Abstract
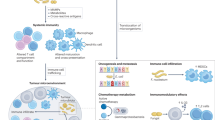
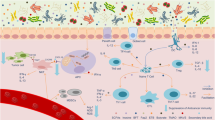
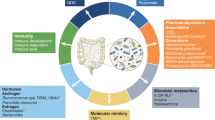
There is currently much interest in defining how the microbiota shapes immune responses in the context of cancer. Various studies in both mice and humans have associated particular commensal species with better (or worse) outcomes in different cancer types and following treatment with cancer immunotherapies. However, the mechanisms involved remain ill-defined and even controversial. In this Viewpoint, Nature Reviews Immunology has invited six eminent scientists in the field to share their thoughts on the key questions and challenges for the field.
The contributors
B. Brett Finlay is a professor in the Michael Smith Laboratories at the University of British Columbia. His research interests are focused on host–microorganism interactions, at the molecular level, and he has published more than 500 articles. He is a co-author of the books Let Them Eat Dirt and The Whole-Body Microbiome.
Romina Goldszmid is an Earl Stadtman Investigator and Head of the Inflammatory Cell Dynamics Section in the Laboratory of Integrative Cancer Immunology, National Cancer Institute, National Institutes of Health (NIH). She received her PhD degree from the University of Buenos Aires, Argentina, and completed her postdoctoral training at the National Institute of Allergy and Infectious Diseases, NIH. She has a long-standing interest in understanding myeloid cell development, differentiation and function in the context of cancer and infections. Current work in her laboratory aims to dissect the myeloid cell repertoire within tumours, determine their contribution to therapy efficacy and unravel the mechanisms by which the microbiota regulates their function.
Kenya Honda is a professor at Keio University School of Medicine and a team leader at the RIKEN Center for Integrative Medical Sciences, Japan. His laboratory has been aiming to elucidate and translate the mutualistic relationship between the gut microbiota and the host immune system using gnotobiotic animal models.
Giorgio Trinchieri is an NIH Distinguished Investigator and Chief of the Laboratory of Integrative Cancer Immunology, Center for Cancer Research, National Cancer Institute, NIH. His research has focused for many years on the interplay between inflammation, innate resistance and adaptive immunity and on the role of pro-inflammatory cytokines and interferons in the regulation of haematopoiesis, innate resistance and immunity against infections and tumours. The present focus of his laboratory is on the role of inflammation, innate resistance, immunity and the commensal microbiota in carcinogenesis, cancer progression and prevention of or therapy for cancer.
Jennifer Wargo is a professor of surgical oncology and genomic medicine at the University of Texas MD Anderson Cancer Center. She leads the Program for Innovative Microbiome and Translational Research (PRIME-TR) and is internationally recognized for her contributions to cancer research in immunotherapy and the microbiome.
Laurence Zitvogel, full professor at Paris-Saclay University, is a research director at INSERM and Scientific Director of the immuno-oncology program at Gustave Roussy, France. She has contributed to the field of cancer immunology and immunotherapy, pioneering the concepts of immunogenic cell death and gut microbiota in cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Routy, B. et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Pushalkar, S. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416 (2018).
Groza, D. et al. Bacterial ghosts as adjuvant to oxaliplatin chemotherapy in colorectal carcinomatosis. OncoImmunology 7, e1424676 (2018).
Anker, J. F. et al. Multi-faceted immunomodulatory and tissue-tropic clinical bacterial isolate potentiates prostate cancer immunotherapy. Nat. Commun. 9, 1591 (2018).
Bhatt, A. P., Redinbo, M. R. & Bultman, S. J. The role of the microbiome in cancer development and therapy. CA. Cancer J. Clin. 67, 326–344 (2017).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Linehan, J. L. et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 172, 784–796 (2018).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806 (2019).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Gil-Cruz, C. et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 366, 881–886 (2019).
Viaud, S. et al. The intestinal microbiota modulates the anti cancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Lee, C. et al. NOD2 supports crypt survival and epithelial regeneration after radiation-induced injury. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20174297 (2019).
Litvak, Y. & Bäumler, A. J. Microbiota-nourishing immunity: a guide to understanding our microbial self. Immunity 51, 214–224 (2019).
Koyama, M. et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity 51, 885–898 (2019).
McDonald, D. et al. American gut: an open platform for citizen science microbiome research. mSystems https://doi.org/10.1128/mSystems.00031-18 (2018).
Vetizou, M. & Trinchieri, G. Anti-PD1 in the wonder-gut-land. Cell Res. 28, 263–264 (2018).
