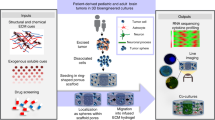
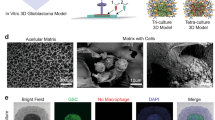
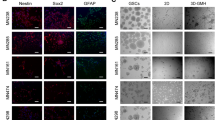
Abstract
Glioblastoma (GBM) is the most aggressive and common form of primary brain cancer. Several decades of research have provided great insight into GBM progression; however, the prognosis remains poor, with a median patient survival time of ~15 months. The tumour microenvironment (TME) of GBM plays a crucial role in mediating tumour progression and thus is being explored as a therapeutic target. Progress in the development of treatments targeting the TME is currently limited by a lack of model systems that can accurately recreate the distinct extracellular matrix composition and anatomic features of the brain, such as the blood–brain barrier and axonal tracts. Biomaterials can be applied to develop synthetic models of the GBM TME to mimic physiological and pathophysiological features of the brain, including cellular and extracellular matrix composition, mechanical properties and topography. In this Review, we summarize key features of the GBM microenvironment and discuss different strategies for the engineering of GBM TME models, including 2D and 3D models featuring chemical and mechanical gradients, interfaces and fluid flow. Finally, we highlight the potential of engineered TME models as platforms for mechanistic discovery and drug screening, as well as preclinical testing and precision medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
24 September 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
04 September 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united states in 2011–2015. Neuro. Oncol. 20, iv1–iv86 (2018).
Koshy, M. et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 107, 207–212 (2012).
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009).
Watanabe, M., Tanaka, R. & Takeda, N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34, 463–469 (1992).
Young, R. M., Jamshidi, A., Davis, G. & Sherman, J. H. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl. Med. 3, 121 (2015).
Sherriff, J. et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br. J. Radiol. 86, 20120414 (2013).
Eyler, C. E. & Rich, J. N. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 26, 2839–2845 (2008).
Franceschi, E. et al. Treatment options for recurrent glioblastoma: pitfalls and future trends. Expert Rev. Anticancer. Ther. 9, 613–619 (2009).
Quail, D. F. & Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341 (2017).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Nakasone, E. S. et al. Imaging tumor–stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21, 488–503 (2012).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Provenzano, P. P., Inman, D. R., Eliceiri, K. W. & Keely, P. J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK–ERK linkage. Oncogene 28, 4326–4343 (2009).
Elahi-Gedwillo, K. Y., Carlson, M., Zettervall, J. & Provenzano, P. P. Antifibrotic therapy disrupts stromal barriers and modulates the immune landscape in pancreatic ductal adenocarcinoma. Cancer Res. 79, 372–386 (2019).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
De Vleeschouwer, S. & Bergers, G. in Glioblastoma Ch. 16 (ed De Vleeschouwer, S.) (Codon Publications, 2017).
Jain, A. et al. Guiding intracortical brain tumour cells to an extracortical cytotoxic hydrogel using aligned polymeric nanofibres. Nat. Mater. 13, 308–316 (2014).
Gritsenko, P. G., Ilina, O. & Friedl, P. Interstitial guidance of cancer invasion. J. Pathol. 226, 185–199 (2012).
Bellail, A. C., Hunter, S. B., Brat, D. J., Tan, C. & Van Meir, E. G. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int. J. Biochem. Cell Biol. 36, 1046–1069 (2004).
van Tellingen, O. et al. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 19, 1–12 (2015).
de Vries, N. A., Beijnen, J. H., Boogerd, W. & van Tellingen, O. Blood–brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev. Neurother. 6, 1199–1209 (2006).
Nimsky, C. et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurg. 56, 130–138 (2005).
Giese, A. & Westphal, M. Glioma invasion in the central nervous system. Neurosurg. 39, 235–252 (1996).
Miller, K., Chinzei, K., Orssengo, G. & Bednarz, P. Mechanical properties of brain tissue in-vivo: experiment and computer simulation. J. Biomech. 33, 1369–1376 (2000).
Budday, S. et al. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 46, 318–330 (2015).
Bernstein, J. J. & Woodard, C. A. Glioblastoma cells do not intravasate into blood vessels. Neurosurg. 36, 124–132 (1995).
Nakod, P. S., Kim, Y. & Rao, S. S. Biomimetic models to examine microenvironmental regulation of glioblastoma stem cells. Cancer Lett. 429, 41–53 (2018).
Lenting, K., Verhaak, R., ter Laan, M., Wesseling, P. & Leenders, W. Glioma: experimental models and reality. Acta Neuropathol. 133, 263–282 (2017).
Xiao, W., Sohrabi, A. & Seidlits, S. K. Integrating the glioblastoma microenvironment into engineered experimental models. Future Sci. OA 3, FSO189 (2017).
Novak, U. & Kaye, A. H. Extracellular matrix and the brain: components and function. J. Clin. Neurosci. 7, 280–290 (2000).
Zimmermann, D. R. & Dours-Zimmermann, M. T. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol. 130, 635–653 (2008).
Bertolotto, A., Magrassi, M. L., Orsi, L., Sitia, C. & Schiffer, D. Glycosaminoglycan changes in human gliomas. A biochemical study. J. Neurooncol. 4, 43–48 (1986).
Chintala, S. K., Sawaya, R., Gokaslan, Z. L., Fuller, G. & Rao, J. S. Immunohistochemical localization of extracellular matrix proteins in human glioma, both in vivo and in vitro. Cancer Lett. 101, 107–114 (1996).
