Abstract
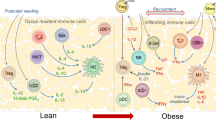
Adipose tissue is a complex dynamic organ with whole-body immunometabolic influence. Much of the work into understanding the role of immune cells in adipose tissue has been in the context of obesity. These investigations have also uncovered a range of typical (immune) and non-typical functions exerted by adipose tissue leukocytes. Here we provide an overview of the adipose tissue immune system, including its role as an immune reservoir in the whole-body response to infection and as a site of parasitic and viral infections. We also describe the functional roles of specialized immunological structures found within adipose tissue. However, our main focus is on the recently discovered ‘non-immune’ functions of adipose tissue immune cells, which include the regulation of adipocyte homeostasis, as well as responses to changing nutrient status and body temperature. In doing so, we outline the therapeutic potential of the adipose tissue immune system in health and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
v. Recklinghausen, F. Ueber Eiter-und Bindegewebskörperchen. Arch. Pathol. Anat. 28, 157–197 (1863).
Ranvier, L. Du dévelopment et de l’accroissement desvaiseaux sanguins. Arch. Physiol. Norm. Pathol. 6, 429–446 (1874).
Wertheimer, E. & Shapiro, B. The physiology of adipose tissue. Physiol. Rev. 28, 451 (1948).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science https://doi.org/10.1126/science.7678183 (1993). This is the first study to identify adipose tissue as a source of inflammation in obesity, which is a direct contributing factor in the development of obesity-associated insulin resistance.
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003). This study identifies that obesity is associated with a profound increase in the numbers of adipose tissue macrophages, which are an important source of adipose tissue inflammation in obesity in both humans and mice.
Kane, H. & Lynch, L. Innate immune control of adipose tissue homeostasis. Trends Immunol. 40, 857–872 (2019).
Trim, W., Turner, J. E. & Thompson, D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front. Immunol. https://doi.org/10.3389/fimmu.2018.00169 (2018).
Thompson, D., Karpe, F., Lafontan, M. & Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 92, 157–191 (2012).
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. https://doi.org/10.1038/nrendo.2017.90 (2017).
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
Shimobayashi, M. et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 128, 1538–1550 (2018).
Lee, M. J., Wu, Y. & Fried, S. K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 34, 1–11 (2013).
Exley, M. A., Hand, L., O’Shea, D. & Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 223, R41–R48 (2014).
Cox, A. R., Chernis, N., Masschelin, P. M. & Hartig, S. M. Immune cells gate white adipose tissue expansion. Endocrinology 160, 1645–1658 (2019).
Priest, C. & Tontonoz, P. Inter-organ cross-talk in metabolic syndrome. Nat. Metab. 1, 1177–1188 (2019).
LaMarche, N. M., Kohlgruber, A. C. & Brenner, M. B. Innate T cells govern adipose tissue biology. J. Immunol. 201, 1827–1834 (2018).
Lynch, L. et al. Regulatory iNKT cells lack PLZF expression and control Treg cell and macrophage homeostasis in adipose tissue. Nat. Immunol. 16, 85–95 (2015). This study shows adipose tissue is home to a resident population of iNKT cells that provide important anti-inflammatory signals within the tissue as well as controlling the number, proliferation and suppressor function of Treg cells.
Kohlgruber, A. C. et al. gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19, 464–474 (2018). This is the first study to identify the importance of adipose tissue γδ T cells in body temperature regulation and in sustaining Treg cell abundance via IL-17A.
Pond, C. M. & Mattacks, C. A. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J. Lipid Res. 36, 2219–2231 (1995). This is one of the earliest studies to investigate the interplay between adipose tissue and the immune system, identifying in guinea pigs that the proximity of lymph nodes to adipose tissues influences both lipolysis and lymphocyte proliferation.
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005). This study identifies how most adipose tissue macrophages coalesce around dead/dying adipocytes in obese mice and humans with obesity so as to scavenge adipocyte debris.
Bénézech, C. et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat. Immunol. 16, 819–828 (2015).
Rangel-Moreno, J. et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 30, 731–743 (2009). This is the first report to elucidate the immunological functions of omental fat-associated lymphoid structures, showing how they promote immunity to peritoneal antigens.
Portis, B. Role of omentum of rabbits, dogs and guinea-pigs in antibody production. J. Infect. Dis. 34, 159–185 (1924).
