. et al. Comparing the efficacy and tolerability of a new daily oral vitamin B12 formulation and intermittent intramuscular vitamin B12 in normalizing low cobalamin levels: a randomized, open-label, parallel-group study. Clin. Ther. 33, 358–371.e2 (2011).
Emisphere. Improved oral delivery with Eligen. Emisphere http://www.emisphere.com/improved-oral-delivery-eligen/ (2019).
Novo Nordisk. Company announcement: Novo Nordisk files for EU regulatory approval of oral semaglutide for the treatment of type 2 diabetes. Novo Nordisk http://hugin.info/2013/R/2242550/885282.pdf (2019).
Novo Nordisk. Novo Nordisk files for US FDA approval of oral semaglutide for blood sugar control and cardiovascular risk reduction in adults with type 2 diabetes. Novo Nordisk https://www.novonordisk-us.com/media/news-releases.html?122958 (2019).
Buckley, S. T. et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 10, eaar7047 (2018). A report indicating that oral delivery of semaglutide using SNAC takes place in the stomach and is confined to tablet vicinity.
Twarog, C. et al. Intestinal permeation enhancers for oral delivery of macromolecules: a comparison between salcaprozate sodium (SNAC) and sodium caprate (C10). Pharmaceutics 11, E78 (2019). In-depth analysis of SNAC and C10 as permeation enhancers for oral delivery.
Gonze, M. D. et al. Orally administered heparin for preventing deep venous thrombosis. Am. J. Surg. 176, 176–178 (1998).
Pratley, R. et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 394, 39–50 (2019).
Tarasenko, T. N., Cusmano-Ozog, K. & McGuire, P. J. Tissue acylcarnitine status in a mouse model of mitochondrial β-oxidation deficiency during metabolic decompensation due to influenza virus infection. Mol. Genet. Metab. 125, 144–152 (2018).
Gilligan, J. P., Maurer, G. R., Railkar, A. M., Daggs, T. A. & Shields, P. P. Room temperature stable oral calcitonin formulation. Patent application WO2018026993A1 (2018).
Doi, N., Tomita, M. & Hayashi, M. Absorption enhancement effect of acylcarnitines through changes in tight junction protein in Caco-2 cell monolayers. Drug Metab. Pharmacokinet. 26, 162–170 (2010).
Whitehead, K. & Mitragotri, S. Mechanistic analysis of chemical permeation enhancers for oral drug delivery. Pharm. Res. 25, 1412–1419 (2008).
Gupta, V., Hwang, B. H., Doshi, N. & Mitragotri, S. A permeation enhancer for increasing transport of therapeutic macromolecules across the intestine. J. Control. Release 172, 541–549 (2013).
Sakai, M., Imai, T., Ohtake, H., Azuma, H. & Otagiri, M. Simultaneous use of sodium deoxycholate and dipotassium glycyrrhizinate enhances the cellular transport of poorly absorbed compounds across Caco-2 cell monolayers. J. Pharm. Pharmacol. 51, 27–33 (2003).
Qiao, J. et al. Oral bioavailability and lymphatic transport of pueraria flavone-loaded self-emulsifying drug-delivery systems containing sodium taurocholate in rats. Pharmaceutics 10, 147 (2018).
Song, K. H., Chung, S. J. & Shim, C. K. Enhanced intestinal absorption of salmon calcitonin (sCT) from proliposomes containing bile salts. J. Control. Release 106, 298–308 (2005).
Lundin, S., Pantzar, N., Hedin, L. & Weström, B. R. Intestinal absorption enhancement by sodium taurodihydrofusidate of a peptide hormone analogue (dDAVP) and a macromolecule (BSA) in vitro and in vivo. Int. J. Pharm. 59, 263–269 (1990).
Moghimipour, E., Ameri, A. & Handali, S. Absorption-enhancing effects of bile salts. Molecules 20, 14451–14473 (2015).
Moghimipour, E., Jalali, A., Sajjadi Tabassi, S. A. & Löbenberg, R. The enhancing effect of sodium glycocholate and sodium salicylate on rats gastro-intestinal permeability to insulin. Iran. J. Pharm. Res. 3, 87–91 (2004).
Gordon, G. S., Moses, A. C., Silver, R. D., Flier, J. S. & Carey, M. C. Nasal absorption of insulin: enhancement by hydrophobic bile salts. Proc. Natl Acad. Sci. USA 82, 7419–7423 (2006).
Bowe, C. L. et al. Design of compounds that increase the absorption of polar molecules. Proc. Natl Acad. Sci. USA 94, 12218–12223 (2002).
Greenwood, J., Adu, J., Davey, A. J., Abbott, N. J. & Bradbury, M. W. B. The effect of bile salts on the permeability and ultrastructure of the perfused, energy-depleted, rat blood–brain barrier. J. Cereb. Blood Flow Metab. 11, 644–654 (1991).
New, R. R. C. Dissolution aids for oral peptide delivery comprising a biguanide. Patent application WO2007093806A1 (2007).