Mahesparan, R. et al. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 105, 49–57 (2003).
Cowman, M. K., Lee, H.-G., Schwertfeger, K. L., McCarthy, J. B. & Turley, E. A. The content and size of hyaluronan in biological fluids and tissues. Front. Immunol. 6, 261 (2015).
Dicker, K. T. et al. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 10, 1558–1570 (2014).
Akiyama, Y. et al. Hyaluronate receptors mediating glioma cell migration and proliferation. J. Neurooncol. 53, 115–127 (2001).
Breyer, R. et al. Disruption of intracerebral progression of rat C6 glioblastoma by in vivo treatment with anti-CD44 monoclonal antibody. J. Neurosurg. 62, 140–149 (2000).
Ponta, H., Sherman, L. & Herrlich, P. A. CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 4, 33–45 (2003).
Delpech, B. et al. Hyaluronan and hyaluronectin in the extracellular matrix of human brain tumour stroma. Eur. J. Cancer 29A, 1012–1017 (1993).
Yoo, K.-C. et al. Proinvasive extracellular matrix remodeling in tumor microenvironment in response to radiation. Oncogene 37, 3317–3328 (2018).
Valkonen, M. et al. Elevated expression of hyaluronan synthase 2 associates with decreased survival in diffusely infiltrating astrocytomas. BMC Cancer 18, 664 (2018).
Tian, X. et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349 (2013).
Chanmee, T., Ontong, P. & Itano, N. Mini-review. Hyaluronan: a modulator of the tumor microenvironment. Cancer Lett. 375, 20–30 (2016).
Chen, J.-W. E. et al. Influence of hyaluronic acid transitions in tumor microenvironment on glioblastoma malignancy and invasive behavior. Front. Mater 5, 39 (2018).
Gladson, C. L. The extracellular matrix of gliomas: modulation of cell function. J. Neuropathol. Exp. Neurol. 58, 1029–1040 (1999).
Ljubimova, J. Y., Fujita, M., Khazenzon, N. M., Ljubimov, A. V. & Black, K. L. Changes in laminin isoforms associated with brain tumor invasion and angiogenesis. Front. Biosci. 11, 81–88 (2006).
Gamble, J. T. et al. Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion. Biochem. Biophys. Res. Commun. 506, 833–839 (2018).
Lathia, J. D. et al. Laminin alpha 2 enables glioblastoma stem cell growth. Ann. Neurol. 72, 766–778 (2012).
Lathia, J. D. et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6, 421–432 (2010).
Shannon, S. et al. Dexamethasone-mediated activation of fibronectin matrix assembly reduces dispersal of primary human glioblastoma cells. PLoS One 10, e0135951 (2015).
Serres, E. et al. Fibronectin expression in glioblastomas promotes cell cohesion, collective invasion of basement membrane in vitro and orthotopic tumor growth in mice. Oncogene 33, 3451–3462 (2014).
Sabari, J. et al. Fibronectin matrix assembly suppresses dispersal of glioblastoma cells. PLoS One 6, e24810 (2011).
Yuan, L. et al. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene 26, 2563–2573 (2007).
Ogawa, K., Oguchi, M., Nakashima, Y. & Yamabe, H. Distribution of collagen Type IV in brain tumors: An immunohistochemical study. J. Neurooncol. 7, 357–366 (1989).
Rojiani, A. M. & Dorovini-Zis, K. Glomeruloid vascular structures in glioblastoma multiforme: an immunohistochemical and ultrastructural study. J. Neurosurg. 85, 1078–1084 (1996).
Pointer, K. B. et al. Association of collagen architecture with glioblastoma patient survival. J Neurosurg 126, 1812–1821 (2017).
Rauch, U. Brain matrix: structure, turnover and necessity. Biochem. Soc. Trans. 35, 656–660 (2007).
Lundell, A. et al. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure 12, 1495–1506 (2004).
Miroshnikova, Y. A. et al. Tissue mechanics promote IDH1-dependent HIF1α–tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 18, 1336–1345 (2016).
Mirzaei, R. et al. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. Oncoimmunology 7, e1478647 (2018).
Sarkar, S., Nuttall, R. K., Liu, S., Edwards, D. R. & Yong, V. W. Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 66, 11771–11780 (2006).
Rascher, G. et al. Extracellular matrix and the blood–brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 104, 85–91 (2002).
Pen, A., Moreno, M. J., Martin, J. & Stanimirovic, D. B. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: microarray study of laser-captured glioblastoma vessels. Glia 55, 559–572 (2007).
Pietras, A. et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell 14, 357–369 (2014).
Wei, J. et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Invest. 129, 137–149 (2018).
Lamour, V. et al. Targeting osteopontin suppresses glioblastoma stem-like cell character and tumorigenicity in vivo. Int. J. Cancer 137, 1047–1057 (2015).
Oyinlade, O. et al. Targeting UDP-α-d-glucose 6-dehydrogenase inhibits glioblastoma growth and migration. Oncogene 37, 2615–2629 (2018).
Chauvet, D. et al. In vivo measurement of brain tumor elasticity using intraoperative shear wave elastography. Eur. J. Ultrasound 37, 584–590 (2015).
Stewart, D. C., Rubiano, A., Dyson, K. & Simmons, C. S. Mechanical characterization of human brain tumors from patients and comparison to potential surgical phantoms. PLoS One 12, e0177561 (2017).
