Abstract
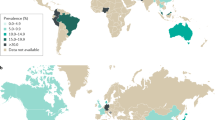
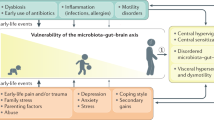
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder that is characterized by abdominal pain and an altered defecation pattern. It affects between 5 and 20% of the general population and can seriously impact quality of life. The pathophysiology of IBS is rather complex and multifactorial including, for example, altered signalling by the gut–brain axis, dysbiosis, abnormal visceral pain signalling and intestinal immune activation. The latter has gained particular interest in recent years, with growing insight into the bidirectional communication between the nervous system and the immune system. In this Review, we detail the current evidence suggesting that immune activation contributes to the pathology seen in patients with IBS and discuss the potential mechanisms involved. Moreover, we describe how immune mediators, particularly those released by mast cells, can directly activate or sensitize pain-transmitting nerves, leading to increased pain signalling and abdominal pain. Finally, we discuss the potential of interventions targeting immune activation as a new therapeutic strategy for patients suffering from IBS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
23 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41577-022-00713-4
References
Lacy, B. E. et al. Bowel disorders. Gastroenterology 150, 1393–1407 (2016).
Lovell, R. M. & Ford, A. C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721 (2012).
Ford, A. C., Sperber, A. D., Corsetti, M. & Camilleri, M. Irritable bowel syndrome. Lancet 396, 1675–1688 (2020).
Drossman, D. A. et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig. Dis. Sci. 38, 1569–1580 (1993).
Shen, T. C. et al. Bidirectional association between asthma and irritable bowel syndrome: two population-based retrospective cohort studies. PLoS ONE 11, e0153911 (2016).
Jones, M. P., Walker, M. M., Ford, A. C. & Talley, N. J. The overlap of atopy and functional gastrointestinal disorders among 23,471 patients in primary care. Aliment. Pharmacol. Ther. 40, 382–391 (2014).
Klem, F. et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 155, 1042–1054 (2017).
Böhn, L., Störsrud, S., Törnblom, H., Bengtsson, U. & Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 108, 634–641 (2013).
Major, G. et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 152, 124–133 (2017).
Barrett, J. S. et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 31, 874–882 (2010).
Staudacher, H. M. & Whelan, K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 66, 1517–1527 (2017).
Hiatt, R. B. & Katz, L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am. J. Gastroenterol. 37, 541–545 (1962).
Brookes, S. J. H., Spencer, N. J., Costa, M. & Zagorodnyuk, V. P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 10, 286–296 (2013).
Yoo, B. B. & Mazmanian, S. K. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46, 910–926 (2017).
Brierley, S. M., Jones, R. C. W., Gebhart, G. F. & Blackshaw, L. A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178 (2004). This study shows that the splanchnic and pelvic nerves contain distinct populations of mechanosensitive afferent neurons and differentially transmit mechanical information from the colon.
Grundy, L., Erickson, A. & Brierley, S. M. Visceral pain. Annu. Rev. Physiol. 81, 261–284 (2019).
Brierley, S. M. & Linden, D. R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 11, 611–627 (2014).
Feng, B. & Gebhart, G. F. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G170–G180 (2011).
Hockley, J. R. F. et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68, 633–644 (2018). This study identifies seven subtypes of sensory neurons innervating the colon of mice using single-cell RNA sequencing, therefore providing the molecular signature of specific neurons projecting to the splanchnic and pelvic nerves.
Ritchie, J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 14, 125–132 (1973).
Mertz, H., Naliboff, B., Munakata, J., Niazi, N. & Mayer, E. A. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 109, 40–52 (1995).
Posserud, I. et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 133, 1113–1123 (2007).
Whitehead, W. E. et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology 98, 1187–1192 (1990).
Lembo, T. et al. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology 107, 1686–1696 (1994).
Törnblom, H., Van Oudenhove, L., Tack, J. & Simrén, M. Interaction between preprandial and postprandial rectal sensory and motor abnormalities in IBS. Gut 63, 1441–1449 (2014).
