Abstract
Effector T cells leave the lymph nodes armed with specialized functional attributes. Their antigenic targets may be located anywhere in the body, posing the ultimate challenge: how to efficiently identify the target tissue, navigate through a complex tissue matrix and, ultimately, locate the immunological insult. Recent advances in real-time in situ imaging of effector T cell migratory behaviour have revealed a great degree of mechanistic plasticity that enables effector T cells to push and squeeze their way through inflamed tissues. This process is shaped by an array of ‘stop’ and ‘go’ guidance signals including target antigens, chemokines, integrin ligands and the mechanical cues of the inflamed microenvironment. Effector T cells must sense and interpret these competing signals to correctly position themselves to mediate their effector functions for complete and durable responses in infectious disease and malignancy. Tuning T cell migration therapeutically will require a new understanding of this complex decision-making process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Masopust, D. & Schenkel, J. M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 13, 309–320 (2013).
Schulz, O., Hammerschmidt, S. I., Moschovakis, G. L. & Forster, R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu. Rev. Immunol. 34, 203–242 (2016).
Te Boekhorst, V., Preziosi, L. & Friedl, P. Plasticity of cell migration in vivo and in silico. Annu. Rev. Cell Dev. Biol. 32, 491–526 (2016).
Friedl, P. & Wolf, K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188, 11–19 (2010).
Vargas, P., Barbier, L., Saez, P. J. & Piel, M. Mechanisms for fast cell migration in complex environments. Curr. Opin. Cell Biol. 48, 72–78 (2017).
Lam, P. Y. & Huttenlocher, A. Interstitial leukocyte migration in vivo. Curr. Opin. Cell Biol. 25, 650–658 (2013).
Lammermann, T. & Germain, R. N. The multiple faces of leukocyte interstitial migration. Semin. Immunopathol. 36, 227–251 (2014).
Yamada, K. M. & Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752 (2019).
Kameritsch, P. & Renkawitz, J. Principles of leukocyte migration strategies. Trends Cell Biol. 30, 818–832 (2020).
Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 17, 679–690 (2017).
Renkawitz, J. et al. Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol. 11, 1438–1443 (2009).
van Helvert, S., Storm, C. & Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 20, 8–20 (2018).
Wolf, K., Muller, R., Borgmann, S., Brocker, E. B. & Friedl, P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3262–3269 (2003).
Harris, T. H. et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486, 545–548 (2012). This paper is the first to suggest that the random interstitial migration pattern of T cells resembles a Levy walk, a pattern thought to increase the chances of finding rare targets.
Lim, K. et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349, aaa4352 (2015). This study demonstrates that neutrophils can leave chemokine-rich trails that guide virus-specific CD8+ T cell migration in the infected airway.
Egen, J. G. et al. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28, 271–284 (2008).
Overstreet, M. G. et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat. Immunol. 14, 949–958 (2013). This paper identifies the matrix-binding αV integrin as critical for CD4+ T cell interstitial migration within inflamed skin.
Renkawitz, J. et al. Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature 568, 546–550 (2019). The study shows that the ‘nucleus-first configuration’, a distinctive feature of amoeboid cells, facilitates rapid navigation of dendritic cells along the path of least resistance.
Bufi, N. et al. Human primary immune cells exhibit distinct mechanical properties that are modified by inflammation. Biophys. J. 108, 2181–2190 (2015).
McGregor, A. L., Hsia, C. R. & Lammerding, J. Squish and squeeze — the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 40, 32–40 (2016).
Davidson, P. M., Denais, C., Bakshi, M. C. & Lammerding, J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell Mol. Bioeng. 7, 293–306 (2014).
Salvermoser, M., Begandt, D., Alon, R. & Walzog, B. Nuclear deformation during neutrophil migration at sites of inflammation. Front. Immunol. 9, 2680 (2018).
Olins, A. L. et al. The human granulocyte nucleus: unusual nuclear envelope and heterochromatin composition. Eur. J. Cell Biol. 87, 279–290 (2008).
Salvermoser, M. et al. Myosin 1f is specifically required for neutrophil migration in 3D environments during acute inflammation. Blood 131, 1887–1898 (2018).
Thiam, H. R. et al. Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nat. Commun. 7, 10997 (2016).
Thompson, S. B. et al. Formin-like 1 mediates effector T cell trafficking to inflammatory sites to enable T cell-mediated autoimmunity. eLife 9, e58046 (2020).