He, Y. et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 24, 1532–1535 (2018).
Helmink, B. A. et al. The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388 (2019).
Gharaibeh, R. Z. & Jobin, C. Microbiota and cancer immunotherapy: in search of microbial signals. Gut 68, 385–388 (2018).
Batten, M. et al. Low intestinal microbial diversity is associated with severe immune-related adverse events and lack of response to neoadjuvant combination anti-PD1, anti-CTLA4 immunotherapy. Cancer Res. https://doi.org/10.1158/1538-7445.AM2019-2822 (2019).
Peled, J. U. et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 382, 822–834 (2020).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Stevenson, A. et al. Host-microbe interactions mediating antitumorigenic effects of MRX0518, a gut microbiota-derived bacterial strain, in breast, renal and lung carcinoma. J. Clin. Oncol. 36, e15006 (2018).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Wang, Y. et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 24, 1804–1808 (2018).
Shepherd, E. S. et al. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 557, 434–438 (2018).
Patnode, M. L. et al. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell 179, 59–73 (2019).
Staley, C. et al. Durable long-term bacterial engraftment following encapsulated fecal microbiota transplantation to treat clostridium difficile infection. MBio 10, e01586–e01619 (2019).
Kaiser, J. Fecal transplants could help patients on cancer immunotherapy drugs. Science https://doi.org/10.1126/science.aax5960 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03341143 (2020).
McQuade, J. L. et al. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 20, e77–e91 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03353402 (2019).
Baruch, E. N. et al. Abstract CT042: fecal microbiota transplantation (FMT) and re-induction of anti-PD-1 therapy in refractory metastatic melanoma patients - preliminary results from a phase I clinical trial (NCT03353402). Cancer Res. https://doi.org/10.1158/1538-7445.AM2019-CT042 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03341143 (2020).
Maleki, S. Combination of fecal microbiota transplantation from healthy donors with anti-PD1 immunotherapy in treatment-naïve advanced or metastatic melanoma patients. SITC https://sitc.sitcancer.org/2019/abstracts/titles/index.php?filter=Clinical+Trial+In+Progress (2019).
Derosa, L. et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 29, 1437–1444 (2018).
Chalabi, M. et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.01.006 (2020).
Pflug, N. et al. Efficacy of antineoplastic treatment is associated with the use of antibiotics that modulate intestinal microbiota. Oncoimmunology 5, e1150399 (2016).
Kaderbhai, C. et al. Antibiotic use does not appear to influence response to nivolumab. Anticancer Res. 37, 3195–3200 (2017).
Shono, Y. et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 8, 339ra371 (2016).
Taur Y., et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 55, 905-914.
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Wilson, B. E., Routy, B., Nagrial, A. & Chin, V. T. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol. Immunother. 69, 343–354 (2020).
Pinato, D. J. et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 5, 1774–1778 (2019).
Huang, X.-Z. et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology 8, e1665973 (2019).
Elkrief, A. et al. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor. Ann. Oncol. 30, 1572–1579 (2019).
Hill, C. et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Suez, J. et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174, 1406–1423 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
National Center for Complementary and Integrative Health. Statistics from the National Health Interview Survey. NIH https://www.nccih.nih.gov/health/statistics-from-the-national-health-interview-survey (2017).
Spencer, C. N. et al. Abstract 2838: the gut microbiome (GM) and immunotherapy response are influenced by host lifestyle factors. Cancer Res. https://doi.org/10.1158/1538-7445.AM2019-2838 (2019).
Arthur, J. C. et al. VSL#3 probiotic modifies mucosal microbial composition but does not reduce colitis-associated colorectal cancer. Sci. Rep. 3, 2868 (2013).
Suez, J. et al. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Acknowledgements
K.H. was funded through AMED LEAP under grant number JP18gm0010003. R.G. is supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute. The L.Z. laboratory is supported by RHU Torino Lumière (ANR-16-RHUS-0008), the ONCOBIOME project (European Union Horizon 2020 programme), the Seerave Foundation, the French Agence Nationale de la Recherche (Ileobiome), the French Ligue Contre le Cancer (Équipe Labelisées programme), the French Association pour la Recherche sur le Cancer, Cancéropôle Ile-de-France, the French Fondation pour la Recherche Médicale, a donation by Elior and Dassault Systems, the European Research Council, Fondation Carrefour, the French Institut National du Cancer INSERM (HTE programme), LabEx Immuno-Oncology, SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE) and the CARE network (directed by X. Mariette, Assistance Publique — Hôpitaux de Paris, