Abstract
Each day, the gastrointestinal tract encounters an influx of microbial and nutrient-derived signals and its physiological activities often adhere to a circadian rhythm. As such, group 3 innate lymphoid cells (ILC3s) that reside in the intestinal mucosa must function within a highly dynamic environment. In this Progress article, we highlight a series of recent reports that have characterized the circadian clock in ILC3s. We discuss how these studies have illustrated the roles of environmental cues and clock genes in regulating ILC3 biology and consider the implications for intestinal immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
O’Neill, J. S. & Reddy, A. B. Circadian clocks in human red blood cells. Nature 469, 498–503 (2011).
Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998).
Hogenesch, J. B., Gu, Y. Z., Jain, S. & Bradfield, C. A. The basic-helix–loop–helix–PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl Acad. Sci. USA 95, 5474–5479 (1998).
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Kume, K. et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205 (1999).
Shearman, L. P. et al. Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019 (2000).
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Sato, T. K. et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43, 527–537 (2004).
Bugge, A. et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes. Dev. 26, 657–667 (2012).
Cho, H. et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012).
Mitsui, S., Yamaguchi, S., Matsuo, T., Ishida, Y. & Okamura, H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes. Dev. 15, 995–1006 (2001).
Ueda, H. R. et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 37, 187–192 (2005).
Bechtold, D. A. & Loudon, A. S. I. Hypothalamic clocks and rhythms in feeding behaviour. Trends Neurosci. 36, 74–82 (2013).
Asher, G. & Sassone-Corsi, P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92 (2015).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Robinette, M. L. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317 (2015).
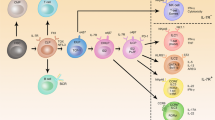
Vonarbourg, C. et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33, 736–751 (2010).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Klose, C. S. N. et al. A T-bet gradient controls the fate and function of CCR6–RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Rankin, L. C. et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat. Immunol. 14, 389–395 (2013).
Pickard, J. M. et al. Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness. Nature 514, 638 (2014).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Pham, T. A. N. et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe 16, 504–516 (2014).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282 (2008).
Sawa, S. et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 12, 320–326 (2011).
Aparicio-Domingo, P. et al. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J. Exp. Med. 212, 1783–1791 (2015).
Lindemans, C. A. et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564 (2015).
Lee, J. S. et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13, 144–152 (2012).
Song, C. et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J. Exp. Med. 212, 1869–1882 (2015).
Mao, K. et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554, 255–259 (2018).
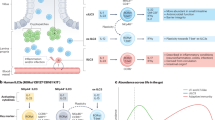
Godinho-Silva, C. et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature 574, 254–258 (2019).
Teng, F. et al. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci. Immunol. 4, eaax1215 (2019).
Wang, Q. et al. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci. Immunol. 4, eaay7501 (2019).
Seillet, C. et al. The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat. Immunol. 21, 168–177 (2020).
Arjona, A. & Sarkar, D. K. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 174, 7618–7624 (2005).
Logan, R. W., Wynne, O., Levitt, D., Price, D. & Sarkar, D. K. Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1–/– mutant mice. J. Interf. Cytokine Res. 33, 108–114 (2013).
Manoogian, E. N. C. & Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 39, 59–67 (2017).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes. Dev. 14, 2950–2961 (2000).
Aton, S. J., Colwell, C. S., Harmar, A. J., Waschek, J. & Herzog, E. D. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 8, 476–483 (2005).
Talbot, J. et al. Feeding-dependent VIP neuron–ILC3 circuit regulates the intestinal barrier. Nature 579, 575–580 (2020).
Iwasaki, M., Akiba, Y. & Kaunitz, J. D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: focus on the gastrointestinal system. F1000Res. 8, 1–13 (2019).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Thaiss, C. A. et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167, 1495–1510.e12 (2016).
Wang, Y. et al. The intestinal microbiota regulates body composition through NFIL 3 and the circadian clock. Science 357, 912–916 (2017).
Kuang, Z. et al. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 365, 1428–1434 (2019).
Kinnebrew, M. A. et al. Interleukin 23 production by intestinal CD103+CD11b+ dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287 (2012).