Ciasca, G. et al. Nano-mechanical signature of brain tumours. Nanoscale 8, 19629–19643 (2016).
Ulrich, T. A. et al. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167–4174 (2009).
Thomas, T. W. & DiMilla, P. A. Spreading and motility of human glioblastoma cells on sheets of silicone rubber depend on substratum compliance. Med. Biol. Eng. Comput. 38, 360–370 (2000).
Grundy, T. J. et al. Differential response of patient-derived primary glioblastoma cells to environmental stiffness. Sci. Rep. 6, 23353 (2016).
Wong, S. Y. et al. Constitutive activation of myosin-dependent contractility sensitizes glioma tumor-initiating cells to mechanical inputs and reduces tissue invasion. Cancer Res. 75, 1113–1122 (2015).
Ruiz-Ontañon, P. et al. Cellular plasticity confers migratory and invasive advantages to a population of glioblastoma-initiating cells that infiltrate peritumoral tissue. Stem Cells 31, 1075–1085 (2013).
Kim, Y. & Kumar, S. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol. Cancer Res. 12, 1416–1429 (2014).
Umesh, V., Rape, A. D., Ulrich, T. A. & Kumar, S. Microenvironmental stiffness enhances glioma cell proliferation by stimulating epidermal growth factor receptor signaling. PLoS One 9, e101771 (2014).
Mammoto, T. et al. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am. J. Pathol. 183, 1293–1305 (2013).
Seano, G. et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 3, 230–245 (2019).
Watkins, S. et al. Disruption of astrocyte–vascular coupling and the blood–brain barrier by invading glioma cells. Nat. Commun. 5, 4196 (2014).
Candiello, J. et al. Biomechanical properties of native basement membranes. FEBS J. 274, 2897–2908 (2007).
Charles, N. & Holland, E. C. The perivascular niche microenvironment in brain tumor progression. Cell Cycle 9, 3012–3021 (2010).
Giese, A. et al. Migration of human glioma cells on myelin. Neurosurg. 38, 755–764 (1996).
Hensel, T., Amberger, V. & Schwab, M. A metalloprotease activity from C6 glioma cells inactivates the myelin-associated neurite growth inhibitors and can be neutralized by antibodies. Br. J. Cancer 78, 1564–1572 (1998).
Amberger, V. R., Hensel, T., Ogata, N. & Schwab, M. E. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 58, 149–158 (1998).
Oellers, P., Schröer, U., Senner, V., Paulus, W. & Thanos, S. ROCKs are expressed in brain tumors and are required for glioma-cell migration on myelinated axons. Glia 57, 499–509 (2009).
Wang, J. et al. Invasion of white matter tracts by glioma stem cells is regulated by a NOTCH1–SOX2 positive-feedback loop. Nat. Neurosci. 22, 91–105 (2019).
Balzer, E. M. et al. Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J. 26, 4045–4056 (2012).
Monzo, P. et al. Mechanical confinement triggers glioma linear migration dependent on formin FHOD3. Mol. Biol. Cell 27, 1246–1261 (2016).
Heldin, C.-H., Rubin, K., Pietras, K., Ostman, A. & Östman, A. High interstitial fluid pressure — an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004).
Swartz, M. A. & Fleury, M. E. Interstitial flow and its effects in soft tissues. Annu. Rev. Biomed. Eng. 9, 229–256 (2007).
Abbott, N. J. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 45, 545–552 (2004).
Geer, C. P. & Grossman, S. A. Interstitial fluid flow along white matter tracts: A potentially important mechanism for the dissemination of primary brain tumors. J. Neurooncol. 32, 193–201 (1997).
Kingsmore, K. M. et al. Interstitial flow differentially increases patient-derived glioblastoma stem cell invasion via CXCR4, CXCL12, and CD44-mediated mechanisms. Integr. Biol. 8, 1246–1260 (2016).
Munson, J. M., Bellamkonda, R. V. & Swartz, M. A. Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res. 73, 1536–1546 (2013).
Cornelison, R. C., Brennan, C. E., Kingsmore, K. M. & Munson, J. M. Convective forces increase CXCR4-dependent glioblastoma cell invasion in GL261 murine model. Sci. Rep. 8, 17057 (2018).
Monteiro, A., Hill, R., Pilkington, G. & Madureira, P. The role of hypoxia in glioblastoma invasion. Cells 6, 45 (2017).
Lee, C. G. et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 60, 5565–5570 (2000).
Figueroa, J. et al. Exosomes from glioma-associated mesenchymal stem cells increase the tumorigenicity of glioma stem-like cells via transfer of miR-1587. Cancer Res. 77, 5808–5819 (2017).
Hossain, A. et al. Mesenchymal stem cells isolated from human gliomas increase proliferation and maintain stemness of glioma stem cells through the IL-6/gp130/STAT3 pathway. Stem Cells 33, 2400–2415 (2015).
Brandao, M., Simon, T., Critchley, G. & Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 67, 779–790 (2019).
Poon, C. C., Sarkar, S., Yong, V. W. & Kelly, J. J. P. Glioblastoma-associated microglia and macrophages: targets for therapies to improve prognosis. Brain 140, 1548–1560 (2017).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015).
Infanger, D. W. et al. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 73, 7079–7089 (2013).
Soda, Y. et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl. Acad. Sci. USA 108, 4274–4280 (2011).