Diabetology, Technology. Axcess oral delivery system. Diabetology http://www.diabetology.co.uk/technology/ (2019).
Diabetology, Projects. Capsulin, Combulin, Oral GLP-1. Broad product pipeline. Diabetology http://www.diabetology.co.uk/projects/ (2019).
Proxima Concepts. Group development & licensee companies. Proxima Concepts http://www.oralcalcitonin.com/group.htm (2019).
Williams, G. M., Iatropoulos, M. J. & Whysner, J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem. Toxicol. 37, 1027–1038 (1999).
Whitehead, K., Karr, N. & Mitragotri, S. Safe and effective permeation enhancers for oral drug delivery. Pharm. Res. 25, 1782–1788 (2008).
Bzik, V. A. & Brayden, D. J. An assessment of the permeation enhancer, 1-phenyl-piperazine (PPZ), on paracellular flux across rat intestinal mucosae in Ussing chambers. Pharm. Res. 33, 2506–2516 (2016).
Lamson, N. G., Cusimano, G., Suri, K., Zhang, A. & Whitehead, K. A. The pH of piperazine derivative solutions predicts their utility as transepithelial permeation enhancers. Mol. Pharm. 13, 578–585 (2016).
Fein, K. C., Lamson, N. G. & Whitehead, K. A. Structure–function analysis of phenylpiperazine derivatives as intestinal permeation enhancers. Pharm. Res. 34, 1320–1329 (2017).
Dickson, A. J., Vorce, S. P., Holler, J. M. & Lyons, T. P. Detection of 1-benzylpiperazine, 1-(3-trifluoromethylphenyl)-piperazine, and 1-(3-chlorophenyl)-piperazine in 3,4-methylenedioxymethamphetamine-positive urine samples. J. Anal. Toxicol. 34, 464–469 (2010).
Cummings, C. S. et al. ATRP-grown protein–polymer conjugates containing phenylpiperazine selectively enhance transepithelial protein transport. J. Control. Release 255, 270–278 (2017).
Egorova, K. S. & Ananikov, V. P. Toxicity of ionic liquids: eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 7, 336–360 (2014).
Egorova, K. S., Gordeev, E. G. & Ananikov, V. P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 117, 7132–7189 (2017).
Banerjee, A. et al. Ionic liquids for oral insulin delivery. Proc. Natl Acad. Sci. USA 115, 7296–7301 (2018).
Petkovic, M. et al. Novel biocompatible cholinium-based ionic liquids — toxicity and biodegradability. Green Chem. 12, 643–649 (2010).
Williams, H. D. et al. Ionic liquids provide unique opportunities for oral drug delivery: structure optimization and in vivo evidence of utility. Chem. Commun. 50, 1688–1690 (2014).
Ma, C., Laaksonen, A., Liu, C., Lu, X. & Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 47, 8685–8720 (2018).
McQueen, L. & Lai, D. Ionic liquid aqueous two-phase systems from a pharmaceutical perspective. Front. Chem. 7, 135 (2019).
Kaper, J. B., Morris, J. G. Jr & Levine, M. M. Cholera. Clin. Microbiol. Rev. 8, 48–86 (1995).
Uzzau, S., Cappuccinelli, P. & Fasano, A. Expression of Vibrio cholerae zonula occludens toxin and analysis of its subcellular localization. Microb. Pathog. 27, 377–385 (1999).
Fasano, A. & Uzzau, S. Modulation of intestinal tight junctions by zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Invest. 99, 1158–1164 (1997). One of the first reports to use zonula occludens toxin to deliver biologic drugs via enteral administration.
Goldblum, S. E. et al. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 25, 144–158 (2011).
Takahashi, A. et al. Mutated C-terminal fragments of Clostridium perfringens enterotoxin have increased affinity to claudin-4 and reversibly modulate tight junctions in vitro. Biochem. Biophys. Res. Commun. 410, 466–470 (2011).
Maher, S., Wang, X., Bzik, V., McClean, S. & Brayden, D. J. Evaluation of intestinal absorption and mucosal toxicity using two promoters. II. Rat instillation and perfusion studies. Eur. J. Pharm. Sci. 38, 301–311 (2009).
Applied Molecular Transport. Platform technology. Applied Molecular Transport https://www.appliedmt.com/platform-technology/ (2019).
Bai, J. P. F., Chang, L. L. & Guo, J. H. Effects of polyacrylic polymers on the degradation of insulin and peptide drugs by chymotrypsin and trypsin. J. Pharm. Pharmacol. 48, 17–21 (1996).
Roy, S., Pal, K., Anis, A., Pramanik, K. & Prabhakar, B. Polymers in mucoadhesive drug-delivery systems: a brief note. Des. Monomers Polym. 12, 483–495 (2009).
Alexander, A., Ajazuddin, M., Swarna, M., Sharma, M. & Tripathi, D. Polymers and permeation enhancers: specialized components of mucoadhesives. Stamford J. Pharm. Sci. 4, 91–95 (2011).