Degroote, R. L. et al. Formin like 1 expression is increased on CD4+ T lymphocytes in spontaneous autoimmune uveitis. J. Proteom. 154, 102–108 (2017).
Odoardi, F. et al. T cells become licensed in the lung to enter the central nervous system. Nature 488, 675–679 (2012).
Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 (2010).
Lammermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Reversat, A. et al. Cellular locomotion using environmental topography. Nature 582, 582–585 (2020). This paper is the first demonstration that T cells can transmit forces by coupling the retrograde flow of actin to a geometrically irregular environment, in the complete absence of any adhesion.
Stolp, B. et al. Salivary gland macrophages and tissue-resident CD8+ T cells cooperate for homeostatic organ surveillance. Sci. Immunol. 5, eaaz4371 (2020).
Martens, R. et al. Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Nat. Commun. 11, 1114 (2020).
Griffith, J. W., Sokol, C. L. & Luster, A. D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 32, 659–702 (2014).
Lammermann, T. & Kastenmuller, W. Concepts of GPCR-controlled navigation in the immune system. Immunol. Rev. 289, 205–231 (2019).
Nourshargh, S. & Alon, R. Leukocyte migration into inflamed tissues. Immunity 41, 694–707 (2014).
Hogg, N., Patzak, I. & Willenbrock, F. The insider’s guide to leukocyte integrin signalling and function. Nat. Rev. Immunol. 11, 416–426 (2011).
Shulman, Z. et al. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat. Immunol. 13, 67–76 (2011).
Handel, T. M., Johnson, Z., Crown, S. E., Lau, E. K. & Proudfoot, A. E. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu. Rev. Biochem. 74, 385–410 (2005).
Mihov, D. & Spiess, M. Glycosaminoglycans: sorting determinants in intracellular protein traffic. Int. J. Biochem. Cell Biol. 68, 87–91 (2015).
Stoler-Barak, L. et al. Blood vessels pattern heparan sulfate gradients between their apical and basolateral aspects. PLoS ONE 9, e85699 (2014).
Wang, J. & Kubes, P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165, 668–678 (2016).
Steinert, E. M. et al. Cutting edge: evidence for nonvascular route of visceral organ immunosurveillance by T cells. J. Immunol. 201, 337–342 (2018).
Thompson, S. et al. Regulation of chemokine function: the roles of GAG-binding and post-translational nitration. Int. J. Mol. Sci. 18, 1692 (2017).
Proudfoot, A. E. et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl Acad. Sci. USA 100, 1885–1890 (2003). This paper demonstrates that GAG binding mediates chemokine tethering and immobilization, which is a prerequisite of chemokine activation.
Gangavarapu, P. et al. The monomer–dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. J. Leukoc. Biol. 91, 259–265 (2012).
Salanga, C. L. & Handel, T. M. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: the role of structural dynamics in function. Exp. Cell Res. 317, 590–601 (2011).
Graham, G. J., Handel, T. M. & Proudfoot, A. E. I. Leukocyte adhesion: reconceptualizing chemokine presentation by glycosaminoglycans. Trends Immunol. 40, 472–481 (2019).
Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 (2010).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Sun, Z., Costell, M. & Fassler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 21, 25–31 (2019).
Hons, M. et al. Chemokines and integrins independently tune actin flow and substrate friction during intranodal migration of T cells. Nat. Immunol. 19, 606–616 (2018).
Hallmann, R. et al. The regulation of immune cell trafficking by the extracellular matrix. Curr. Opin. Cell Biol. 36, 54–61 (2015).
Hyun, Y. M. et al. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J. Exp. Med. 209, 1349–1362 (2012).
Wang, S. et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 203, 1519–1532 (2006).
Wu, C. et al. Endothelial basement membrane laminin α5 selectively inhibits T lymphocyte extravasation into the brain. Nat. Med. 15, 519–527 (2009).
Sixt, M. et al. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 153, 933–946 (2001).
Zhang, X. et al. The endothelial basement membrane acts as a checkpoint for entry of pathogenic T cells into the brain. J. Exp. Med. 217, e20191339 (2020). This study highlights the functional importance of spatial patterning of ECM components, showing that the expression of laminin at the interface between the endothelial monolayer and the underlying basement membrane is critical to control pathogenicity of infiltrating T cells.