Simrén, M. et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut 67, 255–262 (2018).
Boué, J. et al. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4+ T cells in mice. Gastroenterology 146, 166–175 (2014).
Hughes, P. A. et al. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58, 1333–1341 (2009).
Hughes, P. A. et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 62, 1456–1465 (2013).
Cenac, N. et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 117, 636–647 (2007).
Barbara, G. et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132, 26–37 (2007).
Buhner, S. et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137, 1425–1434 (2009).
Cremon, C. et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am. J. Gastroenterol. 106, 1290–1298 (2011).
Cibert-Goton, V. et al. Pain severity correlates with biopsy-mediated colonic afferent activation but not psychological scores in patients with IBS-D. Clin. Transl. Gastroenterol. 12, e00313 (2021).
Ji, R. R., Samad, T. A., Jin, S. X., Schmoll, R. & Woolf, C. J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36, 57–68 (2002).
Linley, J. E., Rose, K., Ooi, L. & Gamper, N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflug. Arch. Eur. J. Physiol. 459, 657–669 (2010).
Pattison, L. A., Krock, E., Svensson, C. I. & Smith, E. S. J. Cell–cell interactions in joint pain: rheumatoid arthritis and osteoarthritis. Pain 162, 714–717 (2021).
Hucho, T. & Levine, J. D. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55, 365–376 (2007).
Gold, M. S. & Gebhart, G. F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257 (2010).
Van Wanrooij, S. J. M. M. et al. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? Am. J. Gastroenterol. 109, 99–109 (2014).
Valdez-Morales, E. E. et al. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am. J. Gastroenterol. 108, 1634–1643 (2013).
Wouters, M. M. et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150, 875–887.e9 (2016). This study shows that sensitization of TRPV1 is mediated by H1R in IBS and treatment with ebastine, a H1R antagonist, reduces VHS, symptoms and abdominal pain in patients with IBS.
Balemans, D. et al. Evidence for long-term sensitization of the bowel in patients with post-infectious-IBS. Sci. Rep. 7, 13606 (2017).
Balemans, D. et al. Histamine-mediated potentiation of TRPA1 and TRPV4 signaling in submucosal neurons in IBS patients. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G338–G349 (2019).
Cenac, N. et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 59, 481–488 (2010).
Rolland-Fourcade, C. et al. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut 66, 1767–1778 (2017).
Jimenez-Vargas, N. N. et al. Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc. Natl Acad. Sci. USA 115, E7438–E7447 (2018).
Amadesi, S. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 24, 4300–4312 (2004).
Dai, Y. et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Invest. 117, 1979–1987 (2007).
Grant, A. D. et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 578, 715–733 (2007).
Dothel, G. et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 148, 1002–1011 (2015).
Yu, Y. B. et al. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut 61, 685–694 (2012).
Brizuela, M., Castro, J., Harrington, A. M. & Brierley, S. M. Pruritogenic mechanisms and gut sensation: putting the “irritant” into irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G1131–G1141 (2021).
Gwee, K. A. et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 44, 400–406 (1999).
Spiller, R. C. et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47, 804–811 (2000).
Bashashati, M. et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol. Motil. 30, e13192 (2018).
Bashashati, M. et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol. Motil. 26, 1036–1048 (2014).
Bennet, S. M. P. P. et al. Global cytokine profiles and association with clinical characteristics in patients with irritable bowel syndrome. Am. J. Gastroenterol. 111, 1165–1176 (2016).
Bennet, S. M. P. P. et al. Systemic cytokines are elevated in a subset of patients with irritable bowel syndrome but largely unrelated to symptom characteristics. Neurogastroenterol. Motil. 30, 1–13 (2018).
Aguilera-Lizarraga, J. et al. Expression of immune-related genes in rectum and colon descendens of irritable bowel syndrome patients is unrelated to clinical symptoms. Neurogastroenterol. Motil. 31, e13579 (2019).
Barbara, G. et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702 (2004). This seminal study shows that mucosal infiltration of colonic mast cells in close proximity to nerve fibres and the release of mediators may play an important role in the development of abdominal pain in patients with IBS.
