Abstract
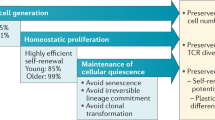
Age-related T cell dysfunction can lead to failure of immune tolerance mechanisms, resulting in aberrant T cell-driven cytokine and cytotoxic responses that ultimately cause tissue damage. In this Review, we discuss the role of T cells in the onset and progression of age-associated conditions, focusing on cardiovascular disorders, metabolic dysfunction, neuroinflammation and defective tissue repair and regeneration. We present different mechanisms by which T cells contribute to inflammageing and might act as modulators of age-associated diseases, including through enhanced pro-inflammatory and cytotoxic activity, defective clearance of senescent cells or regulation of the gut microbiota. Finally, we propose that ‘resetting’ immune system tolerance or targeting pathogenic T cells could open up new therapeutic opportunities to boost resilience to age-related diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019).
Horwitz, D. A., Fahmy, T. M., Piccirillo, C. A. & La Cava, A. Rebalancing immune homeostasis to treat autoimmune diseases. Trends Immunol. 40, 888–908 (2019).
Metchnikoff, I. I. The Prolongation of Life: Optimistic Studies (Springer, 2004).
Goronzy, J. J. & Weyand, C. M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 19, 573–583 (2019).
Elyahu, Y. et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci. Adv. 5, eaaw8330 (2019).
Akbar, A. N. & Henson, S. M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 11, 289–295 (2011).
Yu, Y. R. et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat. Immunol. 21, 1540–1551 (2020).
Callender, L. A. et al. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell 19, e13067 (2020).
Ucar, D. et al. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med. 214, 3123–3144 (2017).
Callender, L. A., Carroll, E. C., Bober, E. A. & Henson, S. M. Divergent mechanisms of metabolic dysfunction drive fibroblast and T-cell senescence. Ageing Res. Rev. 47, 24–30 (2018).
Lanna, A., Henson, S. M., Escors, D. & Akbar, A. N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 15, 965–972 (2014).
Callender, L. A. et al. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 17, e12675 (2018).
Pereira, B. I. et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 21, 684–694 (2020).
Rodriguez, I. J. et al. Immunosenescence study of T cells: a systematic review. Front. Immunol. 11, 604591 (2021).
Yoshida, S. et al. The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nat. Commun. 11, 2482 (2020).
Shirakawa, K. et al. Obesity accelerates T cell senescence in visceral adipose tissue. J. Clin. Invest. 126, 4626–4639 (2016).
Yi, H. S. et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 10, 249 (2019).
Mogilenko, D. A. et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+CD8+ T cells as conserved hallmark of inflammaging. Immunity 54, 99–115.e12 (2020).
Covre, L. P., De Maeyer, R. P. H., Gomes, D. C. O. & Akbar, A. N. The role of senescent T cells in immunopathology. Aging Cell 19, e13272 (2020).
Derhovanessian, E. et al. Hallmark features of immunosenescence are absent in familial longevity. J. Immunol. 185, 4618–4624 (2010).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Biol. Sci. Med. Sci. 69, S4–S9 (2014).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32, 44–55 (2020).
Baixauli, F. et al. Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab. 22, 485–498 (2015).
Ekiz, H. A. et al. T cell-expressed microRNA-155 reduces lifespan in a mouse model of age-related chronic inflammation. J. Immunol. 204, 2064–2075 (2020).
Faust, H. J. et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Invest. 130, 5493–5507 (2020).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Hashimoto, K. et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc. Natl Acad. Sci. USA 116, 24242–24251 (2019).
Petersen, C. et al. T cell-mediated regulation of the microbiota protects against obesity. Science 365, eaat9351 (2019).
Pérez, M. M. et al. Interleukin-17/interleukin-17 receptor axis elicits intestinal neutrophil migration, restrains gut dysbiosis and lipopolysaccharide translocation in high-fat diet-induced metabolic syndrome model. Immunology 156, 339–355 (2019).
Gisterå, A. & Hansson, G. K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 13, 368–380 (2017).
Saigusa, R., Winkels, H. & Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 17, 387–401 (2020).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019).
Frostegård, J. et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (TH1) and macrophage-stimulating cytokines. Atherosclerosis 145, 33–43 (1999).
Buono, C. et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA 102, 1596–1601 (2005).
Tsilingiri, K. et al. Oxidized low-density lipoprotein receptor in lymphocytes prevents atherosclerosis and predicts subclinical disease. Circulation 139, 243–255 (2019).
Sato, K. et al. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 203, 239–250 (2006).
Ramos, G. C. et al. Myocardial aging as a T-cell-mediated phenomenon. Proc. Natl Acad. Sci. USA 114, E2420–E2429 (2017).