Frasca, D. & Blomberg, B. B. Adipose tissue: a tertiary lymphoid organ: does it change with age? Gerontology https://doi.org/10.1159/000502036 (2019).
Meza-Perez, S. & Randall, T. D. Immunological functions of the omentum. Trends Immunol. 38, 526–536 (2017).
Carlow, D. A., Gold, M. R. & Ziltener, H. J. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J. Immunol. 183, 1155–1165 (2009).
Perez-Shibayama, C. et al. Fibroblastic reticular cells initiate immune responses in visceral adipose tissues and secure peritoneal immunity. Sci. Immunol. 3, eaar4539 (2018).
Molofsky, A. B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Moro, K. et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Jackson-Jones, L. H. et al. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat. Commun. 7, 12651 (2016).
Jackson-Jones, L. H. et al. Stromal cells covering omental fat-associated lymphoid clusters trigger formation of neutrophil aggregates to capture peritoneal contaminants. Immunity 52, 700–715.e706 (2020).
Shimotsuma, M. et al. Morpho-physiological function and role of omental milky spots as omentum-associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology 26, 90–101 (1993).
Macdougall, C. E. & Longhi, M. P. Adipose tissue dendritic cells in steady-state. Immunology 156, 228–234 (2019).
GeurtsvanKessel, C. H. et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus–infected mice. J. Exp. Med. 206, 2339–2349 (2009).
Ansel, K. M., Harris, R. B. S. & Cyster, J. G. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16, 67–76 (2002).
Ha, S. A. et al. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 203, 2541–2550 (2006).
Shen, L. et al. B-1a lymphocytes attenuate insulin resistance. Diabetes 64, 593–603 (2015).
Buechler, M. B. et al. A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages. Immunity 51, 119–130.e115 (2019).
Mora, J. R. & von Andrian, U. H. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin. Immunol. 21, 28–35 (2009).
Liu, M., Silva-Sanchez, A., Randall, T. D. & Meza-Perez, S. Specialized immune responses in the peritoneal cavity and omentum. J. Leukoc. Biol. https://doi.org/10.1002/JLB.5MIR0720-271RR (2020).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Bertola, A. et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61, 2238–2247 (2012).
Han, S. J. et al. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity 47, 1154–1168.e1156 (2017). This is the first study to show that adipose tissue is a reservoir for memory T cells, after infection, identifying white adipose tissue as a potentially major contributor to immunological memory.
Honce, R. & Schultz-Cherry, S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front. Immunol. 10, 1071 (2019).
Misumi, I. et al. Obesity expands a distinct population of T cells in adipose tissue and increases vulnerability to infection. Cell Rep. 27, 514–524.e515 (2019).
Sheridan, P. A. et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 36, 1072–1077 (2012).
Porsche, C. E., Delproposto, J. B., Patrick, E., Zamarron, B. F. & Lumeng, C. N. Adipose tissue dendritic cell signals are required to maintain T cell homeostasis and obesity-induced expansion. Mol. Cell. Endocrinol. 505, 110740 (2020).
Combs, T. P. et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J. Biol. Chem. 280, 24085–24094 (2005).
Caljon, G. et al. The dermis as a delivery site of trypanosoma brucei for tsetse flies. PLoS Pathog. 12, e1005744 (2016).
Damouche, A. et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog. 11, e1005153 (2015).
Tanowitz, H. B., Scherer, P. E., Mota, M. M. & Figueiredo, L. M. Adipose tissue: a safe haven for parasites? Trends Parasitol. 33, 276–284 (2017).
Trindade, S. et al. Trypanosoma brucei parasites occupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe 19, 837–848 (2016).
Spalding, K. L. et al. Dynamics of fat cell turnover in humans. Nature 453, 783–787 (2008).
Anhê, F. F. et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2, 233–242 (2020).
Bourgeois, C. et al. Specific biological features of adipose tissue, and their impact on HIV persistence. Front. Microbiol. https://doi.org/10.3389/fmicb.2019.02837 (2019).
Agarwal, N. et al. HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation. Sci. Transl. Med. 5, 213ra164 (2013).
Nagajyothi, F. et al. Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity 16, 1992–1997 (2008).