Marvola, M., Nykänen, P., Rautio, S., Isonen, N. & Autere, A. M. Enteric polymers as binders and coating materials in multiple-unit site-specific drug delivery systems. Eur. J. Pharm. Sci. 7, 259–267 (1999).
Fang, Y. et al. Eudragit L/HPMCAS blend enteric-coated lansoprazole pellets: enhanced drug stability and oral bioavailability. AAPS PharmSciTech 15, 513–521 (2014).
Bando, H. & McGinity, J. W. Relationship between drug dissolution and leaching of plasticizer for pellets coated with an aqueous Eudragit S100:L100 dispersion. Int. J. Pharm. 323, 11–17 (2006).
Liu, F., Merchant, H. A., Kulkarni, R. P., Alkademi, M. & Basit, A. W. Evolution of a physiological pH 6.8 bicarbonate buffer system: application to the dissolution testing of enteric coated products. Eur. J. Pharm. Biopharm. 78, 151–157 (2011).
Siepmann, F., Siepmann, J., Walther, M., MacRae, R. & Bodmeier, R. Aqueous HPMCAS coatings: effects of formulation and processing parameters on drug release and mass transport mechanisms. Eur. J. Pharm. Biopharm. 63, 262–269 (2006).
Kamei, N., Aoyama, Y., Khafagy, E. S., Henmi, M. & Takeda-Morishita, M. Effect of different intestinal conditions on the intermolecular interaction between insulin and cell-penetrating peptide penetratin and on its contribution to stimulation of permeation through intestinal epithelium. Eur. J. Pharm. Biopharm. 94, 42–51 (2015).
Kamei, N. et al. Applicability and limitations of cell-penetrating peptides in noncovalent mucosal drug or carrier delivery systems. J. Pharm. Sci. 105, 747–753 (2016).
Kamei, N., Shigei, C., Hasegawa, R. & Takeda-Morishita, M. Exploration of the key factors for optimizing the in vivo oral delivery of insulin by using a noncovalent strategy with cell-penetrating peptides. Biol. Pharm. Bull. 41, 239–246 (2018).
Garcia, J., Fernández-Blanco, Á., Teixidó, M., Sánchez-Navarro, M. & Giralt, E. D-polyarginine lipopeptides as intestinal permeation enhancers. ChemMedChem 13, 2045–2052 (2018).
Zhang, D., Wang, J. & Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 229, 130–139 (2016).
Khafagy, E. S. et al. Efficiency of cell-penetrating peptides on the nasal and intestinal absorption of therapeutic peptides and proteins. Int. J. Pharm. 381, 49–55 (2009).
Ways, T. M. M., Lau, W. M. & Khutoryanskiy, V. V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 10, E267 (2018).
Agulló, E., Rodríguez, M. S., Ramos, V. & Albertengo, L. Present and future role of chitin and chitosan in food. Macromol. Biosci. 3, 521–530 (2003).
Sogias, I. A., Williams, A. C. & Khutoryanskiy, V. V. Why is chitosan mucoadhesive? Biomacromolecules 9, 1837–1842 (2008).
Thanou, M. M. et al. Effects of N-trimethyl chitosan chloride, a novel absorption enhancer, on Caco-2 intestinal epithelia and the ciliary beat frequency of chicken embryo trachea. Int. J. Pharm. 185, 73–82 (1999).
Thanou, M., Verhoef, J. C., Marbach, P. & Junginger, H. E. Intestinal absorption of octreotide N-trimethyl chitosan chloride (TMC) ameliorates the permeability and absorption properties of the somatostatin analogue in vitro and in vivo. J. Pharm. Sci. 89, 951–957 (2000).
Shitrit, Y. & Bianco-Peled, H. Acrylated chitosan for mucoadhesive drug delivery systems. Int. J. Pharm. 517, 247–255 (2017).
Thanou, M., Nihot, M. T., Jansen, M., Verhoef, J. C. & Junginger, H. E. Mono-N-carboxymethyl chitosan (MCC), a polyampholytic chitosan derivative, enhances the intestinal absorption of low molecular weight heparin across intestinal epithelia in vitro and in vivo. J. Pharm. Sci. 90, 38–46 (2001).
Kast, C. E. & Bernkop-Schnürch, A. Thiolated polymers — thiomers: development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials 22, 2345–2352 (2001).
Bernkop-Schnürch, A. Thiomers: a new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 57, 1569–1582 (2005).
Bernkop-Schnürch, A., Kast, C. E. & Guggi, D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems. J. Control. Release 93, 95–103 (2003).
Hanif, M., Zaman, M. & Qureshi, S. Thiomers: a blessing to evaluating era of pharmaceuticals. Int. J. Polym. Sci. 2015, 146329 (2015).
Iqbal, J. et al. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials 33, 1528–1535 (2012).