Hardee, M. E. & Zagzag, D. Mechanisms of glioma-associated neovascularization. Am. J. Pathol. 181, 1126–1141 (2012).
Davis, M. E. Glioblastoma: overview of disease and treatment. Clin. J. Oncol. Nurs. 20, S2–S8 (2016).
Brat, D. J. et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 64, 920–927 (2004).
Lim, S. et al. Glioblastoma-secreted soluble CD44 activates tau pathology in the brain. Exp. Mol. Med. 50, 5 (2018).
Lacroix, M. et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 95, 190–198 (2001).
Shinoda, J. et al. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. J. Neurosurg. 99, 597–603 (2003).
Bregy, A. et al. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 13, 1453–1461 (2013).
Perry, J., Chambers, A., Spithoff, K. & Laperriere, N. Gliadel wafers in the treatment of malignant glioma: a systematic review. Curr. Oncol. 14, 189–194 (2007).
Stupp, R. et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma. JAMA 318, 2306–2316 (2017).
Davies, A. M., Weinberg, U. & Palti, Y. Tumor treating fields: a new frontier in cancer therapy. Ann. N. Y. Acad. Sci. 1291, 86–95 (2013).
Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82 (2007). This paper demonstrated that interactions between endothelial cells and GSCs regulate the self-renewal and tumour-initiating capacity of GSCs, therefore acting as a perivascular niche.
Borovski, T., De Sousa E Melo, F., Vermeulen, L. & Medema, J. P. Cancer stem cell niche: the place to be. Cancer Res. 71, 634–639 (2011).
Silver, D. J. & Lathia, J. D. Revealing the glioma cancer stem cell interactome, one niche at a time. J. Pathol. 244, 260–264 (2018).
Brooks, M. D., Sengupta, R., Snyder, S. C. & Rubin, J. B. Hitting them where they live: targeting the glioblastoma perivascular stem cell niche. Curr. Pathobiol. Rep. 1, 101–110 (2013).
Shiraki, Y. et al. Significance of perivascular tumour cells defined by CD109 expression in progression of glioma. J. Pathol. 243, 468–480 (2017).
Wolf, K. J., Lee, S. & Kumar, S. A 3D topographical model of parenchymal infiltration and perivascular invasion in glioblastoma. APL Bioeng. 2, 031903 (2018).
Ngo, M. T. & Harley, B. A. C. Perivascular signals alter global gene expression profile of glioblastoma and response to temozolomide in a gelatin hydrogel. Biomater. 198, 122–134 (2019).
Zhu, T. S. et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 71, 6061–6072 (2011).
Bao, S. et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66, 7843–7848 (2006).
Charles, N. et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 6, 141–152 (2010).
Tilghman, J. et al. HMMR maintains the stemness and tumorigenicity of glioblastoma stem-like cells. Cancer Res. 74, 3168–3179 (2014).
Chanmee, T., Ontong, P., Kimata, K. & Itano, N. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front. Oncol. 5, 180 (2015).
Ferrandez, E., Gutierrez, O., Segundo, D. S. & Fernandez-Luna, J. L. NFκB activation in differentiating glioblastoma stem-like cells is promoted by hyaluronic acid signaling through TLR4. Sci. Rep. 8, 6341 (2018).
Chen, J. & Kumar, S. Biophysical regulation of cancer stem/initiating cells: Implications for disease mechanisms and translation. Curr. Opin. Biomed. Eng. 1, 87–95 (2017).
Barnes, J. M. et al. A tension-mediated glycocalyx–integrin feedback loop promotes mesenchymal-like glioblastoma. Nat. Cell Biol. 20, 1203–1214 (2018).
Iwadate, Y. Epithelial–mesenchymal transition in glioblastoma progression. Oncol. Lett. 11, 1615–1620 (2016).
Lau, J. et al. STAT3 blockade inhibits a radiation-induced proneural-to-mesenchymal transition in glioma. Cancer Res. 75, 4302–4311 (2015).
Bhat, K. P. L. et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24, 331–346 (2013).
Xiao, W. et al. Brain-mimetic 3D culture platforms allow investigation of cooperative effects of extracellular matrix features on therapeutic resistance in glioblastoma. Cancer Res. 78, 1358–1370 (2018).
Soeda, A. et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene 28, 3949–3959 (2009).
Colwell, N. et al. Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro. Oncol. 19, 887–896 (2017).
Heddleston, J. M., Li, Z., McLendon, R. E., Hjelmeland, A. B. & Rich, J. N. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 8, 3274–3284 (2009).
Gupta, K. & Burns, T. C. Radiation-induced alterations in the recurrent glioblastoma microenvironment: therapeutic implications. Front. Oncol. 8, 503 (2018).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Rath, B. H., Wahba, A., Camphausen, K. & Tofilon, P. J. Coculture with astrocytes reduces the radiosensitivity of glioblastoma stem-like cells and identifies additional targets for radiosensitization. Cancer Med. 4, 1705–1716 (2015).
De Pascalis, I. et al. Endothelial trans-differentiation in glioblastoma recurring after radiotherapy. Mod. Pathol. 31, 1361–1366 (2018).
Mao, L. et al. Enhancement of invadopodia activity in glioma cells by sublethal doses of irradiation and temozolomide. J. Neurosurg. 129, 598–610 (2018).