Wang, X., Rodda, L. B., Bannard, O. & Cyster, J. G. Integrin-mediated interactions between B cells and follicular dendritic cells influence germinal center B cell fitness. J. Immunol. 192, 4601–4609 (2014).
Schrock, D. C. et al. Pivotal role for αV integrins in Tfh GC positioning and support of the germinal center response. 116, 4462–4470 (2019).
Ray, S. J. et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20, 167–179 (2004).
Reilly, E. C. et al. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl Acad. Sci. USA 117, 12306–12314 (2020).
Wilson, E. H. et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity 30, 300–311 (2009).
Moreau, H. D., Piel, M., Voituriez, R. & Lennon-Dumenil, A. M. Integrating physical and molecular insights on immune cell migration. Trends Immunol. 39, 632–643 (2018).
Nicolas-Boluda, A. & Donnadieu, E. Obstacles to T cell migration in the tumor microenvironment. Comp. Immunol. Microbiol. Infect. Dis. 63, 22–30 (2019).
Salmon, H. et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910 (2012). This study demonstrates that the density and orientation of the stromal ECM play key roles in controlling the migration of T cells.
Zhu, C., Chen, W., Lou, J., Rittase, W. & Li, K. Mechanosensing through immunoreceptors. Nat. Immunol. 20, 1269–1278 (2019).
Trichet, L. et al. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl Acad. Sci. USA 109, 6933–6938 (2012).
Fernandes, N. R. J. et al. CD4+ T cell interstital migration controlled by fibronectin in the inflamed skin. Front. Immunol. 11, 1501 (2020).
Wang, W. Y., Davidson, C. D., Lin, D. & Baker, B. M. Actomyosin contractility-dependent matrix stretch and recoil induces rapid cell migration. Nat. Commun. 10, 1186 (2019).
Sarris, M. & Sixt, M. Navigating in tissue mazes: chemoattractant interpretation in complex environments. Curr. Opin. Cell Biol. 36, 93–102 (2015).
Krummel, M. F., Bartumeus, F. & Gerard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 16, 193–201 (2016).
Bromley, S. K., Peterson, D. A., Gunn, M. D. & Dustin, M. L. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J. Immunol. 165, 15–19 (2000).
Dustin, M. L. & Choudhuri, K. Signaling and polarized communication across the T cell immunological synapse. Annu. Rev. Cell Dev. Biol. 32, 303–325 (2016).
Baker, C. M. et al. Opposing roles for RhoH GTPase during T-cell migration and activation. Proc. Natl Acad. Sci. USA 109, 10474–10479 (2012).
Brunner-Weinzierl, M. C. & Rudd, C. E. CTLA-4 and PD-1 control of T-cell motility and migration: implications for tumor immunotherapy. Front. Immunol. 9, 2737 (2018).
Schneider, H. et al. Reversal of the TCR stop signal by CTLA-4. Science 313, 1972–1975 (2006).
Sarris, M. et al. Inflammatory chemokines direct and restrict leukocyte migration within live tissues as glycan-bound gradients. Curr. Biol. 22, 2375–2382 (2012). The study highlights that, by interacting with extracellular heparan sulfate proteoglycans, chemokines can establish robust surface-bound gradients that mediate both recruitment and retention of leukocytes at sites of infection.
Hickman, H. D. et al. CXCR3 chemokine receptor enables local CD8+ T cell migration for the destruction of virus-infected cells. Immunity 42, 524–537 (2015). This paper is a powerful in vivo demonstration that chemokine signals guide the ability of CD8+ T cells to locate virus-infected cells within the infected tissue and thereby exert antiviral effector functions.
Egen, J. G. et al. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity 34, 807–819 (2011).
Shakhar, G. et al. Stable T cell–dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat. Immunol. 6, 707–714 (2005).
Honda, T. et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 40, 235–247 (2014). This dynamic analysis of T cell interactions with APCs in the inflamed tissue reveals that the pattern of transient arrest and rapid resumption of migration is controlled by a TCR-dependent negative-feedback loop mediated by effector T cell upregulation of PD1, which may protect the host from immunopathology.
Moreau, H. D. et al. Dynamic in situ cytometry uncovers T cell receptor signaling during immunological synapses and kinapses in vivo. Immunity 37, 351–363 (2012).
Cyster, J. G. & Schwab, S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Sadik, C. D. & Luster, A. D. Lipid–cytokine–chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukoc. Biol. 91, 207–215 (2012).