Aguilera-Lizarraga, J. et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 590, 151–156 (2021). This study characterizes a peripheral mechanism that underlies food-induced abdominal pain upon loss of local oral tolerance, mediated by food-antigen specific IgE-dependent activation of mast cells in the colon.
Vicario, M. et al. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut 64, 1379–1388 (2015).
Fritscher-Ravens, A. et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 147, 1012–1020.e3 (2014). This study uses confocal later endomicroscopy to show that the intestinal mucosa of patients with IBS undergoes profound structural remodelling upon exposure to food antigens.
Fritscher-Ravens, A. et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 157, 109–118 (2019).
Faria, A. M. C. & Weiner, H. L. Oral tolerance. Immunol. Rev. 206, 232–259 (2005).
Fonseca, D. M. Da et al. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163, 354–366 (2015).
Bouziat, R. et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356, 44–50 (2017). This study shows that a reovirus infection can interfere with the development of oral tolerance to dietary antigens by suppressing peripheral Treg cells and promoting TH1-type immunity against these food antigens.
Caminero, A., Meisel, M., Jabri, B. & Verdu, E. F. Mechanisms by which gut microorganisms influence food sensitivities. Nat. Rev. Gastroenterol. Hepatol. 16, 7–18 (2019).
Yu, W., Freeland, D. M. H. & Nadeau, K. C. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 16, 751–765 (2016).
Kassinen, A. et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133, 24–33 (2007).
Hadizadeh, F. et al. Faecal microbiota composition associates with abdominal pain in the general population. Gut 67, 778–779 (2018).
Frost, F. et al. Functional abdominal pain and discomfort (IBS) is not associated with faecal microbiota composition in the general population. Gut 68, 1131–1133 (2019).
Hugerth, L. W. et al. No distinct microbiome signature of irritable bowel syndrome found in a Swedish random population. Gut 69, 1076–1084 (2020).
Jeffery, I. B. et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 (2012).
Pittayanon, R. et al. Gut microbiota in patients with irritable bowel syndrome — a systematic review. Gastroenterology 157, 97–108 (2019).
Schoepfer, A. M., Schaffer, T., Seibold-Schmid, B., Müller, S. & Seibold, F. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol. Motil. 20, 1110–1118 (2008).
Zhou, S. Y. et al. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Invest. 128, 267–280 (2018).
Vervier, K. et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut https://doi.org/10.1136/gutjnl-2021-325177 (2021).
Mars, R. A. T. et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 182, 1460–1473 (2020).
Botschuijver, S. et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology 153, 1026–1039 (2017).
Larauche, M., Mulak, A. & Taché, Y. Stress and visceral pain: from animal models to clinical therapies. Exp. Neurol. 233, 49–67 (2012).
Van Den Wijngaard, R. M. et al. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol. Motil. 21, 1107–e94 (2009).
Coughlan, S. et al. The gut virome in irritable bowel syndrome differs from that of controls. Gut Microbes 13, 1–15 (2021).
Mihindukulasuriya, K. A. et al. Multi-omics analyses show disease, diet, and transcriptome interactions with the virome. Gastroenterology 161, 1194–1207.e8 (2021).
Odenwald, M. A. & Turner, J. R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14, 9–21 (2017).
Shen, L., Weber, C. R., Raleigh, D. R., Yu, D. & Turner, J. R. Tight junction pore and leak pathways: a dynamic duo. Annu. Rev. Physiol. 73, 289–309 (2011).
Vivinus-Nébot, M. et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am. J. Gastroenterol. 107, 75–81 (2012).
Hanning, N. et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Ther. Adv. Gastroenterol. 14, 1756284821993586 (2021).
Khoshbin, K. et al. Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults. Gastroenterology 161, 463–475.e13 (2021).
Piche, T. et al. Impaired Intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58, 196–201 (2009).
Dunlop, S. P. et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 101, 1288–1294 (2006).