Tsidulko, A. Y. et al. Conventional anti-glioblastoma chemotherapy affects proteoglycan composition of brain extracellular matrix in rat experimental model in vivo. Front. Pharmacol. 9, 1104 (2018).
Yoshida, D., Piepmeier, J. M., Bergenheim, T., Henriksson, R. & Teramoto, A. Suppression of matrix metalloproteinase-2-mediated cell invasion in U87MG, human glioma cells by anti-microtubule agent: in vitro study. Br. J. Cancer 77, 21–25 (1998).
Sawyers, C. Targeted cancer therapy. Nature 432, 294–297 (2004).
Higgins, M. J. & Baselga, J. Targeted therapies for breast cancer. J. Clin. Invest. 121, 3797–3803 (2011).
Westphal, M., Maire, C. L. & Lamszus, K. EGFR as a target for glioblastoma treatment: an unfulfilled promise. CNS Drugs 31, 723–735 (2017).
Butowski, N. et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an ivy foundation early phase clinical trials consortium phase II study. Neuro. Oncol. 18, 557–564 (2016).
Friedman, H. S. et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 27, 4733–4740 (2009).
Kreisl, T. N. et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 27, 740–745 (2009).
Wenger, K. J. et al. Bevacizumab as a last-line treatment for glioblastoma following failure of radiotherapy, temozolomide and lomustine. Oncol. Lett. 14, 1141–1146 (2017).
Pàez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Momeny, M. et al. Blockade of vascular endothelial growth factor receptors by tivozanib has potential anti-tumour effects on human glioblastoma cells. Sci. Rep. 7, 44075 (2017).
Batchelor, T. T. et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 28, 2817–2823 (2010).
Batchelor, T. T. et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J. Clin. Oncol. 31, 3212–3218 (2013).
Kreisl, T. N. et al. Continuous daily sunitinib for recurrent glioblastoma. J. Neurooncol. 111, 41–48 (2013).
Neal, J. & Wakelee, H. AMG-386, a selective angiopoietin-1/-2-neutralizing peptibody for the potential treatment of cancer. Curr. Opin. Mol. Ther. 12, 487–495 (2010).
Reardon, D. A. et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J. Natl. Compr. Canc. Netw. 9, 414–427 (2011).
Fang, H. & DeClerck, Y. A. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 73, 4965–4977 (2013).
Papadopoulos, K. P. et al. A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin. Cancer Res. 14, 7110–7115 (2008).
Albertella, M. R. et al. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin. Cancer Res. 14, 1096–1104 (2008).
Patterson, L. H. & McKeown, S. R. AQ4N: a new approach to hypoxia-activated cancer chemotherapy. Br. J. Cancer 83, 1589–1593 (2000).
Jain, K. K. A critical overview of targeted therapies for glioblastoma. Front. Oncol. 8, 419 (2018).
Carbonell, W. S., DeLay, M., Jahangiri, A., Park, C. C. & Aghi, M. K. β1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 73, 3145–3154 (2013).
Scaringi, C., Minniti, G., Caporello, P. & Enrici, R. M. Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results. Anticancer. Res. 32, 4213–4223 (2012).
Stupp, R. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1100–1108 (2014).
Tucci, M., Stucci, S. & Silvestris, F. Does cilengitide deserve another chance? Lancet. Oncol. 15, e584–e585 (2014).
Vogelbaum, M. A. & Aghi, M. K. Convection-enhanced delivery for the treatment of glioblastoma. Neuro. Oncol. 17, ii3–ii8 (2015).
Brown, M. C. et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med. 9, eaan4220 (2017).
Desjardins, A. et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018).
Rape, A., Ananthanarayanan, B. & Kumar, S. Engineering strategies to mimic the glioblastoma microenvironment. Adv. Drug Deliv. Rev. 79–80, 172–183 (2014).
Stylli, S. S., Luwor, R. B., Ware, T. M. B., Tan, F. & Kaye, A. H. Mouse models of glioma. J. Clin. Neurosci. 22, 619–626 (2015).
Joo, K. M. et al. Patient-specific orthotopic glioblastoma xenograft models recapitulate the histopathology and biology of human glioblastomas in situ. Cell Rep. 3, 260–273 (2013).
Simeonova, I. & Huillard, E. In vivo models of brain tumors: roles of genetically engineered mouse models in understanding tumor biology and use in preclinical studies. Cell. Mol. Life Sci. 71, 4007–4026 (2014).
Ismail Kola, J. L. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–715 (2004).
Wu, M. & Swartz, M. A. Modeling tumor microenvironments in vitro. J. Biomech. Eng. 136, 021011 (2014).
Pathak, A. & Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. USA 109, 10334–10339 (2012).
Diao, W. et al. Behaviors of glioblastoma cells in in vitro microenvironments. Sci. Rep. 9, 85 (2019).
Fernandez-Fuente, G., Mollinedo, P., Grande, L., Vazquez-Barquero, A. & Fernandez-Luna, J. L. Culture dimensionality influences the resistance of glioblastoma stem-like cells to multikinase inhibitors. Mol. Cancer Ther. 13, 1664–1672 (2014).
Rape, A. D., Zibinsky, M., Murthy, N. & Kumar, S. A synthetic hydrogel for the high-throughput study of cell–ECM interactions. Nat. Commun. 6, 8129 (2015).
Ananthanarayanan, B., Kim, Y. & Kumar, S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix–mimetic hyaluronic acid hydrogel platform. Biomater. 32, 7913–7923 (2011).