Tager, A. M. et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat. Immunol. 4, 982–990 (2003).
Qi, H. T follicular helper cells in space–time. Nat. Rev. Immunol. 16, 612–625 (2016).
Cyster, J. G., Dang, E. V., Reboldi, A. & Yi, T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 14, 731–743 (2014).
Yan, H. et al. Plexin B2 and semaphorin 4C guide T cell recruitment and function in the germinal center. Cell Rep. 19, 995–1007 (2017).
Moriyama, S. et al. Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. J. Exp. Med. 211, 1297–1305 (2014).
Lu, P., Shih, C. & Qi, H. Ephrin B1-mediated repulsion and signaling control germinal center T cell territoriality and function. Science 356, eaai9264 (2017).
Muppidi, J. R., Lu, E. & Cyster, J. G. The G protein-coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination. J. Exp. Med. 212, 2213–2222 (2015).
Lu, E., Wolfreys, F. D., Muppidi, J. R., Xu, Y. & Cyster, J. G. S-Geranylgeranyl-l-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature 567, 244–248 (2019).
Muller, A. J. et al. CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity 37, 147–157 (2012).
Mandl, J. N. et al. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naive CD4+ and CD8+ T cells. Proc. Natl Acad. Sci. USA 109, 18036–18041 (2012).
Gebhardt, T. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011).
Leon, B. & Lund, F. E. Compartmentalization of dendritic cell and T-cell interactions in the lymph node: anatomy of T-cell fate decisions. Immunol. Rev. 289, 84–100 (2019).
Kurachi, M. et al. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 208, 1605–1620 (2011).
Hu, J. K., Kagari, T., Clingan, J. M. & Matloubian, M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc. Natl Acad. Sci. USA 108, E118–E127 (2011).
Groom, J. R. et al. CXCR3 chemokine receptor–ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 37, 1091–1103 (2012).
Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014).
Sallusto, F. & Lanzavecchia, A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177, 134–140 (2000).
Tough, D. F., Rioja, I., Modis, L. K. & Prinjha, R. K. Epigenetic regulation of T cell memory: recalling therapeutic implications. Trends Immunol. 41, 29–45 (2020).
Gerard, A. et al. Detection of rare antigen-presenting cells through T cell-intrinsic meandering motility, mediated by Myo1g. Cell 158, 492–505 (2014). This study identifies MYO1G as a T cell-intrinsic regulator of the meandering search pattern of migrating T cells that appears to enhance the detection of rare cognate APCs.
Laakso, J. M., Lewis, J. H., Shuman, H. & Ostap, E. M. Myosin I can act as a molecular force sensor. Science 321, 133–136 (2008).
Mrass, P. et al. ROCK regulates the intermittent mode of interstitial T cell migration in inflamed lungs. Nat. Commun. 8, 1010 (2017).
Gaylo-Moynihan, A. et al. Programming of distinct chemokine-dependent and -independent search strategies for TH1 and TH2 cells optimizes function at inflamed sites. Immunity 51, 298–309 e296 (2019). This study reveals that TH cell differentiation imparts distinct effector subset-specific migration patterns and particular dependencies on migrational cues: TH1 cells explored the inflamed tissue in a GPCR- and chemokine-dependent fashion, whereas TH2 cells scanned the tissue independent of GPCR signals.
Du, F. et al. Inflammatory TH17 cells express integrin αvβ3 for pathogenic function. Cell Rep. 16, 1339–1351 (2016).
Osborn, J. F. et al. Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8+ T cells. Sci. Immunol. 2, eaan6049 (2017).
Steinert, E. M. et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015).
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332 (2013).
Ariotti, S. et al. Subtle CXCR3-dependent chemotaxis of CTLs within infected tissue allows efficient target localization. J. Immunol. 195, 5285–5295 (2015).
Castellino, F. et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell–dendritic cell interaction. Nature 440, 890–895 (2006).
Petrie Aronin, C. E. et al. Migrating myeloid cells sense temporal dynamics of chemoattractant concentrations. Immunity 47, 862–874.e3 (2017).
Witt, C. M., Raychaudhuri, S., Schaefer, B., Chakraborty, A. K. & Robey, E. A. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 3, e160 (2005).
Majumdar, R., Sixt, M. & Parent, C. A. New paradigms in the establishment and maintenance of gradients during directed cell migration. Curr. Opin. Cell Biol. 30, 33–40 (2014).