Iqbal, J., Sakloetsakun, D. & Bernkop-Schnürch, A. Thiomers: inhibition of cytochrome P450 activity. Eur. J. Pharm. Biopharm. 78, 361–365 (2011).
Wang, X., Iqbal, J., Rahmat, D. & Bernkop-Schnürch, A. Preactivated thiomers: permeation enhancing properties. Int. J. Pharm. 438, 217–224 (2012).
Ijaz, M. & Bernkop-Schnürch, A. Preactivated thiomers: their role in drug delivery. Expert Opin. Drug Deliv. 12, 1269–1281 (2015).
Date, A. A., Hanes, J. & Ensign, L. M. Nanoparticles for oral delivery: design, evaluation and state-of-the-art. J. Control. Release 240, 504–526 (2016).
Houchin, M. L. & Topp, E. M. Chemical degradation of peptides and proteins in PLGA: a review of reactions and mechanisms. J. Pharm. Sci. 97, 2395–2404 (2008).
Vaishya, R. D., Mandal, A., Gokulgandhi, M., Patel, S. & Mitra, A. K. Reversible hydrophobic ion-paring complex strategy to minimize acylation of octreotide during long-term delivery from PLGA microparticles. Int. J. Pharm. 489, 237–245 (2015).
Vila, A., Sánchez, A., Tobío, M., Calvo, P. & Alonso, M. J. Design of biodegradable particles for protein delivery. J. Control. Release 78, 15–24 (2002).
He, P. et al. Poly(ester amide) blend microspheres for oral insulin delivery. Int. J. Pharm. 455, 259–266 (2013).
Damgé, C., Socha, M., Ubrich, N. & Maincent, P. Poly(ε-caprolactone)/Eudragit nanoparticles for oral delivery of aspart-insulin in the treatment of diabetes. J. Pharm. Sci. 99, 879–889 (2010).
Mathiowitz, E. et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature 386, 410–414 (1997).
Geary, R. S. & Wade Schlameus, H. Vancomycin and insulin used as models for oral delivery of peptides. J. Control. Release 23, 65–74 (1993).
Allémann, E., Leroux, J.-C. & Gurny, R. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv. Drug Deliv. Rev. 34, 171–189 (1998).
Kumari, A., Yadav, S. K. & Yadav, S. C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 75, 1–18 (2010).
van de Weert, M., Hennink, W. E. & Jiskoot, W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 17, 1159–1167 (2000).
Kapoor, S., Hegde, R. & Bhattacharyya, A. J. Influence of surface chemistry of mesoporous alumina with wide pore distribution on controlled drug release. J. Control. Release 140, 34–39 (2009).
Amirthalingam, E. et al. Macrocyclic imidazolium-based amphiphiles for the synthesis of gold nanoparticles and delivery of anionic drugs. J. Colloid Interface Sci. 437, 132–139 (2015).
Joshi, H. M., Bhumkar, D. R., Joshi, K., Pokharkar, V. & Sastry, M. Gold nanoparticles as carriers for efficient transmucosal insulin delivery. Langmuir 22, 300–305 (2006).
Bhumkar, D. R., Joshi, H. M., Sastry, M. & Pokharkar, V. B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 24, 1415–1426 (2007).
Deng, W., Wang, H., Wu, B. & Zhang, X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm. Sin. B 9, 74–86 (2019).
Deng, W. et al. Selenium nanoparticles as versatile carriers for oral delivery of insulin: Insight into the synergic antidiabetic effect and mechanism. Nanomedicine 13, 1965–1974 (2017).
Florek, J., Caillard, R. & Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery—current status and perspective of MSNs drug carriers. Nanoscale 9, 15252–15277 (2017).
Diaz, A. et al. Nanoencapsulation of insulin into zirconium phosphate for oral delivery applications. Biomacromolecules 11, 2465–2470 (2010).
Safari, M., Kamari, Y., Ghiaci, M., Sadeghi-aliabadi, H. & Mirian, M. Synthesis and characterization of insulin/zirconium phosphate@TiO2hybrid composites for enhanced oral insulin delivery applications. Drug Dev. Ind. Pharm. 43, 862–870 (2017).
Han, L. et al. Synthesis and performance of functionalized α-zirconium phosphate modified with octadecyl isocyanate. J. Nanomater. 2018, 5873871 (2018).
Rachmiel, M. et al. OR14-1 pharmacodynamics, safety, tolerability, and efficacy of oral insulin formulation (Oshadi Icp) among young adults with type 1 diabetes: a summary of clinical studies phases I, Ib, and Ii. J. Endocr. Soc. 3, OR14–1 (2019).
Kulthe, S. S., Choudhari, Y. M., Inamdar, N. N. & Mourya, V. Polymeric micelles: authoritative aspects for drug delivery. Des. Monomers Polym. 15, 465–521 (2012).
Sadoqi, M., Lau-Cam, C. A. & Wu, S. H. Investigation of the micellar properties of the tocopheryl polyethylene glycol succinate surfactants TPGS 400 and TPGS 1000 by steady state fluorometry. J. Colloid Interface Sci. 333, 585–589 (2009).