Vergnolle, N. Clinical relevance of proteinase activated receptors (PARs) in the gut. Gut 54, 867–874 (2005).
Vergnolle, N., Wallace, J. L., Bunnett, N. W. & Hollenberg, M. D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 22, 146–152 (2001).
Gecse, K. et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57, 591–599 (2008).
Annaházi, A. et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am. J. Gastroenterol. 108, 1322–1331 (2013).
Róka, R. et al. A pilot study of fecal serine-protease activity: a pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 5, 550–555 (2007).
Tooth, D. et al. Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: origin and effect of gut transit. Gut 63, 753–760 (2014).
Wilcz-Villega, E. M., McClean, S. & O’Sullivan, M. A. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am. J. Gastroenterol. 108, 1140–1151 (2013).
Tulic, M. K. et al. Presence of commensal house dust mite allergen in human gastrointestinal tract: a potential contributor to intestinal barrier dysfunction. Gut 65, 757–766 (2016).
Iliev, I. D. et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58, 1481–1489 (2009).
Hammad, H. & Lambrecht, B. N. Barrier epithelial cells and the control of type 2 immunity. Immunity 43, 29–40 (2015).
Von Moltke, J., Ji, M., Liang, H. E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016).
Liew, F. Y., Girard, J. P. & Turnquist, H. R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 16, 676–689 (2016).
Hepworth, M. R., Maurer, M. & Hartmann, S. Regulation of type 2 immunity to helminths by mast cells. Gut Microbes 3, 476–481 (2012).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Nakajima, S., Kabata, H., Kabashima, K. & Asano, K. Anti-TSLP antibodies: targeting a master regulator of type 2 immune responses. Allergol. Int. 69, 197–203 (2020).
Ito, T. et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202, 1213–1223 (2005).
Ma, H., Qiu, Y. & Yang, H. Intestinal intraepithelial lymphocytes: maintainers of intestinal immune tolerance and regulators of intestinal immunity. J. Leukoc. Biol. 109, 339–347 (2021).
Matricon, J. et al. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 36, 1009–1031 (2012).
Frossard, C. P., Asigbetse, K. E., Burger, D. & Eigenmann, P. A. Gut T cell receptor-γδ+ intraepithelial lymphocytes are activated selectively by cholera toxin to break oral tolerance in mice. Clin. Exp. Immunol. 180, 118–130 (2015).
Junker, Y. et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of Toll-like receptor 4. J. Exp. Med. 209, 2395–2408 (2012).
Zevallos, V. F. et al. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 152, 1100–1113 (2017).
Greger, J. L. & Sutherland, J. E. Aluminum exposure and metabolism. Crit. Rev. Clin. Lab. Sci. 34, 439–474 (1997).
Esquerre, N. et al. Aluminum ingestion promotes colorectal hypersensitivity in rodents. Cell Mol. Gastroenterol. Hepatol. 7, 185–196 (2019).
Bischoff, S. C. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 7, 93–104 (2007).
Galli, S. J., Nakae, S. & Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 6, 135–142 (2005).
Krystel-Whittemore, M., Dileepan, K. N. & Wood, J. G. Mast cell: a multi-functional master cell. Front. Immunol. 6, 620 (2016).
Albert-Bayo, M. et al. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells 8, 135 (2019).
Abraham, S. N. & St. John, A. L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10, 440–452 (2010).
Redegeld, F. A., Yu, Y., Kumari, S., Charles, N. & Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 282, 87–113 (2018).
Galli, S. J. & Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 18, 693–704 (2012).
Chiu, I. M., Von Hehn, C. A. & Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067 (2012).
McNeil, B. D. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 137–141 (2015).
Meixiong, J. et al. Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 50, 1163–1171 (2019).
Green, D. P., Limjunyawong, N., Gour, N., Pundir, P. & Dong, X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101, 412–420 (2019).
Qin, H. Y., Cheng, C. W., Tang, X. D. & Bian, Z. X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 20, 14126–14131 (2014).
Castagliuolo, I. et al. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am. J. Physiol. Gastrointest. Liver Physiol. 271, G884–G892 (1996).