Wolf, K. J. & Kumar, S. Hyaluronic acid: incorporating the bio into the material. ACS Biomater. Sci. Eng. [epub ahead of print], (2019).
Schanté, C. E., Zuber, G., Herlin, C. & Vandamme, T. F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 85, 469–489 (2011).
Kaphle, P., Li, Y. & Yao, L. The mechanical and pharmacological regulation of glioblastoma cell migration in 3D matrices. J. Cell. Physiol. 234, 3948–3960 (2019).
Ulrich, T. A., Jain, A., Tanner, K., MacKay, J. L. & Kumar, S. Probing cellular mechanobiology in three-dimensional culture with collagen–agarose matrices. Biomater. 31, 1875–1884 (2010).
Ulrich, T. A., Lee, T. G., Shon, H. K., Moon, D. W. & Kumar, S. Microscale mechanisms of agarose-induced disruption of collagen remodeling. Biomater. 32, 5633–5642 (2011).
Yang, Y. et al. Influence of chondroitin sulfate and hyaluronic acid on structure, mechanical properties, and glioma invasion of collagen I gels. Biomater. 32, 7932–7940 (2011).
Yang, Y., Motte, S. & Kaufman, L. J. Pore size variable type I collagen gels and their interaction with glioma cells. Biomater. 31, 5678–5688 (2010).
Ylivinkka, I. et al. Motility of glioblastoma cells is driven by netrin-1 induced gain of stemness. J. Exp. Clin. Cancer Res. 36, 9 (2017).
Kumar, K. K. et al. Glioma stem cell invasion through regulation of the interconnected ERK, integrin α 6 and N-cadherin signaling pathway. Cell. Signal. 24, 2076–2084 (2012).
Wang, C., Tong, X., Jiang, X. & Yang, F. Effect of matrix metalloproteinase-mediated matrix degradation on glioblastoma cell behavior in 3D PEG-based hydrogels. J. Biomed. Mater. Res. A 105, 770–778 (2017).
Ma, N. K. L. et al. Collaboration of 3D context and extracellular matrix in the development of glioma stemness in a 3D model. Biomater. 78, 62–73 (2016).
Martínez-Ramos, C. & Lebourg, M. Three-dimensional constructs using hyaluronan cell carrier as a tool for the study of cancer stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 103, 1249–1257 (2015).
Heffernan, J. M. et al. PNIPAAm-co-Jeffamine® (PNJ) scaffolds as in vitro models for niche enrichment of glioblastoma stem-like cells. Biomater. 143, 149–158 (2017).
Kievit, F. M. et al. Modeling the tumor microenvironment using chitosan-alginate scaffolds to control the stem-like state of glioblastoma cells. Biomater. Sci. 4, 610–613 (2016).
Koh, I. et al. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci. Rep. 8, 4608 (2018).
Yi, H.-G. et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. [epub ahead of print], (2019). This paper describes a 3D-printed, spatially organized model of perivascular invasion that includes brain-derived matrix and patient-derived tumour cells.
Weiswald, L.-B., Bellet, D. & Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 17, 1–15 (2015).
Lee, J. et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9, 391–403 (2006).
Timmins, N. E. & Nielsen, L. K. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol. Med. 140, 141–151 (2007).
Mirab, F., Kang, Y. J. & Majd, S. Preparation and characterization of size-controlled glioma spheroids using agarose hydrogel microwells. PLoS One 14, e0211078 (2019).
Zhang, X.-P. et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol. Cell. Biochem. 307, 101–108 (2007).
Herrera-Perez, R. M. et al. Presence of stromal cells in a bioengineered tumor microenvironment alters glioblastoma migration and response to STAT3 inhibition. PLoS One 13, e0194183 (2018).
Florczyk, S. J. et al. Porous chitosan–hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 34, 10143–10150 (2013).
Herrera-Perez, M., Voytik-Harbin, S. L. & Rickus, J. L. Extracellular matrix properties regulate the migratory response of glioblastoma stem cells in three-dimensional culture. Tissue Eng. Part A 21, 2572–2582 (2015).
Pedron, S., Becka, E. & Harley, B. A. C. C. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials 34, 7408–7417 (2013).
Wang, C., Tong, X. & Yang, F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol. Pharm. 11, 2115–2125 (2014).
Heffernan, J. M., Overstreet, D. J., Le, L. D., Vernon, B. L. & Sirianni, R. W. Bioengineered scaffolds for 3D analysis of glioblastoma proliferation and invasion. Ann. Biomed. Eng. 43, 1965–1977 (2014).
Pedron, S., Hanselman, J. S., Schroeder, M. A., Sarkaria, J. N. & Harley, B. A. C. Extracellular hyaluronic acid influences the efficacy of EGFR tyrosine kinase inhibitors in a biomaterial model of glioblastoma. Adv. Healthc. Mater. 6, 1700529 (2017).
Pedron, S. et al. Hyaluronic acid-functionalized gelatin hydrogels reveal extracellular matrix signals temper the efficacy of erlotinib against patient-derived glioblastoma specimens. Biomaterials 219, 119371 (2019).
Shin, H. Fabrication methods of an engineered microenvironment for analysis of cell–biomaterial interactions. Biomaterials 28, 126–133 (2007).
Brown, T. E. & Anseth, K. S. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem. Soc. Rev. 46, 6532–6552 (2017).