Xie, S. et al. Targeted folate-conjugated pluronic P85/poly(lactide-co-glycolide) polymersome for the oral delivery of insulin. Nanomedicine 13, 2527–2544 (2018).
Batrakova, E. V. & Kabanov, A. V. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 130, 98–106 (2008).
Yang, H. et al. Glucose-responsive complex micelles for self-regulated release of insulin under physiological conditions. Soft Matter 9, 8589–8599 (2013).
Zhang, Z. H. et al. N-octyl-N-arginine chitosan micelles as an oral delivery system of insulin. J. Biomed. Nanotechnol. 9, 601–609 (2013).
Akbarzadeh, A. et al. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 8, 102 (2013).
Attama, A. A., Momoh, M. A. & Builders, P. F. in Recent Advances in Novel Drug Carrier Systems Ch. 5 (ed Sezer, A. D.) (IntechOpen, 2012).
Johnston, M. J. W. et al. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim. Biophys. Acta 1768, 1121–1127 (2007).
Wagner, A. & Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 591325 (2011).
Nisini, R., Poerio, N., Mariotti, S., De Santis, F. & Fraziano, M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 9, 155 (2018).
Ball, R. L., Bajaj, P. & Whitehead, K. A. Oral delivery of siRNA lipid nanoparticles: fate in the GI tract. Sci. Rep. 8, 2178 (2018).
Almeida, A. J. & Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 59, 478–490 (2007).
Sharma, A. & Sharma, U. S. Liposomes in drug delivery: progress and limitations. Int. J. Pharm. 154, 123–140 (1997).
Ahn, H. & Park, J. H. Liposomal delivery systems for intestinal lymphatic drug transport. Biomater. Res. 20, 36 (2016).
Goodman, B. E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 34, 44–53 (2010).
He, H. et al. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 9, 36–48 (2019).
Edgar, J. R. Q&A: What are exosomes, exactly? BMC Biol. 14, 46 (2016).
Hessvik, N. P. & Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 75, 193–208 (2018).
Kaparakis-Liaskos, M. & Ferrero, R. L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 15, 375–387 (2015).
Goes, A. & Fuhrmann, G. Biogenic and biomimetic carriers as versatile transporters to treat infections. ACS Infect. Dis. 4, 881–892 (2018).
Betker, J. L., Angle, B. M., Graner, M. W. & Anchordoquy, T. J. The potential of exosomes from cow milk for oral delivery. J. Pharm. Sci. 108, 1496–1505 (2019). Highlights the potential of exosomes for oral delivery and that they are absorbed in the gastrointestinal tract via neonatal Fc receptors.
Munagala, R., Aqil, F., Jeyabalan, J. & Gupta, R. C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 371, 48–61 (2016).
Manca, S. et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 8, 11321 (2018).
Yang, M. X. et al. Crystalline monoclonal antibodies for subcutaneous delivery. Proc. Natl Acad. Sci. USA 100, 6934–6939 (2003).
Halban, P. A., Mutkoski, R., Dodson, G. & Orci, L. Resistance of the insulin crystal to lysosomal proteases: implications for pancreatic B-cell crinophagy. Diabetologia 30, 348–353 (1987).
Margolin, A. L. Novel crystalline catalysts. Trends Biotechnol. 14, 223–230 (1996).
Margolin, A. L. & Navia, M. A. Protein crystals as novel catalytic materials. Angew. Chem. Int. Ed. Engl. 40, 2204–2222 (2001).
Mass High Tech Staff, Boston Business Journal. Calif. biotech takes over now-defunct Altus’ IP, assets. The Business Journals https://www.bizjournals.com/boston/blog/mass-high-tech/2010/05/calif-biotech-takes-over-now-defunct-altus.html (2010).
Ajinomoto Althea, Inc. Ajinomoto Co., Inc. Completes Acquisition of Althea Technologies, Inc. Cision PR Newswire https://www.prnewswire.com/news-releases/ajinomoto-co-inc-completes-acquisition-of-althea-technologies-inc-201549041.html (2013).
Hetrick, E. M., Sperry, D. C., Nguyen, H. K. & Strege, M. A. Characterization of a novel cross-linked lipase: impact of cross-linking on solubility and release from drug product. Mol. Pharm. 11, 1189–1200 (2014).
Ignatious, F., Sun, L., Lee, C. P. & Baldoni, J. Electrospun nanofibers in oral drug delivery. Pharm. Res. 27, 576–588 (2010).
Wang, J. et al. Manufacturing of polymer continuous nanofibers using a novel co-extrusion and multiplication technique. Polymer 55, 673–685 (2014).
Li, D. & Xia, Y. Electrospinning of nanofibers: reinventing the wheel? Adv. Mater. 16, 1151–1170 (2004).
Xie, J. et al. Mussel inspired protein-mediated surface modification to electrospun fibers and their potential biomedical applications. J. Biomed. Mater. Res. A 100, 929–938 (2012).