Savage, A. K., Liang, H.-E. & Locksley, R. M. The development of steady-state activation hubs between adult LTi ILC3s and primed macrophages in small intestine. J. Immunol. 199, 1912–1922 (2017).
He, W. et al. Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte subsets to tissues. Immunity 49, 1175–1190.e7 (2018).
Woldt, E. et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 19, 1039–1046 (2013).
Sengupta, S. et al. The circadian gene Rev-erbα improves cellular bioenergetics and provides preconditioning for protection against oxidative stress. Free. Radic. Biol. Med. 93, 177–189 (2016).
Duez, H. et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbα. Gastroenterology 135, 689–698 (2008).
Le Martelot, G. et al. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 7, 1–12 (2009).
Sitaula, S., Zhang, J., Ruiz, F. & Burris, T. P. Rev-erb regulation of cholesterologenesis. Biochem. Pharmacol. 131, 68–77 (2017).
Santori, F. R. et al. Identification of natural RORγ ligands that regulate the development of lymphoid cells. Cell Metab. 21, 286–298 (2015).
Soroosh, P. et al. Oxysterols are agonist ligands of RORγt and drive TH17 cell differentiation. Proc. Natl Acad. Sci. USA 111, 12163–12168 (2014).
Saleh, M. M. et al. Colitis-induced TH17 cells increase the risk for severe subsequent Clostridium difficile infection. Cell Host Microbe 25, 756–765.e5 (2019).
Nakagawa, T. et al. Endogenous IL-17 as a factor determining the severity of Clostridium difficile infection in mice. J. Med. Microbiol. 65, 821–827 (2016).
Yu, H. et al. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin. Vaccine Immunol. 24, 1–11 (2017).
Balsalobre, A. et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 (2000).
Seshadri, S., Pope, R. L. & Zenewicz, L. A. Glucocorticoids inhibit group 3 innate lymphocyte IL-22 production. J. Immunol. 201, 1267–1274 (2018).
Kohsaka, A. et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 6, 414–421 (2007).
Mendoza, J., Pévet, P. & Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 586, 5901–5910 (2008).
Kim, H. Y. et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 20, 54–61 (2014).
Sasaki, T. et al. Innate lymphoid cells in the induction of obesity. Cell Rep. 28, 202–217.e7 (2019).
Huang, Y. et al. IL-25-responsive, lineage-negative KLRG1hi cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169 (2015).
Acknowledgements
The authors thank S. Gilfillan and B. Bhattarai for helpful feedback. This work was supported by National Institutes of Health (NIH) grants AI095542, DE025884, AI134236 and AI134035 (to M.C.) and T32 GM007200 (to Q.W.). M.C. receives research support from Pfizer, Crohn’s & Colitis Foundation and an anonymous donor in New York.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.C. receives research support from Pfizer. Q.W. declares no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks C. Scheiermann, H. Spits and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Entrainment
-
Synchronization of the endogenous clock to the period of an external oscillation (zeitgeber).
- Phase advance
-
A shift in the organism’s circadian rhythm such that it starts earlier. For the studies reviewed here, this is achieved through advance in the light schedule or earlier onset of light.
- Suprachiasmatic nucleus
-
(SCN). A group of neurons in the ventral hypothalamus that serve as the circadian pacemaker.
- Zeitgebers
-
(Literally, ‘timegivers’). Any external time cues capable of entraining an organism.
- Zeitgeber time
-
(ZT). A unit of time defined with reference to entraining environmental cues. In many cases, the zeitgeber time is designated in reference to light, with ZT0 denoting onset of light and ZT12 denoting onset of darkness.
Rights and permissions
About this article
Cite this article
Wang, Q., Colonna, M. Keeping time in group 3 innate lymphoid cells. Nat Rev Immunol 20, 720–726 (2020). https://doi.org/10.1038/s41577-020-0397-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-0397-z
This article is cited by
-
Microbial circadian clocks: host-microbe interplay in diel cycles
BMC Microbiology (2023)
-
Gegen Qinlian Decoction treatment of asymptomatic hyperuricemia by targeting circadian immune function
Chinese Medicine (2023)