Cortese, B., Gigli, G. & Riehle, M. Mechanical gradient cues for guided cell motility and control of cell behavior on uniform substrates. Adv. Funct. Mater. 19, 2961–2968 (2009).
Pedron, S., Becka, E. & Harley, B. A. Spatially gradated hydrogel platform as a 3D engineered tumor microenvironment. Adv. Mater. 27, 1567–1572 (2015). A microfluidic-based mixing tool was developed and applied to generate 3D materials with gradients of matrix and cellular composition, facilitating rapid investigation of TME parameters on tumour progression.
Rao, S. S. et al. Inherent interfacial mechanical gradients in 3D hydrogels influence tumor cell behaviors. PLoS One 7, e35852 (2012).
Pedron, S. & Harley, B. A. C. Impact of the biophysical features of a 3D gelatin microenvironment on glioblastoma malignancy. J. Biomed. Mater. Res. A 101, 3404–3415 (2013).
Gritsenko, P., Leenders, W. & Friedl, P. Recapitulating in vivo-like plasticity of glioma cell invasion along blood vessels and in astrocyte-rich stroma. Histochem. Cell Biol. 148, 1–12 (2017).
Rape, A. D. & Kumar, S. A composite hydrogel platform for the dissection of tumor cell migration at tissue interfaces. Biomaterials 35, 8846–8853 (2014).
Beliveau, A., Thomas, G., Gong, J., Wen, Q. & Jain, A. Aligned nanotopography promotes a migratory state in glioblastoma multiforme tumor cells. Sci. Rep. 6, 26143 (2016).
Kievit, F. M. et al. Aligned chitosan–polycaprolactone polyblend nanofibers promote the migration of glioblastoma cells. Adv. Healthc. Mater. 2, 1651–1659 (2013).
Sharma, P., Sheets, K., Elankumaran, S. & Nain, A. S. The mechanistic influence of aligned nanofibers on cell shape, migration and blebbing dynamics of glioma cells. Integr. Biol. 5, 1036–1044 (2013).
Grodecki, J. et al. Glioma–astrocyte interactions on white matter tract–mimetic aligned electrospun nanofibers. Biotechnol. Prog. 31, 1406–1415 (2015).
Rao, S. S. et al. Mimicking white matter tract topography using core–shell electrospun nanofibers to examine migration of malignant brain tumors. Biomaterials 34, 5181–5190 (2013).
Agudelo-Garcia, P. A. et al. Glioma cell migration on three-dimensional nanofiber scaffolds is regulated by substrate topography and abolished by inhibition of STAT3 signaling. Neoplasia 13, 831–840 (2011).
Sung, K. E. & Beebe, D. J. Microfluidic 3D models of cancer. Adv. Drug Deliv. Rev. 79–80, 68–78 (2014).
Ayuso, J. M. et al. Glioblastoma on a microfluidic chip: Generating pseudopalisades and enhancing aggressiveness through blood vessel obstruction events. Neuro. Oncol. 19, 503–513 (2017). This paper applied a microfluidic model to test how pseudopalisades form, which had previously only been inferred from in vivo data.
Truong, D. et al. A three-dimensional (3D) organotypic microfluidic model for glioma stem cells — Vascular interactions. Biomaterials 198, 63–77 (2019).
Chonan, Y., Taki, S., Sampetrean, O., Saya, H. & Sudo, R. Endothelium-induced three-dimensional invasion of heterogeneous glioma initiating cells in a microfluidic coculture platform. Integr. Biol. 9, 762–773 (2017).
Xiao, Y. et al. Ex vivo dynamics of human glioblastoma cells in a microvasculature-on-a-chip system correlates with tumor heterogeneity and subtypes. Adv. Sci. 6, 1801531 (2019).
Akay, M. et al. Drug screening of human GBM spheroids in brain cancer chip. Sci. Rep. 8, 15423 (2018).
Han, J. et al. Rapid emergence and mechanisms of resistance by U87 glioblastoma cells to doxorubicin in an in vitro tumor microfluidic ecology. Proc. Natl. Acad. Sci. USA 113, 14283–14288 (2016).
Fan, Y. et al. Engineering a brain cancer chip for high-throughput drug screening. Sci. Rep. 6, 25062 (2016).
Jie, M. et al. Evaluation of drug combination for glioblastoma based on an intestine–liver metabolic model on microchip. Analyst 142, 3629–3638 (2017).
Dai, X., Ma, C., Lan, Q. & Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 8, 045005 (2016).
Wang, X. et al. Bioprinting of glioma stem cells improves their endotheliogenic potential. Colloids Surf. B Biointerfaces 171, 629–637 (2018).
Heinrich, M. A. et al. 3D-Bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv. Mater. 31, 1806590 (2019).
Hubert, C. G. et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 76, 2465–2477 (2016). The method used in this paper to derive and culture patient cells and matrix minimally disturbed cell–matrix interactions, preserved tumour cell heterogeneity and resulted in an accurate recapitulation of patient tumour response in an orthotopic xenograft culture.
Hattori, N. Cerebral organoids model human brain development and microcephaly. Mov. Disord. 29, 185–185 (2014).
Nayernia, Z. et al. The relationship between brain tumor cell invasion of engineered neural tissues and in vivo features of glioblastoma. Biomaterials 34, 8279–8290 (2013).
Huang, Y., Agrawal, B., Clark, P. A., Williams, J. C. & Kuo, J. S. Evaluation of cancer stem cell migration using compartmentalizing microfluidic devices and live cell imaging. J. Vis. Exp. 58, e3297 (2011).