Vanheule, V., Metzemaekers, M., Janssens, R., Struyf, S. & Proost, P. How post-translational modifications influence the biological activity of chemokines. Cytokine 109, 29–51 (2018).
Borroni, E. M., Mantovani, A., Locati, M. & Bonecchi, R. Chemokine receptors intracellular trafficking. Pharmacol. Ther. 127, 1–8 (2010).
Mantovani, A., Bonecchi, R. & Locati, M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat. Rev. Immunol. 6, 907–918 (2006).
Bonecchi, R. & Graham, G. J. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front. Immunol. 7, 224 (2016).
Lau, S. et al. A negative-feedback loop maintains optimal chemokine concentrations for directional cell migration. Nat. Cell Biol. 22, 266–273 (2020).
Molon, B. et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 208, 1949–1962 (2011).
Nieto, M. et al. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J. Exp. Med. 186, 153–158 (1997).
Hyun, Y. M., Chung, H. L., McGrath, J. L., Waugh, R. E. & Kim, M. Activated integrin VLA-4 localizes to the lamellipodia and mediates T cell migration on VCAM-1. J. Immunol. 183, 359–369 (2009).
Vroon, A., Heijnen, C. J. & Kavelaars, A. GRKs and arrestins: regulators of migration and inflammation. J. Leukoc. Biol. 80, 1214–1221 (2006).
Schwarz, J. et al. Dendritic cells interpret haptotactic chemokine gradients in a manner governed by signal-to-noise ratio and dependent on GRK6. Curr. Biol. 27, 1314–1325 (2017).
Kehrl, J. H. The impact of RGS and other G-protein regulatory proteins on Gαi-mediated signaling in immunity. Biochem. Pharmacol. 114, 40–52 (2016).
Bromley, S. K., Mempel, T. R. & Luster, A. D. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9, 970–980 (2008).
Campbell, J. J., Foxman, E. F. & Butcher, E. C. Chemoattractant receptor cross talk as a regulatory mechanism in leukocyte adhesion and migration. Eur. J. Immunol. 27, 2571–2578 (1997).
Heit, B., Tavener, S., Raharjo, E. & Kubes, P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159, 91–102 (2002).
Lin, F. & Butcher, E. C. Modeling the role of homologous receptor desensitization in cell gradient sensing. J. Immunol. 181, 8335–8343 (2008).
Wu, D. & Lin, F. Modeling cell gradient sensing and migration in competing chemoattractant fields. PLoS ONE 6, e18805 (2011).
Zidar, D. A., Violin, J. D., Whalen, E. J. & Lefkowitz, R. J. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl Acad. Sci. USA 106, 9649–9654 (2009).
Coombs, C. et al. Chemokine receptor trafficking coordinates neutrophil clustering and dispersal at wounds in zebrafish. Nat. Commun. 10, 5166 (2019). This elegant study visualizes the differential membrane trafficking of chemokine receptors in neutrophils and links chemokine receptor availability to the accumulation and subsequent dispersal of neutrophils at a wound site, ensuring an effective and self-resolving response to tissue damage.
Sun, H. et al. Distinct chemokine signaling regulates integrin ligand specificity to dictate tissue-specific lymphocyte homing. Dev. Cell 30, 61–70 (2014).
Prentice-Mott, H. V. et al. Biased migration of confined neutrophil-like cells in asymmetric hydraulic environments. Proc. Natl Acad. Sci. USA 110, 21006–21011 (2013).
Moses, M. E., Cannon, J. L., Gordon, D. M. & Forrest, S. Distributed adaptive search in T cells: lessons from ants. Front. Immunol. 10, 1357 (2019).
Torabi-Parizi, P. et al. Pathogen-related differences in the abundance of presented antigen are reflected in CD4+ T cell dynamic behavior and effector function in the lung. J. Immunol. 192, 1651–1660 (2014).
Afonso, P. V. et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091 (2012).
Lammermann, T. et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 (2013). This study shows that neutrophil production of LTB4 and cell surface integrins were required for long-distance neutrophil warming in the extravascular space of a damaged tissue.
Lim, K. et al. In situ neutrophil efferocytosis shapes T cell immunity to influenza infection. Nat. Immunol. 21, 1046–1057 (2020).
Leon, B. et al. Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol. 13, 681–690 (2012). Together with Groom et al. (2012), this study was the first to define a chemokine-controlled intranodal repositioning event that was critical for the completion of TH cell differentiation in the lymph node.
Goldberg, M. F. et al. Salmonella persist in activated macrophages in T cell-sparse granulomas but are contained by surrounding CXCR3 ligand-positioned TH1 cells. Immunity 49, 1090–1102.e7 (2018).
Richmond, J. M. et al. Keratinocyte-derived chemokines orchestrate T-cell positioning in the epidermis during vitiligo and may serve as biomarkers of disease. J. Invest. Dermatol. 137, 350–358 (2017).
Groom, J. R. & Luster, A. D. CXCR3 in T cell function. Exp. Cell Res. 317, 620–631 (2011).
Sung, J. H. et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell 150, 1249–1263 (2012).
Kastenmuller, W. et al. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity 38, 502–513 (2013).
Carrasco, Y. R. & Batista, F. D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Gerner, M. Y., Torabi-Parizi, P. & Germain, R. N. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42, 172–185 (2015).
Woodruff, M. C. et al. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J. Exp. Med. 211, 1611–1621 (2014).
Baptista, A. P. et al. The chemoattractant receptor Ebi2 drives intranodal naive CD4+ T cell peripheralization to promote effective adaptive immunity. Immunity 50, 1188–1201.e6 (2019).
McFarland, A. P. & Colonna, M. Sense and immuno-sensibility: innate lymphoid cell niches and circuits. Curr. Opin. Immunol. 62, 9–14 (2020).
Dahlgren, M. W. et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 50, 707–722.e6 (2019). This study implicates adventitial stromal cells in the support of conserved niches for type 2 lymphocyte expansion and function in the lung.
Klose, C. S. N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017).
Moriyama, S. et al. β2-Adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 (2018).
Dahlgren, M. W. & Molofsky, A. B. Adventitial cuffs: regional hubs for tissue immunity. Trends Immunol. 40, 877–887 (2019).
Veres, T. Z. et al. Allergen-induced CD4+ T cell cytokine production within airway mucosal dendritic cell-T cell clusters drives the local recruitment of myeloid effector cells. J. Immunol. 198, 895–907 (2017).
Filipe-Santos, O. et al. A dynamic map of antigen recognition by CD4 T cells at the site of Leishmania major infection. Cell Host Microbe 6, 23–33 (2009).
Siracusa, F. et al. Nonfollicular reactivation of bone marrow resident memory CD4 T cells in immune clusters of the bone marrow. Proc. Natl Acad. Sci. USA 115, 1334–1339 (2018).
Natsuaki, Y. et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat. Immunol. 15, 1064–1069 (2014). This work defines an induced perivascular niche in the skin where dendritic cells form clusters with effector T cells to promote in situ T cell proliferation and activation.
Huang, L. R. et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8+ T cells and successful immunotherapy against chronic viral liver infection. Nat. Immunol. 14, 574–583 (2013).
Wakim, L. M., Woodward-Davis, A. & Bevan, M. J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl Acad. Sci. USA 107, 17872–17879 (2010).
Iijima, N. & Iwasaki, A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346, 93–98 (2014). This study is one of the first to identify a TRM tissue niche, where chemokines secreted by local macrophages maintained vaginal CD4 TRM cells in memory lymphocyte clusters, independent of circulating memory T cells.
Nordenfelt, P. et al. Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. Nat. Commun. 8, 2047 (2017).
Carman, C. V. & Springer, T. A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 167, 377–388 (2004).
Guillou, L. et al. T-lymphocyte passive deformation is controlled by unfolding of membrane surface reservoirs. Mol. Biol. Cell 27, 3574–3582 (2016).
Song, J., Wu, C., Zhang, X. & Sorokin, L. M. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1β-induced peritonitis. J. Immunol. 190, 401–410 (2013).
Proost, P. et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood 110, 37–44 (2007).
Proost, P. et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 98, 3554–3561 (2001).
Ludwig, A., Schiemann, F., Mentlein, R., Lindner, B. & Brandt, E. Dipeptidyl peptidase IV (CD26) on T cells cleaves the CXC chemokine CXCL11 (I-TAC) and abolishes the stimulating but not the desensitizing potential of the chemokine. J. Leukoc. Biol. 72, 183–191 (2002).
Oravecz, T. et al. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J. Exp. Med. 186, 1865–1872 (1997).