Beigier-Bompadre, M. et al. Mycobacterium tuberculosis infection modulates adipose tissue biology. PLoS Pathog. 13, e1006676 (2017).
Cabalén, M. E. et al. Chronic Trypanosoma cruzi infection potentiates adipose tissue macrophage polarization toward an anti-inflammatory M2 phenotype and contributes to diabetes progression in a diet-induced obesity model. Oncotarget 7, 13400–13415 (2016).
Nagajyothi, F. et al. Mechanisms of trypanosoma cruzi persistence in chagas disease. Cell Microbiol. 14, 634–643 (2012).
Kuan, E. L. et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node–homing adipose tissue dendritic cells. J. Immunol. 194, 5200 (2015).
Ivanov, S. et al. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J. Clin. Invest. 126, 1581–1591 (2016).
Macdougall, C. E. et al. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 27, 588–601.e584 (2018).
Eisele, E. & Siliciano, R. F. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37, 377–388 (2012).
Lilienfeld, D. E., Vlahov, D., Tenney, J. H. & McLaughlin, J. S. Obesity and diabetes as risk factors for postoperative wound infections after cardiac surgery. Am. J. Infect. Control. 16, 3–6 (1988).
Nagajyothi, F. et al. Crucial role of the central leptin receptor in murine Trypanosoma cruzi (Brazil strain) infection. J. Infect. Dis. 202, 1104–1113 (2010).
Bourlier, V. et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation https://doi.org/10.1161/circulationaha.107.724096 (2008).
Wernstedt Asterholm, I. et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–118 (2014). This report is the first to show how inflammatory signals in adipose tissue are vital for healthy tissue turnover, expansion and remodelling.
Hubler, M. J., Peterson, K. R. & Hasty, A. H. Iron homeostasis: a new job for macrophages in adipose tissue? Trends Endocrinol. Metab. 26, 101–109 (2015).
Travers, R. L., Motta, A. C., Betts, J. A., Bouloumie, A. & Thompson, D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int. J. Obes. 39, 762–769 (2015).
Silva, H. M. et al. Vasculature-associated adipose tissue macrophages dynamically adapt to inflammatory and metabolic challenges. J. Exp. Med. 216, 786–806 (2019).
Lee, Y. H., Petkova, A. P. & Granneman, J. G. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 18, 355–367 (2013).
Korf, H. et al. Depicting the landscape of adipose tissue-specific macrophages and their immunometabolic signatures during obesity. Immunometabolism 2, e200001 (2019).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019).
Fischer, K. et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 23, 623–630 (2017).
Pirzgalska, R. M. et al. Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309 (2017). This is one of three reports in 2017 that identified a unique population of macrophages within adipose tissue that modulate sympathetic tone in adipose tissue through their sequestration and degradation of noradrenaline released by sympathetic nerves.
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119 (2017).
Wolf, Y. et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18, 665–674 (2017). This study demonstrates a unique population of adipose tissue macrophages that directly control innervation within brown adipose tissue by repelling sympathetic axon growth.
Bartness, T. J., Vaughan, C. H. & Song, C. K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 34 (Suppl. 1), S36–S42 (2010).
Griggio, M. A., Richard, D. & Leblanc, J. Effects of fasting and food restriction on sympathetic activity in brown adipose tissue in mice. J. Comp. Physiol. B 162, 602–606 (1992).
Seydoux, J., Assimacopoulos-Jeannet, F., Jeanrenaud, B. & Girardier, L. Alterations of brown adipose tissue in genetically obese (ob/ob) mice. I. Demonstration of loss of metabolic response to nerve stimulation and catecholamines and its partial recovery after fasting or cold adaptation. Endocrinology 110, 432–438 (1982).
Arner, P. Catecholamine-induced lipolysis in obesity. Int. J. Obes. Relat. Metab. Disord. 23 (Suppl. 1), 10–13 (1999).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011). This study is the first to establish the NLRP3 inflammasome in the pathophysiology of obesity-associated inflammation and insulin resistance.
Pang, C. et al. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Endocrinol. Metab. 295, E313–E322 (2008).
Cho, C. H. et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ. Res. 100, e47–e57 (2007).
Lim, H. Y. et al. Hyaluronan receptor LYVE-1-expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity 49, 326–341.e327 (2018).