Jaiturong, P. et al. Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier. Asian J. Pharm. Sci. 13, 239–247 (2018).
Stephansen, K., García-Díaz, M., Jessen, F., Chronakis, I. S. & Nielsen, H. M. Bioactive protein-based nanofibers interact with intestinal biological components resulting in transepithelial permeation of a therapeutic protein. Int. J. Pharm. 495, 58–66 (2015).
Bhujbal, S. & Dash, A. K. Metformin-loaded hyaluronic acid nanostructure for oral delivery. AAPS PharmSciTech 19, 2543–2553 (2018).
Teutonico, D. & Ponchel, G. Patches for improving gastrointestinal absorption: an overview. Drug Discov. Today 16, 991–997 (2011).
Gupta, V. et al. Delivery of exenatide and insulin using mucoadhesive intestinal devices. Ann. Biomed. Eng. 44, 1993–2007 (2016).
Banerjee, A., Lee, J. & Mitragotri, S. Intestinal mucoadhesive devices for oral delivery of insulin. Bioeng. Transl. Med. 1, 338–346 (2016).
Grabovac, V., Föger, F. & Bernkop-Schnürch, A. Design and in vivo evaluation of a patch delivery system for insulin based on thiolated polymers. Int. J. Pharm. 348, 169–174 (2008).
Banerjee, A., Wong, J., Gogoi, R., Brown, T. & Mitragotri, S. Intestinal micropatches for oral insulin delivery. J. Drug Target. 25, 608–615 (2017).
Whitehead, K., Shen, Z. & Mitragotri, S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J. Control. Release 98, 37–45 (2004).
Gupta, V. et al. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J. Control. Release 172, 753–762 (2013).
Ito, Y. et al. Absorption of interferon α from patches in rats. J. Drug Target. 13, 383–390 (2005).
Venkatesan, N. et al. Gastro-intestinal patch system for the delivery of erythropoietin. J. Control. Release 111, 19–26 (2006).
Eiamtrakarn, S. et al. Gastrointestinal mucoadhesive patch system (GI-MAPS) for oral administration of G-CSF, a model protein. Biomaterials 23, 145–152 (2002).
Shen, Z. & Mitragotri, S. Intestinal patches for oral drug delivery. Pharm. Res. 19, 391–395 (2002).
Banerjee, A., Chen, R., Arafin, S. & Mitragotri, S. Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery. J. Control. Release 297, 71–78 (2019).
Rzhevskiy, A. S., Singh, T. R. R., Donnelly, R. F. & Anissimov, Y. G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J. Control. Release 270, 184–202 (2018). Highlights the use of microneedles for drug delivery enhancement.
Furness, G. Interview: Mir Imran, Rani Therapeutics. ONdrugDelivery Magazine 59, 32–35 (July 2015).
Hale, C. Rani Therapeutics completes first-in-human safety study of its robotic biologic pill. FierceBiotech https://www.fiercebiotech.com/medtech/rani-therapeutics-completes-first-human-safety-study-its-robotic-biologic-pill (2019).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019). Reports the ultra-long-lasting oral delivery of molecules using a polymeric scaffold.
Bellinger, A. M. et al. Oral, ultra-long-lasting drug delivery: Application toward malaria elimination goals. Sci. Transl. Med. 8, 365ra157 (2016).
Kirtane, A. R. et al. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Commun. 9, 2 (2018).
Kanasty, R. et al. A pharmaceutical answer to nonadherence: once weekly oral memantine for Alzheimer’s disease. J. Control. Release 303, 34–41 (2019).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071 (2016).
Peppas, N. A., Wood, K. M. & Blanchette, J. O. Hydrogels for oral delivery of therapeutic proteins. Expert Opin. Biol. Ther. 4, 881–887 (2004).
Ichikawa, H. & Peppas, N. A. Novel complexation hydrogels for oral peptide delivery: in vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. J. Biomed. Mater. Res. A 67, 609–617 (2003).
Kamei, N. et al. Complexation hydrogels for intestinal delivery of interferon β and calcitonin. J. Control. Release 134, 98–102 (2009).
Edelman, E. R., Nathan, A., Katada, M., Gates, J. & Karnovsky, M. J. Perivascular graft heparin delivery using biodegradable polymer wraps. Biomaterials 21, 2279–2286 (2000).
Li, Z. et al. Sodium dodecyl sulfate/β-cyclodextrin vesicles embedded in chitosan gel for insulin delivery with pH-selective release. Acta Pharm. Sin. B 6, 344–351 (2016).
Bai, X. et al. Chitosan-based thermo/pH double sensitive hydrogel for controlled drug delivery. Macromol. Biosci. 18, 1700305 (2018).
Slaughter, B. V., Blanchard, A. T., Maass, K. F. & Peppas, N. A. Dynamic swelling behavior of interpenetrating polymer networks in response to temperature and pH. J. Appl. Polym. Sci. 132, 42076 (2015).