Padgett, L. E. et al. Naive CD8+ T cells expressing CD95 increase human cardiovascular disease severity. Arterioscler. Thromb. Vasc. Biol. 40, 2845–2859 (2020).
Kyaw, T. et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in ApoE-deficient mice. Circulation 127, 1028–1039 (2013).
Van Duijn, J. et al. CD8+ T-cells contribute to lesion stabilization in advanced atherosclerosis by limiting macrophage content and CD4+ T-cell responses. Cardiovasc. Res. 115, 729–738 (2019).
Zhou, H. et al. CD43-mediated IFN-γ production by CD8+ T cells promotes abdominal aortic aneurysm in mice. J. Immunol. 190, 5078–5085 (2013).
Nus, M. et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med. 23, 601–610 (2017).
Gaddis, D. E. et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 9, 1095 (2018).
Wigren, M. et al. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler. Thromb. Vasc. Biol. 32, 2000–2007 (2012).
Meng, X. et al. Regulatory T cells in cardiovascular diseases. Nat. Rev. Cardiol. 13, 167–179 (2016).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12, 178–180 (2006).
Yin, M. et al. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 30, 1825–1831 (2010).
Lin, J. et al. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J. Lipid Res. 51, 1208–1217 (2010).
Robertson, A. K. L. et al. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J. Clin. Invest. 112, 1342–1350 (2003).
Sharma, M. et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ. Res. 127, 335–353 (2020).
Meng, X. et al. Regulatory T cells prevent angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E knockout mice. Hypertension 64, 875–882 (2014).
Xia, N. et al. A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction. Circulation 142, 1956–1973 (2020).
Zacchigna, S. et al. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat. Commun. 9, 2432 (2018).
Wolf, D. et al. Pathogenic autoimmunity in atherosclerosis evolves from initially protective apolipoprotein B100-reactive CD4+ T-regulatory cells. Circulation 142, 1279–1293 (2020).
Rieckmann, M. et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J. Clin. Invest. 129, 4922–4936 (2019).
Li, Y. et al. A CD1d-dependent lipid antagonist to NKT cells ameliorates atherosclerosis in ApoE–/– mice by reducing lesion necrosis and inflammation. Cardiovasc. Res. 109, 305–317 (2016).
Tupin, E. et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 199, 417–422 (2004).
Wang, H. X. et al. CD1d-dependent natural killer T cells attenuate angiotensin II-induced cardiac remodelling via IL-10 signalling in mice. Cardiovasc. Res. 115, 83–93 (2019).
Phoksawat, W. et al. IL-17 and IFN-γ productions by CD4+ T cells and T cell subsets expressing NKG2D associated with the number of risk factors for cardiovascular diseases. Mol. Immunol. 122, 193–199 (2020).
Spyridopoulos, I. et al. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell 15, 389–392 (2016).
Wang, H. et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta-analysis of prospective studies up to 2016. J. Am. Heart Assoc. 6, e005025 (2017).
Bergström, I., Backteman, K., Lundberg, A., Ernerudh, J. & Jonasson, L. Persistent accumulation of interferon-γ-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis 224, 515–520 (2012).
Koller, L. et al. CD4+CD28null cells are an independent predictor of mortality in patients with heart failure. Atherosclerosis 230, 414–416 (2013).
Youn, J. C. et al. Increased frequency of CD4+CD57+ senescent T cells in patients with newly diagnosed acute heart failure: exploring new pathogenic mechanisms with clinical relevance. Sci. Rep. 9, 12887 (2019).
Haach, F. et al. Characterization of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell. Immunol. 281, 11–19 (2013).
Liuzzo, G. et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101, 2883–2888 (2000).
Kovalcsik, E., Antunes, R. F., Baruah, P., Kaski, J. C. & Dumitriu, I. E. Proteasome-mediated reduction in proapoptotic molecule bim renders CD4+CD28null T cells resistant to apoptosis in acute coronary syndrome. Circulation 131, 709–720 (2015).
Pan, X., Wu, F., Chen, X. & Chen, D. T cell senescence accelerates angiotensin II-induced target organ damage. Cardiovasc. Res. 117, 271–283 (2021).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Rocha, V. Z. et al. Interferon-γ, a TH1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ. Res. 103, 467–476 (2008).
Jagannathan-Bogdan, M. et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 186, 1162–1172 (2011).
Priceman, S. J. et al. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc. Natl Acad. Sci. USA 110, 13079–13084 (2013).
Lumeng, C. N. et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 187, 6208–6216 (2011).
Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929 (2009).
Deng, T. et al. Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nat. Commun. 8, 15725 (2017).
Bertola, A. et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing TH17 responses in mice and patients. Diabetes 61, 2238–2247 (2012).
Revelo, X. S. et al. Perforin is a novel immune regulator of obesity-related insulin resistance. Diabetes 64, 90–103 (2015).
Stolarczyk, E. et al. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell Metab. 17, 520–533 (2013).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Cipolletta, D., Cohen, P., Spiegelman, B. M., Benoist, C. & Mathis, D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARγ effects. Proc. Natl Acad. Sci. USA 112, 482–487 (2015).
Bapat, S. P. et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528, 137–141 (2015).
Li, C. et al. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive fat-Treg phenotype. Cell 174, 285–299.e12 (2018).
Kolodin, D. et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21, 543–557 (2015).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Kohlgruber, A. C. et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19, 464–474 (2018).
Lynch, L. et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37, 574–587 (2012).
Mehta, P., Nuotio-Antar, A. M. & Smith, C. W. γδ T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J. Leukoc. Biol. 97, 121–134 (2015).
LaMarche, N. M. et al. Distinct iNKT cell populations use IFNγ or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab. 32, 243–258.e6 (2020).
Goldberg, E. L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2, 50–61 (2020).
Lee, Y. H. O. et al. Senescent T cells predict the development of hyperglycemia in humans. Diabetes 68, 156–162 (2019).
Lau, E. Y. M. et al. Type 2 diabetes is associated with the accumulation of senescent T cells. Clin. Exp. Immunol. 197, 205–213 (2019).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Sweeney, M. D., Kisler, K., Montagne, A., Toga, A. W. & Zlokovic, B. V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 21, 1318–1331 (2018).
Kebir, H. et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat. Med. 13, 1173–1175 (2007).
Smolders, J. et al. Tissue-resident memory T cells populate the human brain. Nat. Commun. 9, 4593 (2018).
Herich, S. et al. Human CCR5 high effector memory cells perform CNS parenchymal immune surveillance via GZMK-mediated transendothelial diapedesis. Brain 142, 3411–3427 (2019).
Kunis, G. et al. IFN-γ-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain 136, 3427–3440 (2013).
Brynskikh, A., Warren, T., Zhu, J. & Kipnis, J. Adaptive immunity affects learning behavior in mice. Brain. Behav. Immun. 22, 861–869 (2008).
Kipnis, J., Cohen, H., Cardon, M., Ziv, Y. & Schwartz, M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl Acad. Sci. USA 101, 8180–8185 (2004).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Pasciuto, E. et al. Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell 182, 625–640.e24 (2020).
Filiano, A. J. et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535, 425–429 (2016).
Fan, K. Q. I. et al. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179, 864–879.e19 (2019).
Lima, K. A. De et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 21, 1421–1429 (2020).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019).
Ritzel, R. M. et al. Age-associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J. Immunol. 196, 3318–3330 (2016).
Meng, H. et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc. Natl Acad. Sci. USA 116, 5558–5563 (2019).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020).
Monsonego, A. et al. Increased T cell reactivity to amyloid β protein in older humans. J. Clin. Invest. 112, 415–422 (2003).
Dhanwani, R. et al. T cell responses to neural autoantigens are similar in Alzheimer’s disease patients and age-matched healthy controls. Front. Neurosci. 14, 874 (2020).
Sulzer, D. et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546, 656–661 (2017).
Lindestam Arlehamn, C. S. et al. α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 11, 1875 (2020).
Baruch, K. et al. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 6, 7967 (2015).
Dansokho, C. et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251 (2016).
Ito, M. et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 (2019).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Whibley, N., Tucci, A. & Powrie, F. Regulatory T cell adaptation in the intestine and skin. Nat. Immunol. 20, 386–396 (2019).
Nielsen, M. M., Witherden, D. A. & Havran, W. L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 17, 733–745 (2017).
Sharp, L. L., Jameson, J. M., Cauvi, G. & Havran, W. L. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 6, 73–79 (2005).
Boismenu, R. & Havran, W. L. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science 266, 1253–1255 (1994).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Santiago, A. F. et al. Aging correlates with reduction in regulatory-type cytokines and T cells in the gut mucosa. Immunobiology 216, 1085–1093 (2011).
Weaver, C. T., Elson, C. O., Fouser, L. A. & Kolls, J. K. The TH17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. Mech. Dis. 8, 477–512 (2013).
Pascual-Reguant, A. et al. TH17 cells express ST2 and are controlled by the alarmin IL-33 in the small intestine. Mucosal Immunol. 10, 1431–1442 (2017).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Naik, S. et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015).
