Abstract
The mutational landscape of colorectal cancer (CRC) does not enable predictions to be made about the survival of patients or their response to therapy. Instead, studying the polarization and activation profiles of immune cells and stromal cells in the tumour microenvironment has been shown to be more informative, thus making CRC a prototypical example of the importance of an inflammatory microenvironment for tumorigenesis. Here, we review our current understanding of how colon cancer cells interact with their microenvironment, comprised of immune cells, stromal cells and the intestinal microbiome, to suppress or escape immune responses and how inflammatory processes shape the immune pathogenesis of CRC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 69, 363–385 (2019).
Fearon, E. R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 6, 479–507 (2011).
Shen, L. et al. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc. Natl Acad. Sci. USA 104, 18654–18659 (2007).
Schonkeren, S. L., Thijssen, M. S., Vaes, N., Boesmans, W. & Melotte, V. The emerging role of nerves and glia in colorectal cancer. Cancers 13, 152 (2021).
Pages, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Pages, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018). This paper establishes the concept of an immunoscore for tumours and shows that it is superior to the AJCC/UICC TNM classification in terms of predicting prognosis.
Anitei, M. G. et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin. Cancer Res. 20, 1891–1899 (2014).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Malka, D. et al. Immune scores in colorectal cancer: where are we? Eur. J. Cancer 140, 105–118 (2020).
Sautes-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015). This study establishes comprehensive molecular subtypes of CRC based on transcriptomic data and shows that patients with stroma-rich tumours have poorer survival.
Calon, A. et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 47, 320–329 (2015).
Isella, C. et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 47, 312–319 (2015).
Calon, A. et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019).
Ganesh, K. & Massague, J. Targeting metastatic cancer. Nat. Med. 27, 34–44 (2021).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Schafer, M. & Werner, S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell. Biol. 9, 628–638 (2008).
Newmark, H. L. et al. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 30, 88–92 (2009).
Meira, L. B. et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 118, 2516–2525 (2008).
Canli, O. et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 32, 869–883 e865 (2017). The first genetic in vivo evidence that reactive oxygen species derived from inflammatory cells can trigger DNA damage in epithelial cells.
Janney, A., Powrie, F. & Mann, E. H. Host-microbiota maladaptation in colorectal cancer. Nature 585, 509–517 (2020).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 (2016).
Schmitt, M. et al. Paneth cells respond to inflammation and contribute to tissue regeneration by acquiring stem-like features through SCF/c-Kit signaling. Cell Rep. 24, 2312–2328.e7 (2018).
Pesic, M. & Greten, F. R. Inflammation and cancer: tissue regeneration gone awry. Curr. Opin. Cell Biol. 43, 55–61 (2016).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Eckmann, L. et al. Opposing functions of IKKβ during acute and chronic intestinal inflammation. Proc. Natl Acad. Sci. USA 105, 15058–15063 (2008).
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Shaked, H. et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc. Natl Acad. Sci. USA 109, 14007–14012 (2012).
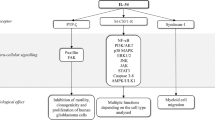
Bollrath, J. & Greten, F. R. IKK/NF-κB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 10, 1314–1319 (2009).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Putoczki, T. L. et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24, 257–271 (2013).
Gronke, K. et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253 (2019).
Ullman, T. A. & Itzkowitz, S. H. Intestinal inflammation and cancer. Gastroenterology 140, 1807–1816 (2011).
Cooks, T. et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 23, 634–646 (2013).
Schulz-Heddergott, R. et al. Therapeutic ablation of gain-of-function mutant p53 in colorectal cancer inhibits stat3-mediated tumor growth and invasion. Cancer Cell 34, 298–314.e7 (2018).
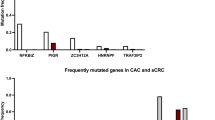
Kakiuchi, N. et al. Frequent mutations that converge on the NFKBIZ pathway in ulcerative colitis. Nature 577, 260–265 (2020).
Nanki, K. et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 577, 254–259 (2020).
Colotta, F., Allavena, P., Sica, A., Garlanda, C. & Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30, 1073–1081 (2009).
Grivennikov, S. I. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin. Immunopathol. 35, 229–244 (2013).
Li, J. et al. Temporal DNA methylation pattern and targeted therapy in colitis-associated cancer. Carcinogenesis 41, 235–244 (2020).
Jackstadt, R. et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell 36, 319–336.e7 (2019).
Varga, J. et al. AKT-dependent NOTCH3 activation drives tumor progression in a model of mesenchymal colorectal cancer. J. Exp. Med. 217, e20191515 (2020).
Wang, D. & DuBois, R. N. Role of prostanoids in gastrointestinal cancer. J. Clin. Invest. 128, 2732–2742 (2018).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Finetti, F. et al. Prostaglandin E2 and cancer: insight into tumor progression and immunity. Biology 9, 434 (2020).
Bonavita, E. et al. Antagonistic inflammatory phenotypes dictate tumor fate and response to immune checkpoint blockade. Immunity 53, 1215–1229.e8 (2020).
Chen, G. et al. Post-transcriptional gene regulation in colitis associated cancer. Front. Genet. 10, 585 (2019).
Ma, Y. et al. Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/β-catenin signalling pathway via suppression of activator protein 2α. Gut 65, 1494–1504 (2016).
Li, X. L. et al. Long noncoding RNA PURPL suppresses basal p53 levels and promotes tumorigenicity in colorectal cancer. Cell. Rep. 20, 2408–2423 (2017).
Rokavec, M. et al. Corrigendum. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 125, 1362 (2015).
Xue, J. et al. LncRNA AB073614 induces epithelial- mesenchymal transition of colorectal cancer cells via regulating the JAK/STAT3 pathway. Cancer Biomark 21, 849–858 (2018).
Ye, C. et al. A novel long non-coding RNA lnc-GNAT1-1 is low expressed in colorectal cancer and acts as a tumor suppressor through regulating RKIP-NF-κB-Snail circuit. J. Exp. Clin. Cancer Res. 35, 187 (2016).
Geng, H. et al. In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology 155, 144–155 (2018).
Kryczek, I. et al. IL-22+CD4+ T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 40, 772–784 (2014).
Rutz, S., Wang, X. & Ouyang, W. The IL-20 subfamily of cytokines–from host defence to tissue homeostasis. Nat. Rev. Immunol. 14, 783–795 (2014).
Zindl, C. L. et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl Acad. Sci. USA 110, 12768–12773 (2013).
Grivennikov, S. I. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258 (2012). This study shows the link between activation of WNT signalling, intestinal barrier defects and the induction of inflammation.
Dmitrieva-Posocco, O. et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity 50, 166–180.e7 (2019).
Schwitalla, S. et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell 23, 93–106 (2013).
Jorgensen, I., Rayamajhi, M. & Miao, E. A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 17, 151–164 (2017).
Kim, E. H., Wong, S. W. & Martinez, J. Programmed necrosis and disease:we interrupt your regular programming to bring you necroinflammation. Cell Death Differ. 26, 25–40 (2019).
Ammirante, M., Shalapour, S., Kang, Y., Jamieson, C. A. & Karin, M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc. Natl Acad. Sci. USA 111, 14776–14781 (2014).
Westendorf, A. M. et al. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting Treg activity. Cell Physiol. Biochem. 41, 1271–1284 (2017).
Flavell, R. A., Sanjabi, S., Wrzesinski, S. H. & Licona-Limon, P. The polarization of immune cells in the tumour environment by TGFβ. Nat. Rev. Immunol. 10, 554–567 (2010).
Nakanishi, Y. et al. Simultaneous loss of both atypical protein kinase C genes in the intestinal epithelium drives serrated intestinal cancer by impairing immunosurveillance. Immunity 49, 1132–1147.e7 (2018).
Ziegler, P. K. et al. Mitophagy in intestinal epithelial cells triggers adaptive immunity during tumorigenesis. Cell 174, 88–101.e16 (2018).
Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434 (2008).
Shalapour, S. et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015).
Shalapour, S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017). References 74 and 75 highlight the role of B cells in tumorigenesis and therapy.
Macpherson, A. J., Yilmaz, B., Limenitakis, J. P. & Ganal-Vonarburg, S. C. IgA function in relation to the intestinal microbiota. Annu. Rev. Immunol. 36, 359–381 (2018).
Mullins, C. S., Gock, M., Krohn, M. & Linnebacher, M. Human colorectal carcinoma infiltrating B lymphocytes are active secretors of the immunoglobulin isotypes A, G, and M. Cancers 11, 776 (2019).
Ghiringhelli, F. & Fumet, J. D. Is there a place for immunotherapy for metastatic microsatellite stable colorectal cancer? Front. Immunol. 10, 1816 (2019).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Price, J. G. et al. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat. Immunol. 16, 1060–1068 (2015).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Diaz, L. A. Jr & Le, D. T. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 373, 1979 (2015).
Le, D. T. et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J. Clin. Oncol. 38, 11–19 (2020).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Ozcan, M., Janikovits, J., von Knebel Doeberitz, M. & Kloor, M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology 7, e1445453 (2018).
Picard, E., Verschoor, C. P., Ma, G. W. & Pawelec, G. Relationships between immune landscapes, genetic subtypes and responses to immunotherapy in colorectal cancer. Front. Immunol. 11, 369 (2020).
Koliaraki, V., Pallangyo, C. K., Greten, F. R. & Kollias, G. Mesenchymal cells in colon cancer. Gastroenterology 152, 964–979 (2017).
Li, H. et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 49, 708–718 (2017).
Ohlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 (2017).
Lee, H. O. et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 52, 594–603 (2020).
Cassetta, L. et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 35, 588–602.e10 (2019).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Li, J. et al. Tumor-associated macrophage infiltration and prognosis in colorectal cancer: systematic review and meta-analysis. Int. J. Colorectal Dis. 35, 1203–1210 (2020).
Mola, S., Pandolfo, C., Sica, A. & Porta, C. The macrophages-microbiota interplay in colorectal cancer (CRC)-related inflammation: prognostic and therapeutic significance. Int. J. Mol. Sci. 21, 6866 (2020).
Llosa, N. J. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 5, 43–51 (2015).
Donadon, M. et al. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J. Exp. Med. 217, e20191847 (2020).
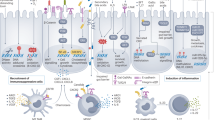
Mizuno, R. et al. The role of tumor-associated neutrophils in colorectal cancer. Int. J. Mol. Sci. 20, 529 (2019).
Sagiv, J. Y. et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 10, 562–573 (2015).
Shaul, M. E. et al. Tumor-associated neutrophils display a distinct N1 profile following TGFbeta modulation: a transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 5, e1232221 (2016).
Fridlender, Z. G. & Albelda, S. M. Tumor-associated neutrophils: friend or foe? Carcinogenesis 33, 949–955 (2012).
Andzinski, L. et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 138, 1982–1993 (2016).
Granot, Z. & Jablonska, J. Distinct functions of neutrophil in cancer and its regulation. Mediators Inflamm. 2015, 701067 (2015).
Richardson, J. J. R., Hendrickse, C., Gao-Smith, F. & Thickett, D. R. Neutrophil extracellular trap production in patients with colorectal cancer vitro. Int. J. Inflam. 2017, 4915062 (2017).
Masucci, M. T., Minopoli, M., Del Vecchio, S. & Carriero, M. V. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front. Immunol. 11, 1749 (2020).
Colangelo, T. et al. Friend or foe? The tumour microenvironment dilemma in colorectal cancer. Biochim. Biophys. Acta. Rev. Cancer 1867, 1–18 (2017).
Jaillon, S. et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 20, 485–503 (2020).
Zitvogel, L., Pietrocola, F. & Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 18, 843–850 (2017).
Schulz, M. D. et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514, 508–512 (2014). This study shows that diet-induced dysbiosis has a greater role than obesity in enhancing tumour progression by suppressing dendritic cell recruitment.
Liu, W. et al. Diet- and genetically-induced obesity produces alterations in the microbiome, inflammation and Wnt pathway in the intestine of Apc(+/1638N) mice: comparisons and contrasts. J. Cancer 7, 1780–1790 (2016).
Wunderlich, C. M. et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat. Commun. 9, 1646 (2018).
Conroy, M. J., Dunne, M. R., Donohoe, C. L. & Reynolds, J. V. Obesity-associated cancer: an immunological perspective. Proc. Nutr. Soc. 75, 125–138 (2016).
James, B. R. et al. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J. Immunol. 189, 1311–1321 (2012).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25, 141–151 (2019).
Albiges, L. et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J. Clin. Oncol. 34, 3655–3663 (2016).
McQuade, J. L. et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19, 310–322 (2018).
De Almeida, C. V., de Camargo, M. R., Russo, E. & Amedei, A. Role of diet and gut microbiota on colorectal cancer immunomodulation. World J. Gastroenterol. 25, 151–162 (2019).
Flemer, B. et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 66, 633–643 (2017).
Feng, Q. et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 6, 6528 (2015).
Martin, H. M. et al. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 127, 80–93 (2004).
Wang, J. & Jia, H. Metagenome-wide association studies: fine-mining the microbiome. Nat. Rev. Microbiol. 14, 508–522 (2016).
Wilson, M. R. et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science 363, eaar7785 (2019).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012). This study demonstrates the link between intestinal inflammation and the expansion of microbial populations with genotoxic properties.
Pleguezuelos-Manzano, C. et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273 (2020). This paper reports that bacteria can induce the same DNA mutation profile ex vivo as that found in patients with CRC.
Kadosh, E. et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 586, 133–138 (2020).
Kasai, C. et al. Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol. Rep. 35, 325–333 (2016).
Viljoen, K. S., Dakshinamurthy, A., Goldberg, P. & Blackburn, J. M. Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS ONE 10, e0119462 (2015).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Kostic, A. D., Chun, E., Meyerson, M. & Garrett, W. S. Microbes and inflammation in colorectal cancer. Cancer Immunol. Res. 1, 150–157 (2013).
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
Casasanta, M. A. et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal. 13, eaba9157 (2020).
Hernandez-Luna, M. A., Lopez-Briones, S. & Luria-Perez, R. The four horsemen in colon cancer. J. Oncol. 2019, 5636272 (2019).
Dejea, C. M. et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597 (2018).
Chung, L. et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe 23, 421 (2018).
Chen, H. et al. Lactobacillus plantarum LPOnlly alters the gut flora and attenuates colitis by inducing microbiome alteration in interleukin10 knockout mice. Mol. Med. Rep. 16, 5979–5985 (2017).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Chang, P. V., Hao, L., Offermanns, S. & Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 111, 2247–2252 (2014).
Tao, R. et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 13, 1299–1307 (2007).
Wu, S. E. et al. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature 586, 108–112 (2020).
Zitvogel, L., Ma, Y., Raoult, D., Kroemer, G. & Gajewski, T. F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 359, 1366–1370 (2018).
Roberti, M. P. et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat. Med. 26, 919–931 (2020).
Hallen-Adams, H. E. & Suhr, M. J. Fungi in the healthy human gastrointestinal tract. Virulence 8, 352–358 (2017).
Liew, W. P. & Mohd-Redzwan, S. Mycotoxin: its impact on gut health and microbiota. Front. Cell Infect. Microbiol. 8, 60 (2018).
Kusunoki, M. et al. Long-term administration of the fungus toxin, sterigmatocystin, induces intestinal metaplasia and increases the proliferative activity of PCNA, p53, and MDM2 in the gastric mucosa of aged Mongolian gerbils. Environ. Health Prev. Med. 16, 224–231 (2011).
Misumi, J. The mechanisms of gastric cancer development produced by the combination of Helicobacter pylori with Sterigmatocystin, a mycotoxin. Nihon. Rinsho 62, 1377–1386 (2004).
Ott, S. J. et al. Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand. J. Gastroenterol. 43, 831–841 (2008).
Leonardi, I. et al. CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi. Science 359, 232–236 (2018).
Shao, T. Y. et al. Commensal Candida albicans positively calibrates systemic Th17 immunological responses. Cell Host Microbe 25, 404–417.e6 (2019).
Malik, A. et al. SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity 49, 515–530.e5 (2018).
Wang, T. et al. The adaptor protein CARD9 protects against colon cancer by restricting mycobiota-mediated expansion of myeloid-derived suppressor cells. Immunity 49, 504–514.e4 (2018). References 150 and 151 highlight the importance of fungi for the development of CRC.
Zhang, L. et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459.e29 (2020).
Li, C. et al. Integrated omics of metastatic colorectal cancer. Cancer Cell 38, 734–747.e9 (2020).
Nusinow, D. P. et al. Quantitative proteomics of the cancer cell line encyclopedia. Cell 180, 387–402.e16 (2020).
Patel, J. N., Fong, M. K. & Jagosky, M. Colorectal cancer biomarkers in the era of personalized medicine. J. Pers. Med. 9, 3 (2019).
Devarasetty, M., Skardal, A., Cowdrick, K., Marini, F. & Soker, S. Bioengineered submucosal organoids for in vitro modeling of colorectal cancer. Tissue Eng. Part A 23, 1026–1041 (2017).
Piccoli, M. et al. Decellularized colorectal cancer matrix as bioactive microenvironment for in vitro 3D cancer research. J. Cell Physiol. 233, 5937–5948 (2018).
Aleman, J. & Skardal, A. A multi-site metastasis-on-a-chip microphysiological system for assessing metastatic preference of cancer cells. Biotechnol. Bioeng. 116, 936–944 (2019).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Manguso, R. T. et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 547, 413–418 (2017).
Papanikolaou, A., Wang, Q. S., Papanikolaou, D., Whiteley, H. E. & Rosenberg, D. W. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis 21, 1567–1572 (2000).
Neufert, C., Becker, C. & Neurath, M. F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2, 1998–2004 (2007).
Moser, A. R. et al. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev. Dyn. 203, 422–433 (1995).
Halberg, R. B. et al. Tumorigenesis in the multiple intestinal neoplasia mouse: redundancy of negative regulators and specificity of modifiers. Proc. Natl Acad. Sci. USA 97, 3461–3466 (2000).
Hinoi, T. et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 67, 9721–9730 (2007).
Boutin, A. T. et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 31, 370–382 (2017).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Roper, J. et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 35, 569–576 (2017).
O’Rourke, K. P. et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 35, 577–582 (2017).
Acknowledgements
Work in the laboratory of F.R.G. is supported by institutional funds from the Georg-Speyer-Haus, by the LOEWE Center Frankfurt Cancer Institute (FCI) funded by the Hessen State Ministry for Higher Education, Research and the Arts [III L 5 – 519/03/03.001 – (0015)], and by the Deutsche Forschungsgemeinschaft (FOR2438: Gr1916/11-1; SFB 815, 1177 and 1292 as well as GRK 2336). The Institute for Tumor Biology and Experimental Therapy, Georg-Speyer-Haus, is funded jointly by the German Federal Ministry of Health and the Ministry of Higher Education, Research and the Arts of the State of Hessen (HMWK).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Peer review information
Nature Reviews Immunology thanks Y. Ben-Neriah and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Stage 4 colorectal cancer
-
The stage at which the colorectal cancer has spread to other parts of the body such as lung, liver, abdominal wall, ovary or distant lymph nodes.