Basan, H., Gümüşderelioğlu, M. & Tevfik Orbey, M. Release characteristics of salmon calcitonin from dextran hydrogels for colon-specific delivery. Eur. J. Pharm. Biopharm. 65, 39–46 (2007).
Ainslie, K. M., Kraning, C. M. & Desai, T. A. Microfabrication of an asymmetric, multi-layered microdevice for controlled release of orally delivered therapeutics. Lab Chip 8, 1042–1047 (2008).
Nielsen, L. H., Keller, S. S. & Boisen, A. Microfabricated devices for oral drug delivery. Lab Chip 18, 2348–2358 (2018).
Mazzoni, C. et al. Polymeric lids for microcontainers for oral protein delivery. Macromol. Biosci. 19, e1900004 (2019).
Jørgensen, J. et al. Microcontainers for oral insulin delivery – in vitro studies of permeation enhancement. Eur. J. Pharm. Biopharm. 143, 98–105 (2019).
von Halling Laier, C. et al. Microcontainers for protection of oral vaccines, in vitro and in vivo evaluation. J. Control. Release 294, 91–101 (2019).
Aran, K. et al. An oral microjet vaccination system elicits antibody production in rabbits. Sci. Transl. Med. 9, eaaf6413 (2017).
Fox, C. B. et al. Fabrication of sealed nanostraw microdevices for oral drug delivery. ACS Nano 10, 5873–5881 (2016).
Kam, K. R. et al. Nanostructure-mediated transport of biologics across epithelial tissue: enhancing permeability via nanotopography. Nano Lett. 13, 164–171 (2013). One of the first reports using surface roughness (texture) with microfabricated devices to improve transport of biologics.
Stewart, T. et al. Calibrated flux measurements reveal a nanostructure-stimulated transcytotic pathway. Exp. Cell Res. 355, 153–161 (2017).
Nemeth, C. L., Lykins, W. R., Tran, H., ElSayed, M. E. H. & Desai, T. A. Bottom-up fabrication of multilayer enteric devices for the oral delivery of peptides. Pharm. Res. 36, 89 (2019).
Abramson, A., Halperin, F., Kim, J. & Traverso, G. Quantifying the value of orally delivered biologic therapies: a cost-effectiveness analysis of oral semaglutide. J. Pharm. Sci. 108, 3138–3145 (2019). Highlights the economics of delivering biologics orally.
Garcia-Castillo, M. D. et al. Mucosal absorption of therapeutic peptides by harnessing the endogenous sorting of glycosphingolipids. eLife 7, e34469 (2018).
Liu, Y. et al. Trehalose glycopolymer enhances both solution stability and pharmacokinetics of a therapeutic protein. Bioconjug. Chem. 28, 836–845 (2017).
Alam, F. et al. Oral delivery of a potent anti-angiogenic heparin conjugate by chemical conjugation and physical complexation using deoxycholic acid. Biomaterials 35, 6543–6552 (2014).
Behrens, C. R. & Liu, B. Methods for site-specific drug conjugation to antibodies. MAbs 6, 46–53 (2014).
Knudsen, L. B. & Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. 10, 155 (2019).
Sarkissian, C. N. et al. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc. Natl Acad. Sci. USA 105, 20894–20899 (2008).
Cummings, C. S. et al. Design of stomach acid-stable and mucin-binding enzyme polymer conjugates. Biomacromolecules 18, 576–586 (2017).
Fuhrmann, K. & Fuhrmann, G. Recent advances in oral delivery of macromolecular drugs and benefits of polymer conjugation. Curr. Opin. Colloid Interface Sci. 31, 67–74 (2017).
US Food and Drug Administration. Generally recognized as safe (GRAS). FDA https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (2018). A useful database of FDA-approved ingredients with generally recognized as safe (GRAS) designation.
Strohl, W. R. & Strohl, L. M. Therapeutic Antibody Engineering: Current and Future Advances Driving the Strongest Growth Area in the Pharmaceutical Industry 1–13 (Woodhead, 2012).
Harloff-Helleberg, S., Nielsen, L. H. & Nielsen, H. M. Animal models for evaluation of oral delivery of biopharmaceuticals. J. Control. Release 268, 57–71 (2017).
von Klein, C. H. The medical features of the Papyrus Ebers. JAMA 45, 1928–1935 (1905).
Sonnedecker, G. & Griffenhagen, G. A history of sugar-coated pills and tablets. J. Am. Pharm. Assoc. 18, 486–488 (1957).
Baldwin, E. A., Hagenmaier, R. & Bai, J. Edible Coatings and Films to Improve Food Quality 2nd edn (CRC, 2002).
Karamitsos, D. T. The story of insulin discovery. Diabetes Res. Clin. Pract. 93, S2–S8 (2011).
Banting, F. G., Best, C. H., Collip, J. B., Campbell, W. R. & Fletcher, A. A. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 12, 141–146 (1922).
Scherer, R. P. Method of and machine for making capsules. US Patent US1970396A (1934).