Barker, C. E. et al. CCL2 nitration is a negative regulator of chemokine-mediated inflammation. Sci. Rep. 7, 44384 (2017).
Loos, T. et al. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood 112, 2648–2656 (2008).
Kiermaier, E. et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science 351, 186–190 (2016).
Denard, B., Han, S., Kim, J., Ross, E. M. & Ye, J. Regulating G protein-coupled receptors by topological inversion. eLife 8, e40234 (2019).
Weber, C., Zernecke, A. & Libby, P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 8, 802–815 (2008).
Gewaltig, J., Kummer, M., Koella, C., Cathomas, G. & Biedermann, B. C. Requirements for CD8 T-cell migration into the human arterial wall. Hum. Pathol. 39, 1756–1762 (2008).
Kyaw, T. et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 127, 1028–1039 (2013).
Kleinschmidt-DeMasters, B. K. & Tyler, K. L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon β-1a for multiple sclerosis. N. Engl. J. Med. 353, 369–374 (2005).
Van Assche, G. et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N. Engl. J. Med. 353, 362–368 (2005).
Fisher, D. T. et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J. Clin. Invest. 121, 3846–3859 (2011).
Moon, E. K. et al. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology 7, e1395997 (2018).
Hu, Z. et al. Mouse IP-10 gene delivered by folate-modified chitosan nanoparticles and dendritic/tumor cells fusion vaccine effectively inhibit the growth of hepatocellular carcinoma in mice. Theranostics 7, 1942–1952 (2017).
Tang, H. et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 (2016).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Peng, S. B. et al. Distinct mobilization of leukocytes and hematopoietic stem cells by CXCR4 peptide antagonist LY2510924 and monoclonal antibody LY2624587. Oncotarget 8, 94619–94634 (2017).
O’Hara, M. H. et al. Safety and pharmacokinetics of CXCR4 peptide antagonist, LY2510924, in combination with durvalumab in advanced refractory solid tumors. J. Pancreat. Cancer 6, 21–31 (2020).
Weinstein, E. J. et al. VCC-1, a novel chemokine, promotes tumor growth. Biochem. Biophys. Res. Commun. 350, 74–81 (2006).
Kuroda, T. et al. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin. Cancer Res. 11, 7629–7636 (2005).
Kang, H., Mansel, R. E. & Jiang, W. G. Genetic manipulation of stromal cell-derived factor-1 attests the pivotal role of the autocrine SDF-1–CXCR4 pathway in the aggressiveness of breast cancer cells. Int. J. Oncol. 26, 1429–1434 (2005).
Campanella, G. S., Medoff, B. D., Manice, L. A., Colvin, R. A. & Luster, A. D. Development of a novel chemokine-mediated in vivo T cell recruitment assay. J. Immunol. Methods 331, 127–139 (2008).
Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012).
Gopinath, S., Lu, P. & Iwasaki, A. Cutting edge: the use of topical aminoglycosides as an effective pull in “prime and pull” vaccine strategy. J. Immunol. 204, 1703–1707 (2020).
Mackay, L. K. et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. USA 109, 7037–7042 (2012).
Gola, A. et al. Prime and target immunization protects against liver-stage malaria in mice. Sci. Transl Med. 10, eaap9128 (2018).
Acknowledgements
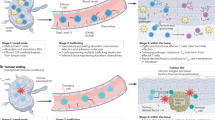
The authors thank members of the ‘Tissue Regulation of T cell Function’ P01 for their input, P. Oakes for discussion and design of the graphics shown in Figure 2 and R. Alon for careful review of the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks J. Schenkel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fowell, D.J., Kim, M. The spatio-temporal control of effector T cell migration. Nat Rev Immunol 21, 582–596 (2021). https://doi.org/10.1038/s41577-021-00507-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-021-00507-0
This article is cited by
-
LFA-1 nanoclusters integrate TCR stimulation strength to tune T-cell cytotoxic activity
Nature Communications (2024)
-
Assessing the generation of tissue resident memory T cells by vaccines
Nature Reviews Immunology (2023)
-
Localization, tissue biology and T cell state — implications for cancer immunotherapy
Nature Reviews Immunology (2023)
-
New genetic and epigenetic insights into the chemokine system: the latest discoveries aiding progression toward precision medicine
Cellular & Molecular Immunology (2023)
-
The effect of chemotaxis on T-cell regulatory dynamics
Journal of Mathematical Biology (2023)