Piccolo, S. R. & Frey, L. J. Clinical and molecular models of glioblastoma multiforme survival. Int. J. Data Min. Bioinform. 7, 245–265 (2013).
Verhaak, R. G. W. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02060890 (2018).
Wang, Y. I., Abaci, H. E. & Shuler, M. L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 114, 184–194 (2017).
van der Helm, M. W., van der Meer, A. D., Eijkel, J. C. T., van den Berg, A. & Segerink, L. I. Microfluidic organ-on-chip technology for blood–brain barrier research. Tissue Barriers 4, e1142493 (2016).
Randazzo, M., Pisapia, J. M., Singh, N. & Thawani, J. P. 3D printing in neurosurgery: A systematic review. Surg. Neurol. Int. 7, S801–S809 (2016).
Ploch, C. C., Mansi, C. S. S. A., Jayamohan, J. & Kuhl, E. Using 3D printing to create personalized brain models for neurosurgical training and preoperative planning. World Neurosurg. 90, 668–674 (2016).
Naftulin, J. S., Kimchi, E. Y. & Cash, S. S. Streamlined, inexpensive 3D printing of the brain and skull. PLoS One 10, e0136198 (2015).
Treiber, J. M. et al. Molecular physiology of contrast enhancement in glioblastomas: an analysis of The Cancer Imaging Archive (TCIA). J. Clin. Neurosci. 55, 86–92 (2018).
Gevaert, O. et al. Glioblastoma multiforme: exploratory radiogenomic analysis by using quantitative image features. Radiology 273, 168–174 (2014).
Chow, D. et al. Imaging genetic heterogeneity in glioblastoma and other glial tumors: review of current methods and future directions. Am. J. Roentgenol. 210, 30–38 (2018).
Lao, J. et al. A deep learning-based radiomics model for prediction of survival in glioblastoma multiforme. Sci. Rep. 7, 10353 (2017).
Dupont, C., Betrouni, N., Reyns, N. & Vermandel, M. On image segmentation methods applied to glioblastoma: state of art and new trends. IRBM 37, 131–143 (2016).
Tamimi, A. F. & Juweid, M. in Glioblastoma Ch. 8 (ed De Vleeschouwer, S.) (Codon Publications, 2017).
Lee, J. H. et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 560, 243–247 (2018).
Khalifa, J. et al. Subventricular zones: new key targets for glioblastoma treatment. Radiat. Oncol. 12, 67 (2017).
Chen, L. et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int. J. Radiat. Oncol. 86, 616–622 (2013).
Ohgaki, H. & Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 170, 1445–1453 (2007).
Gupta, A. & Dwivedi, T. A simplified overview of World Health Organization classification update of central nervous system tumors 2016. J. Neurosci. Rural Pract. 8, 629–641 (2017).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Hartmann, C. et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 120, 707–718 (2010).
Wilson, T. A., Karajannis, M. A. & Harter, D. H. Glioblastoma multiforme: state of the art and future therapeutics. Surg. Neurol. Int. 5, 64 (2014).
Mutter, N. & Stupp, R. Temozolomide: a milestone in neuro-oncology and beyond? Expert Rev. Anticancer Ther. 6, 1187–1204 (2006).
Hegi, M. E. et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin. Cancer Res. 10, 1871–1874 (2004).
Kappelle, A. C. et al. PCV chemotherapy for recurrent glioblastoma multiforme. Neurology 56, 118–120 (2001).
Weller, M., Cloughesy, T., Perry, J. R. & Wick, W. Standards of care for treatment of recurrent glioblastoma — are we there yet? Neuro. Oncol. 15, 4–27 (2013).
Acknowledgements
The authors gratefully acknowledge financial support from the National Science Foundation (Graduate Research Fellowship to K.J.W.) and the National Institutes of Health (Ruth L. Kirschstein Predoctoral Individual National Research Service Award F31CA228317 to K.J.W.; Ruth L. Kirschstein Postdoctoral Individual National Research Service Award F32CA221366 to J.C.; R21EB025017, R01GM122375 and R01DK118940 to S.K.; and R01CA227136 to M.K.A. and S.K.). J.D.C. has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 752097.
Author information
Authors and Affiliations
Contributions
K.J.W., J.C. and J.D.C. researched data for the article. K.J.W. and S.K. made substantial contributions to manuscript writing and the discussion of content. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wolf, K.J., Chen, J., Coombes, J.D. et al. Dissecting and rebuilding the glioblastoma microenvironment with engineered materials. Nat Rev Mater 4, 651–668 (2019). https://doi.org/10.1038/s41578-019-0135-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-019-0135-y
This article is cited by
-
3D bioprinted tumor model: a prompt and convenient platform for overcoming immunotherapy resistance by recapitulating the tumor microenvironment
Cellular Oncology (2024)
-
Reprogramming systemic and local immune function to empower immunotherapy against glioblastoma
Nature Communications (2023)
-
Glioblastoma CD105+ cells define a SOX2− cancer stem cell-like subpopulation in the pre-invasive niche
Acta Neuropathologica Communications (2022)
-
BH3 mimetic drugs cooperate with Temozolomide, JQ1 and inducers of ferroptosis in killing glioblastoma multiforme cells
Cell Death & Differentiation (2022)
-
Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects
Signal Transduction and Targeted Therapy (2022)