Evonik. Precision medication Eudragit. Evonik https://history.evonik.com/sites/geschichte/en/inventions/eudragit/ (2019).
Sun, Y. The creation of synthetic crystalline bovine insulin. Protein Cell 6, 781–783 (2015).
Bangham, A. D., Standish, M. M. & Watkins, J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 13, 238–252 (1965).
Abuchowski, A., van Es, T., Palczuk, N. C. & Davis, F. F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 252, 3578–3581 (1977).
Abuchowski, A., McCoy, J. R., Palczuk, N. C., van Es, T. & Davis, F. F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 252, 3582–3586 (1977).
Itakura, K. et al. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science 198, 1056–1063 (1977).
US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Original new drug application (NDA and BLA) approvals October 1982. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=reportsSearch.process&rptName=2&reportSelectMonth=10&reportSelectYear=1982&nav (2019).
Gasco, M. R. Method for producing solid lipid microspheres having a narrow size distribution. US Patent US5250236A (1993).
Kim, Y.-C., Park, J.-H. & Prausnitz, M. R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 64, 1547–1568 (2012).
US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Cyclosporine. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=050715 (2019).
US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Desmopressin acetate. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=019955 (2019).
US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Exenatide Synthetic. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021773 (2019).
US Securities and Exchange Commission. Emisphere reports first quarter 2015 financial results. US Securities and Exchange Commission https://www.sec.gov/Archives/edgar/data/805326/000119312515190200/d925281dex991.htm (2015).
US Food and Drug Administration. Drugs@FDA: FDA approved drug products Semaglutide. FDA https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=213051 (2019).
Chiasma. Press release: Chiasma completes enrollment of CHIASMA OPTIMAL phase 3 clinical trial of octreotide capsules in patients with acromegaly. Chiasma http://ir.chiasmapharma.com/news-releases/news-release-details/chiasma-completes-enrollment-chiasma-optimal-phase-3-clinical?ID=2369676 (2018).
Oramed Pharmaceuticals, Inc. Press releases: Oramed provides clinical update with meaningful data expected by year-end. Oramed https://www.oramed.com/oramed-provides-clinical-update-with-meaningful-data-expected-by-year-end/ (2019).
Enteris BioPharma. Pipeline: Ovarest. Enteris BioPharma https://enterisbiopharma.com/pipeline/ovarest/ (2019).
Rosenstock, J. et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea : the PIONEER 3 randomized clinical trial. JAMA 321, 1466–1480 (2019).
Yamamoto, A. et al. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm. Res. 11, 1496–1500 (1994).
Morishita, M., Morishita, I., Takayama, K., Machida, Y. & Nagai, T. Novel oral microspheres of insulin with protease inhibitor protecting from enzymatic degradation. Int. J. Pharm. 78, 1–7 (1992).
Morishita, I., Morishita, M., Takayama, K., Machida, Y. & Nagai, T. Hypoglycemic effect of novel oral microspheres of insulin with protease inhibitor in normal and diabetic rats. Int. J. Pharm. 78, 9–16 (1992).
Geho, W. B., Geho, H. C., Lau, J. R. & Gana, T. J. Hepatic-directed vesicle insulin: a review of formulation development and preclinical evaluation. J. Diabetes Sci. Technol. 3, 1451–1459 (2009).
Acknowledgements
S.M. acknowledges funding from Blavatnik Biomedical Accelerator of Harvard University. T.D.B. acknowledges funding from the National Science Foundation (NSF) Graduate Research Fellowship (DGE-1745303). K.A.W. was supported by NSF grant number 1807983.
Author information
Authors and Affiliations
Contributions
S.M., T.D.B. and K.A.W. contributed to discussions of the article content, writing and review or editing of the manuscript before submission. T.D.B. additionally researched data for the article.
Corresponding author
Ethics declarations
Competing interests
S.M. declares that he is a shareholder and director of i2O Therapeutics, which is developing oral-drug-delivery products based on ionic liquids, and acts as a consultant and as a member of the advisory board of Entrega Bio. T.D.B. declares that he is a shareholder and employee of i2O Therapeutics. K.A.W. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brown, T.D., Whitehead, K.A. & Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat Rev Mater 5, 127–148 (2020). https://doi.org/10.1038/s41578-019-0156-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-019-0156-6
This article is cited by
-
Bioinspired oral delivery devices
Nature Reviews Bioengineering (2023)
-
Smart nanoparticles for cancer therapy
Signal Transduction and Targeted Therapy (2023)
-
Bioinspired nanotopographical design of drug delivery systems
Nature Reviews Bioengineering (2023)
-
A cooperative nano-CRISPR scaffold potentiates immunotherapy via activation of tumour-intrinsic pyroptosis
Nature Communications (2023)
-
Preparation, characterization and in vitro evaluation of chitosan nanoparticles for the oral delivery of GLP-1 analog liraglutide
Journal of Thermal Analysis and Calorimetry (2023)