Watanabe, S. & Boucrot, E. Fast and ultrafast endocytosis. Curr. Opin. Cell Biol. 47, 64–71 (2017).
Weinstock, A. et al. Single-cell RNA sequencing of visceral adipose tissue leukocytes reveals that caloric restriction following obesity promotes the accumulation of a distinct macrophage population with features of phagocytic cells. Immunometabolism https://doi.org/10.20900/immunometab20190008 (2019).
Alemán, J. O. et al. Effects of rapid weight loss on systemic and adipose tissue inflammation and metabolism in obese postmenopausal women. J. Endocr. Soc. 1, 625–637 (2017).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. https://doi.org/10.1016/j.cmet.2014.08.010 (2014).
Coats, B. R. et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 20, 3149–3161 (2017).
Xu, X. et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013).
Jaitin, D. A. et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178, 686–698.e614 (2019). This study produces the first single-cell atlas to compare both mouse and human white adipose tissue and identifies a unique population of LAMs that regulate tissue homeostasis in obesity.
Lynch, L. et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37, 574–587 (2012).
Lynch, L. et al. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 39, 1893–1901 (2009).
Lynch, L. Adipose invariant natural killer T cells. Immunology 142, 337–346 (2014).
Huh, J. Y. et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol. Cell Biol. 33, 328–339 (2013).
Rakhshandehroo, M. et al. CD1d-mediated presentation of endogenous lipid antigens by adipocytes requires microsomal triglyceride transfer protein. J. Biol. Chem. 289, 22128–22139 (2014).
Rakhshandehroo, M. et al. Adipocytes harbor a glucosylceramide biosynthesis pathway involved in iNKT cell activation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 1157–1167 (2019).
Park, J. et al. Activation of invariant natural killer T cells stimulates adipose tissue remodeling via adipocyte death and birth in obesity. Genes Dev. 33, 1657–1672 (2019).
LaMarche, N. M. et al. Distinct iNKT cell populations use IFNγ or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab. https://doi.org/10.1016/j.cmet.2020.05.017 (2020).
Wu, L. et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl Acad. Sci. USA 109, E1143–E1152 (2012).
Pamir, N. et al. Granulocyte/macrophage colony-stimulating factor-dependent dendritic cells restrain lean adipose tissue expansion. J. Biol. Chem. 290, 14656–14667 (2015).
Mattacks, C. A., Sadler, D. & Pond, C. M. The control of lipolysis in perinodal and other adipocytes by lymph node and adipose tissue-derived dendritic cells in rats. Adipocytes 1, 43–56 (2005).
Ibrahim, J. et al. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology 143, 1061–1072 (2012).
Herber, D. L. et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 16, 880–886 (2010).
Cao, W. et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J. Immunol. 192, 2920 (2014).
Cipolletta, D. et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue T-reg cells. Nature 486, 549–U151 (2012).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009). This is the first study to identify that lean adipose tissue is host to a unique population of resident Treg cells that protect metabolic homeostasis within the tissue.
Jones, J. R. et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl Acad. Sci. USA 102, 6207–6212 (2005).
Elieh Ali Komi, D., Shafaghat, F. & Christian, M. Crosstalk between mast cells and adipocytes in physiologic and pathologic conditions. Clin. Rev. Allergy Immunol. 58, 388–400 (2020).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009).
Hirai, S. et al. Involvement of mast cells in adipose tissue fibrosis. Am. J. Physiol. Endocrinol. Metab. 306, E247–E255 (2013).
Tanaka, A., Nomura, Y., Matsuda, A., Ohmori, K. & Matsuda, H. Mast cells function as an alternative modulator of adipogenesis through 15-deoxy-delta-12, 14-prostaglandin J2. Am. J. Physiol. Cell Physiol. 301, C1360–C1367 (2011).
Saslow, L. R. et al. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE 9, e91027 (2014).
Grey, N. J., Karl, I. & Kipnis, D. M. Physiologic mechanisms in the development of starvation ketosis in man. Diabetes 24, 10–16 (1975).
Goldberg, E. L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2, 50–61 (2020).
Betz, M. J. & Enerback, S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 14, 77–87 (2018).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019).
Villarroya, J. et al. New insights into the secretory functions of brown adipose tissue. J. Endocrinol. https://doi.org/10.1530/joe-19-0295 (2019).
Mahlakõiv, T. et al. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 4, eaax0416 (2019). This report identifies the importance of stromal cell-derived IL-33 in regulating immune cell homeostasis and anti-inflammatory signals in adipose tissue.
Spallanzani, R. G. et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 4, eaaw3658 (2019).
Hu, B. et al. gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020).
Hu, Y. et al. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 184, 4307–4316 (2010).
Lee, M.-W. et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015). This is the first study to show the importance of ILC2s in the regulation of adipose tissue function and as a source of Met-Enk, directly inducing adipocyte beiging.
Medrikova, D. et al. Brown adipose tissue harbors a distinct sub-population of regulatory T cells. PLoS ONE 10, e0118534 (2015).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Kolodin, D. et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21, 543–557 (2015).
Gnad, T. et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014).
Figueiro, F. et al. Methotrexate up-regulates ecto-5′-nucleotidase/CD73 and reduces the frequency of T lymphocytes in the glioblastoma microenvironment. Purinergic Signal. 12, 303–312 (2016).
Fakhimi, M., Talei, A. R., Ghaderi, A., Habibagahi, M. & Razmkhah, M. Helios, CD73 and CD39 induction in regulatory T cells exposed to adipose derived mesenchymal stem cells. Cell J. 22, 236–244 (2020).
Schuler, P. J. et al. Human CD4+CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 177, 531–543 (2014).
Lynch, L. et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab. 24, 510–519 (2016).
Fabbiano, S. et al. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 24, 434–446 (2016).
Qiu, Y. et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014).
Villarroya, F., Cereijo, R., Villarroya, J., Gavalda-Navarro, A. & Giralt, M. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab. 27, 954–961 (2018).
Garcia-Zepeda, E. A. et al. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat. Med. 2, 449–456 (1996).
Knights, A. J. et al. Eosinophil function in adipose tissue is regulated by Krüppel-like factor 3 (KLF3). Nat. Commun. 11, 2922 (2020).
Löffler, D. et al. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int. J. Obes. 41, 112–119 (2017).
Li, Z.-Y. et al. Subfatin is a novel adipokine and unlike meteorin in adipose and brain expression. CNS Neurosci. Ther. 20, 344–354 (2014).
Rao, R. R. et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014).
Yabut, J. M. et al. Genetic deletion of mast cell serotonin synthesis prevents the development of obesity and insulin resistance. Nat. Commun. 11, 463 (2020).
Zhang, X. et al. Functional inactivation of mast cells enhances subcutaneous adipose tissue browning in mice. Cell Rep. 28, 792–803.e794 (2019).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Moysidou, M. et al. CD8+ T cells in beige adipogenesis and energy homeostasis. JCI Insight 3, e95456 (2018).
Kintscher, U. et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 28, 1304–1310 (2008).
McLaughlin, T. et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 34, 2637–2643 (2014).
Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929 (2009).
Pandolfi, J. B. et al. ATP-induced inflammation drives tissue-resident Th17 cells in metabolically unhealthy obesity. J. Immunol. https://doi.org/10.4049/jimmunol.1502506 (2016).
Cipolletta, D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 142, 517–525 (2014).
Kalin, S. et al. A Stat6/Pten axis links regulatory T cells with adipose tissue function. Cell Metab. 26, 475–492.e477 (2017).
Toubal, A. et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat. Commun. 11, 3755 (2020).
Magalhaes, I. et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J. Clin. Invest. 125, 1752–1762 (2015).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. https://doi.org/10.1016/j.cmet.2019.10.006 (2019).
Frasca, D. et al. Obesity decreases B cell responses in young and elderly individuals. Obesity 24, 615–625 (2016).
Frasca, D. & Blomberg, B. B. Adipose tissue inflammation induces B cell inflammation and decreases B cell function in aging. Front. Immunol. https://doi.org/10.3389/fimmu.2017.01003 (2017).
Srikakulapu, P. & McNamara, C. A. B lymphocytes and adipose tissue inflammation. Arterioscler. Thromb. Vasc. Biol. 40, 1110–1122 (2020).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011). This is the first study to identify B cells as a key player in the inflammatory dysregulation that occurs within adipose tissue in obesity, and that they also secrete autoantibodies that impair insulin signalling.