Söderholm, J. D. et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 123, 1099–1108 (2002).
Theoharides, T. C. et al. Mast cells and inflammation. Biochim. Biophys. Acta 1822, 21–33 (2012).
Kempuraj, D. et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology 145, 43–48 (2004).
Camilleri, M. Diagnosis and treatment of irritable bowel syndrome: a review. JAMA 325, 865–877 (2021).
Dunlop, S. P. et al. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 18, 77–84 (2003).
Jalanka, J. et al. Colonic gene expression and fecal microbiota in diarrhea-predominant irritable bowel syndrome: increased Toll-like receptor 4 but minimal inflammation and no response to mesalazine. J. Neurogastroenterol. Motil. 27, 279–291 (2021).
Barbara, G. et al. Randomised controlled trial of mesalazine in IBS. Gut 65, 82–90 (2016).
Lam, C. et al. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut 65, 91–99 (2016).
Lobo, B. et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: a pilot study. U. Eur. Gastroenterol. J. 5, 887–897 (2017).
Klooker, T. K. et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 59, 1213–1221 (2010).
Pearson, J. S., Niven, R. M., Meng, J., Atarodi, S. & Whorwell, P. J. Immunoglobulin E in irritable bowel syndrome: another target for treatment? A case report and literature review. Ther. Adv. Gastroenterol. 8, 270–277 (2015).
Rothenberg, M. E. An allergic basis for abdominal pain. N. Engl. J. Med. 384, 2156–2158 (2021).
Black, C. J. et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut 69, 74–82 (2020).
Ford, A. C., Harris, L. A., Lacy, B. E., Quigley, E. M. M. & Moayyedi, P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 48, 1044–1060 (2018).
Pimentel, M. et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 364, 22–32 (2011).
O’Toole, P. W. & Shanahan, F. Transplanting microbes for irritable bowels or irritated microbes or both? Gastroenterology 160, 15–17 (2021).
Shanahan, F., Ghosh, T. S. & O’Toole, P. W. The healthy microbiome — what is the definition of a healthy gut microbiome? Gastroenterology 160, 483–494 (2021).
Xu, D. et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am. J. Gastroenterol. 114, 1043–1050 (2019).
McGuire, C. et al. Ex vivo study of human visceral nociceptors. Gut 67, 86–96 (2018).
Jiang, W. et al. ‘First-in-man’: characterising the mechanosensitivity of human colonic afferents. Gut 60, 281–282 (2011).
Lyte, M. & Cryan, J. F. Microbial Endocrinology: The Microbiota–Gut–Brain Axis in Health and Cognitive Function. Advances in Experimental Medicine and Biology (Springer, 2014).
Acknowledgements
J.A.-L. is supported by a FWO postdoctoral fellowship (12X9820N). G.E.B. is funded by the European Research Council (ERC) Advanced Grant (833816-NEUMACS) and the KU Leuven internal funding grant C1 (C14/18/086).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for article, discussion of content, the writing and the review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks S. Abraham, E. Mayer and R. Spiller for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aguilera-Lizarraga, J., Hussein, H. & Boeckxstaens, G.E. Immune activation in irritable bowel syndrome: what is the evidence?. Nat Rev Immunol 22, 674–686 (2022). https://doi.org/10.1038/s41577-022-00700-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-022-00700-9
This article is cited by
-
Gut liver brain axis in diseases: the implications for therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Proenkephalin deletion in hematopoietic cells induces intestinal barrier failure resulting in clinical feature similarities with irritable bowel syndrome in mice
Communications Biology (2023)
-
Persisting symptoms after Cryptosporidium hominis outbreak: a 10-year follow-up from Östersund, Sweden
Parasitology Research (2023)
-
New insights into irritable bowel syndrome pathophysiological mechanisms: contribution of epigenetics
Journal of Gastroenterology (2023)
-
Pitfalls and Traps in the Surgical Evaluation of Patients with Irritable Bowel Syndrome (IBS)
Journal of Gastrointestinal Surgery (2023)