Jameson, J. et al. A role for skin γδ T cells in wound repair. Science 296, 747–749 (2002).
Toulon, A. et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206, 743–750 (2009).
Keyes, B. E. et al. Impaired epidermal to dendritic T cell signaling slows wound repair in aged skin. Cell 167, 1323–1338.e14 (2016).
Nosbaum, A. et al. Cutting edge: regulatory T cells facilitate cutaneous wound healing. J. Immunol. 196, 2010–2014 (2016).
Villalta, S. A. et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci. Transl Med. 6, 258ra142 (2014).
Kuswanto, W. et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 44, 355–367 (2016).
Mock, J. R. et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol. 7, 1440–1451 (2014).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Arpaia, N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015).
Zaiss, D. M. W. et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 38, 275–284 (2013).
Dial, C. F., Tune, M. K., Doerschuk, C. M. & Mock, J. R. Foxp31 regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am. J. Respir. Cell Mol. Biol. 57, 162–173 (2017).
Hui, S. P. et al. Zebrafish regulatory T cells mediate organ-specific regenerative programs. Dev. Cell 43, 659–672.e5 (2017).
Linehan, J. L. et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 172, 784–796.e18 (2018).
Covre, L. P. et al. Circulating senescent T cells are linked to systemic inflammation and lesion size during human cutaneous leishmaniasis. Front. Immunol. 10, 3001 (2019).
Milling, S. Ageing dangerously; homing of senescent CD8 T cells in cutaneous leishmaniasis. Immunology 159, 355–356 (2020).
Bucher, C. H. et al. Experience in the adaptive immunity impacts bone homeostasis, remodeling, and healing. Front. Immunol. 10, 797 (2019).
Ali, N. et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129.e11 (2017).
Fu, X. et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 25, 655–673 (2015).
Biton, M. et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175, 1307–1320.e22 (2018).
Fu, Y. Y. et al. T cell recruitment to the intestinal stem cell compartment drives immune-mediated intestinal damage after allogeneic transplantation. Immunity 51, 90–103.e3 (2019).
Takashima, S. et al. T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage. Sci. Immunol. 4, eaay8556 (2019).
Schreurs, R. R. C. E. et al. Human fetal TNF-α-cytokine-producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 50, 462–476.e8 (2019).
DeJong, E. N., Surette, M. G. & Bowdish, D. M. E. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 28, 180–189 (2020).
Bunker, J. J. & Bendelac, A. IgA responses to microbiota. Immunity 49, 211–224 (2018).
Hirota, K. et al. Plasticity of TH17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14, 372–379 (2013).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Rezende, R. M. et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat. Commun. 9, 3151 (2018).
Wang, S. et al. MyD88 adaptor-dependent microbial sensing by regulatory T cells promotes mucosal tolerance and enforces commensalism. Immunity 43, 289–303 (2015).
Neumann, C. et al. c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host–microbiota homeostasis. Nat. Immunol. 20, 471–481 (2019).
Sáez de Guinoa, J. et al. CD 1d-mediated lipid presentation by CD 11c+ cells regulates intestinal homeostasis. EMBO J. 37, e97537 (2018).
Kubinak, J. L. et al. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17, 153–163 (2015).
Kühn, F. et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 5, e134049 (2020).
Sato, S., Kiyono, H. & Fujihashi, K. Mucosal immunosenescence in the gastrointestinal tract: a mini-review. Gerontology 61, 336–342 (2015).
Stebegg, M. et al. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun. 10, 2443 (2019).
Sage, P. T., Tan, C. L., Freeman, G. J., Haigis, M. & Sharpe, A. H. Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep. 12, 163–171 (2015).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466.e4 (2017).
Clark, R. I. et al. Distinct shifts in microbiota composition during drosophila aging impair intestinal function and drive mortality. Cell Rep. 12, 1656–1667 (2015).
Vujkovic-Cvijin, I. et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat. Commun. 11, 2448 (2020).
Brenchley, J. M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Zeng, M. Y. et al. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44, 647–658 (2016).
Perruzza, L. et al. T follicular helper cells promote a beneficial gut ecosystem for host metabolic homeostasis by sensing microbiota-derived extracellular ATP. Cell Rep. 18, 2566–2575 (2017).
Pomié, C. et al. Triggering the adaptive immune system with commensal gut bacteria protects against insulin resistance and dysglycemia. Mol. Metab. 5, 392–403 (2016).
Garidou, L. et al. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. 22, 100–112 (2015).
Ang, Q. Y. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal TH17 cells. Cell 181, 1263–1275.e16 (2020).
Martins, L. M. S. et al. Interleukin-23 promotes intestinal T helper type 17 immunity and ameliorates obesity-associated metabolic syndrome in a murine high-fat diet model. Immunology 154, 624–636 (2018).
Wang, X. et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514, 237–241 (2014).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Fatkhullina, A. R. et al. An interleukin-23–interleukin-22 axis regulates intestinal microbial homeostasis to protect from diet-induced atherosclerosis. Immunity 49, 943–957.e9 (2018).
Yamashita, T. et al. Intestinal immunity and gut microbiota as therapeutic targets for preventing atherosclerotic cardiovascular diseases. Circ. J. 79, 1882–1890 (2015).
Benakis, C. et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22, 516–523 (2016).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Lutgens, E. et al. Immunotherapy for cardiovascular disease. Eur. Heart J. 40, 3937–3946 (2019).
Baruch, K. et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 22, 135–137 (2016).
Lee, A. H. & Dixit, V. D. Dietary regulation of immunity. Immunity 53, 510–523 (2020).
Dahan, S., Segal, Y. & Shoenfeld, Y. Dietary factors in rheumatic autoimmune diseases: a recipe for therapy? Nat. Rev. Rheumatol. 13, 348–358 (2017).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Delgoffe, G. M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Pollizzi, K. N. & Powell, J. D. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 36, 13–20 (2015).
Bárcena, C. et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242 (2019).
Ahmadi, S. et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 5, e132055 (2020).
Nagpal, R. et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr. Heal. Aging 4, 267–285 (2018).
Acknowledgements
The authors thank M. N. Navarro and G. Soto-Heredero for helpful comments on the manuscript. This study was supported by the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI19/855), the European Regional Development Fund (ERDF) and the European Commission through H2020-EU.1.1, European Research Council grant ERC-2016-StG 715322-EndoMitTalk and the Madrid Government (Comunidad de Madrid-Spain) under the Multiannual Agreement with Universidad Autónoma de Madrid in the line of action encouraging youth research doctors, in the context of the V PRICIT (Regional Programme of Research and Technological Innovation) (SI1/PJI/2019-00073). M.M. is supported by the Miguel Servet Program (CP 19/014, Fundación de Investigación del Hospital 12 de Octubre). M.M.G.H. and E.G.-R. are supported by an FPU grant (FPU19/02576) and a Juan de la Cierva grant (IJC2018-036850-I), respectively, both from Ministerio de Ciencia, Innovación y Universidades (Spain).
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to this work. Conceptualization: M.M., E.C. and G.D.-M.; writing — original draft preparation: E.C., E.G.-R., M.M.G.H., G.D.-M., J.F.A. and M.M.; preparation of figures: M.M.G.H.; review and editing: E.C., E.G.-R., M.M.G.H. and M.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks D. Winer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- T effector memory CD45RA+ (TEMRA) cells
-
A subset of human memory T cells. TEMRA cells re-express the naive T cell-associated marker CD45RA and display multiple characteristics associated with senescence.
- Inflammageing
-
Low-grade chronic inflammation in the absence of infection that appears in association with ageing.
- Senescence-associated secretory phenotype
-
(SASP). Cellular response associated with the irreversible arrest of cell proliferation and consisting of the release of cytokines, chemokines, proteases and growth factors that affect nearby cells in a paracrine manner.
- Senescence surveillance
-
Immune-mediated clearance of senescent cells.
- Dysbiosis
-
Abnormal shifts in the microbiota composition and in the associated microbiota-derived metabolites.
- Bacterial translocation
-
The leakage of viable bacteria and/or their by-products from the intestinal lumen to peripheral tissues, such as the mesenteric lymph nodes, the adipose tissue or the liver.
Rights and permissions
About this article
Cite this article
Carrasco, E., Gómez de las Heras, M.M., Gabandé-Rodríguez, E. et al. The role of T cells in age-related diseases. Nat Rev Immunol 22, 97–111 (2022). https://doi.org/10.1038/s41577-021-00557-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-021-00557-4
This article is cited by
-
CD4 T-cell aging exacerbates neuroinflammation in a late-onset mouse model of amyotrophic lateral sclerosis
Journal of Neuroinflammation (2024)
-
Immunophenotypes in psychosis: is it a premature inflamm-aging disorder?
Molecular Psychiatry (2024)
-
Insights into vaccines for elderly individuals: from the impacts of immunosenescence to delivery strategies
npj Vaccines (2024)
-
T-cell lymphocytes’ aging clock: telomeres, telomerase and aging
Biogerontology (2024)
-
The neuroimmune axis of Alzheimer’s disease
Genome Medicine (2023)