Frasca, D. et al. Identification and characterization of adipose tissue-derived human antibodies with “anti-self” specificity. Front. Immunol. https://doi.org/10.3389/fimmu.2020.00392 (2020).
Ghosh, A. R. et al. Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation. Diabetes 65, 3440–3452 (2016).
Boulenouar, S. et al. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity 46, 273–286 (2017).
Wang, H. et al. Adipose group 1 innate lymphoid cells promote adipose tissue fibrosis and diabetes in obesity. Nat. Commun. 10, 3254 (2019).
Oldenhove, G. et al. PD-1 is involved in the dysregulation of type 2 innate lymphoid cells in a murine model of obesity. Cell Rep. 25, 2053–2060.e2054 (2018).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018). This study establishes how the lipotoxic environment in obesity causes a metabolic reprogramming of natural killer cells, impairing their immunosurveillance capabilities, establishing another link between obesity and cancer.
Theurich, S. et al. IL-6/Stat3-dependent induction of a distinct, obesity-associated NK cell subpopulation deteriorates energy and glucose homeostasis. Cell Metab. 26, 171–184.e176 (2017).
Lee, B.-C. et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab. 23, 685–698 (2016).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011). This is the first study to identify the importance of eosinophils in maintaining adipose tissue metabolic and inflammatory homeostasis via their production of IL-4 and IL-13.
Lee, E.-H. et al. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci. Rep. 8, 9894 (2018).
Bolus, W. R., Kennedy, A. J. & Hasty, A. H. Obesity-induced reduction of adipose eosinophils is reversed with low-calorie dietary intervention. Physiol. Rep. 6, e13919 (2018).
Brigger, D. et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat. Metab. 2, 688–702 (2020).
Watanabe, Y. et al. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J. 33, 11821–11835 (2019).
Talukdar, S. et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 (2012). This study identifies neutrophils as the first leukocytes to infiltrate adipose tissue in response to a high-fat diet, thereby exacerbating inflammatory and metabolic dysfunction.
Weisberg, S. P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 (2006).
Amano, S. U. et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. https://doi.org/10.1016/j.cmet.2013.11.017 (2014).
Ye, J. & Keller, J. N. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging 2, 361–368 (2010).
Speakman, J. R. The evolution of body fatness: trading off disease and predation risk. J. Exp. Biol. 221, jeb167254 (2018).
Ganeshan, K. et al. Energetic trade-offs and hypometabolic states promote disease tolerance. Cell 177, 399–413.e312 (2019).
Lord, G. M. et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901 (1998). This study identifies leptin as a potent modulator of T cell function, identifying a direct communication network between adipocytes and the immune system.
Kovacikova, M. et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int. J. Obes. https://doi.org/10.1038/ijo.2010.112 (2011).
Jordan, S. et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114.e1117 (2019).
Nagai, M. et al. Fasting-refeeding impacts immune cell dynamics and mucosal immune responses. Cell 178, 1072–1087.e1014 (2019).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101.e1015 (2019).
Cain, D. W. & Cidlowski, J. A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 17, 233–247 (2017).
Kosteli, A. et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120, 3466–3479 (2010).
Fazeli, P. K. et al. Prolonged fasting drives a program of metabolic inflammation in human adipose tissue. Mol. Metab. 42, 101082 (2020).
Nei, M., Xu, P. & Glazko, G. Estimation of divergence times from multiprotein sequences for a few mammalian species and several distantly related organisms. Proc. Natl Acad. Sci. USA 98, 2497 (2001).
Shay, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl Acad. Sci. USA 110, 2946 (2013).
Laparra, A. et al. The frequencies of immunosuppressive cells in adipose tissue differ in human, non-human primate, and mouse models. Front. Immunol. 10, 117–117 (2019).
Harman-Boehm, I. et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 92, 2240–2247 (2007).
Mestas, J. & Hughes, C. C. W. Of Mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731 (2004).
Haley, P. J. Species differences in the structure and function of the immune system. Toxicology 188, 49–71 (2003).
Valiathan, R., Ashman, M. & Asthana, D. Effects of ageing on the immune system: infants to elderly. Scand. J. Immunol. 83, 255–266 (2016).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Tao, L. & Reese, T. A. Making mouse models that reflect human immune responses. Trends immunol. 38, 181–193 (2017).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Giordano, A., Frontini, A. & Cinti, S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 15, 405–424 (2016).