- Aneuploidy
-
The presence of an abnormal number of chromosomes in a cell.
- Loss of heterozygosity
-
Describes a cross-chromosomal event that results in loss of the entire gene and the surrounding chromosomal region.
- Microsatellite instability
-
(MSI). The phenotypic indication of non-functional DNA mismatch repair that results in genetic hypermutability.
- Mismatch repair
-
A cellular system that recognizes and corrects DNA errors resulting from wrongly paired DNA bases that occur, for example, during DNA replication, DNA recombination or DNA damage.
- Serrated pathway
-
An alternative pathway of genetic alterations occurring independently of APC mutations that is often initiated by the activation of the RAS–RAF–MEK–ERK–MAPK axis and is frequently characterized by a CpG island methylation pathway phenotype and subsequently by high-level DNA microsatellite instability, especially when located proximally and associated with BRAF mutations.
- AJCC/UICC TNM classification
-
The AJCC/UICC staging system is a classification system developed by the American Joint Committee on Cancer and the Union Internationale Contre le Cancer for describing the extent of disease progression in patients with cancer using a system that scores for tumour size, lymph nodes affected and the presence of metastases.
- Myeloid-derived suppressor cells
-
(MDSCs). A heterogeneous group of cells from the myeloid lineage that have immunosuppressive properties.
- Immune-checkpoint blockade
-
A therapeutic concept that aims to block negative feedback signalling to immune cells to enhance an immune response against tumours.
- AOM/DSS model
-
A mouse model of inflammation-associated colorectal cancer, in which intestinal tumorigenesis is triggered by injection of the mutagen azoxymethane (AOM) and subsequent cycles of inflammation induced by dextran sulfate sodium (DSS).
- WNT pathway
-
An evolutionarily conserved pathway that is important for embryonic development and carcinogenesis, which is activated in more than 90% of colorectal cancers owing to mutations in its signalling components.
- Mitophagy
-
Selective degradation of mitochondria by autophagy.
- Immunoediting
-
Evolution of tumours with the result that tumour cells are no longer effectively recognized and suppressed by the immune system, resulting in the emergence of immune-resistant tumour cell variants.
- Apc-mutant models
-
Mouse models of intestinal cancer in which tumorigenesis is driven by a loss of function of APC, resulting in the hyperactivation of the WNT pathway and the hyperproliferation of affected cells.
- Apc Min/+ mouse model
-
A mouse model of intestinal cancer in which the Apc gene has a truncation mutation at codon 850, resulting in multiple intestinal neoplasia (Min).
Rights and permissions
About this article
Cite this article
Schmitt, M., Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol 21, 653–667 (2021). https://doi.org/10.1038/s41577-021-00534-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-021-00534-x
This article is cited by
-
Musashi-2 potentiates colorectal cancer immune infiltration by regulating the post-translational modifications of HMGB1 to promote DCs maturation and migration
Cell Communication and Signaling (2024)
-
The oncogenic mechanisms of the Janus kinase-signal transducer and activator of transcription pathway in digestive tract tumors
Cell Communication and Signaling (2024)
-
A multi-dimensional approach to unravel the intricacies of lactylation related signature for prognostic and therapeutic insight in colorectal cancer
Journal of Translational Medicine (2024)
-
MIIP downregulation drives colorectal cancer progression through inducing peri-cancerous adipose tissue browning
Cell & Bioscience (2024)
-
Regulation of tumor metastasis and CD8+ T cells infiltration by circRNF216/miR-576-5p/ZC3H12C axis in colorectal cancer
Cellular & Molecular Biology Letters (2024)