Carpentier, A. C. et al. Brown adipose tissue energy metabolism in humans. Front. Endocrinol. https://doi.org/10.3389/fendo.2018.00447 (2018).
Ekelund, U. et al. Physical activity but not energy expenditure is reduced in obese adolescents: a case-control study. Am. J. Clin. Nutr. 76, 935–941 (2002).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Dutton, G. R. et al. 25-year weight gain in a racially balanced sample of U.S. adults: the CARDIA study. Obesity 24, 1962–1968 (2016).
Sanghvi, A., Redman, L. M., Martin, C. K., Ravussin, E. & Hall, K. D. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am. J. Clin. Nutr. 102, 353–358 (2015).
Muller, M. J., Enderle, J. & Bosy-Westphal, A. Changes in energy expenditure with weight gain and weight loss in humans. Curr. Obes. Rep. 5, 413–423 (2016).
Muller, M. J. et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am. J. Clin. Nutr. 102, 807–819 (2015).
Acknowledgements
This work was supported by NIH grant R01 AI134861, ERC Starting Grant (679173), SFI FRL3865 and an American Diabetes Association grant (1-16-JDF-061).
Author information
Authors and Affiliations
Contributions
W.V.T. and L.L. contributed equally to the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks Sean Hartig, Cecile Benezec and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Parabiotic
-
The surgical attachment of two living organisms (mice) that allows the connection of circulatory systems.
- Innate-like B cell
-
An unconventional B cell that responds to innate signals such as Toll-like receptor agonists and microbial pathogens. Innate-like B cells produce regulatory cytokines (for example, IL-10) and large amounts of natural antibodies (primarily IgM) in the steady state, and provide early responses to infectious challenges.
- Crowning macrophages
-
Large clusters of macrophages that coalesce around dead or dying adipocytes to facilitate the clearance and sequestration of cellular debris and lipids.
- Matrix-induced neogenesis
-
The process by which, in the case of adipose tissue, macrophages interact with the extracellular matrix proteins that facilitate the recruitment, proliferation and differentiation of adipocyte progenitor cells, thereby leading to adipose tissue remodelling and adipogenesis.
- Adipocyte browning and thermogenesis
-
Adipocyte browning is the adaptive process white adipocytes undergo in response to often environmentally derived signals such as cold exposure whereupon they increase their rate of mitochondrial biogenesis and mitochondrial respiration. Adipocyte thermogenesis is driven by the uncoupling of oxidative metabolism from ATP production, resulting in proton leakage across the mitochondrial inner membrane, eventually leading to heat production.
- NLRP3 inflammasome
-
A multimeric protein complex that acts as an intracellular sensor for a wide-range of environmental and endogenous danger signals. Activation of the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome initiates inflammation-induced cell death and triggers the release of IL-1β and IL-18.
- Ketogenic diet
-
A high-fat, low-carbohydrate diet that drives a reliance on fats over carbohydrates for whole-body energetic needs. The resultant lack of available carbohydrates brings about the biochemical production of ketone bodies from fatty acids by the liver, inducing a metabolic state called ‘ketosis’.
- Vγ6+δ1+ T cells
-
A subpopulation of γδ T cells characterized by their distinct T cell receptor γ and δ variable chains that specifically home (by an unknown mechanism) to, among other sites, adipose tissue.
Rights and permissions
About this article
Cite this article
Trim, W.V., Lynch, L. Immune and non-immune functions of adipose tissue leukocytes. Nat Rev Immunol 22, 371–386 (2022). https://doi.org/10.1038/s41577-021-00635-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-021-00635-7
This article is cited by
-
Glucocorticoids increase adiposity by stimulating Krüppel-like factor 9 expression in macrophages
Nature Communications (2024)
-
Cytokine production by bovine adipose tissue stromal vascular fraction cells upon Neospora caninum stimulation
Scientific Reports (2024)
-
Calorie restriction-induced leptin reduction and T-lymphocyte activation in blood and adipose tissue in men with overweight and obesity
International Journal of Obesity (2024)
-
Metabolic Derangement by Arsenic: a Review of the Mechanisms
Biological Trace Element Research (2024)
-
Adipocyte p53 coordinates the response to intermittent fasting by regulating adipose tissue immune cell landscape
Nature Communications (2024)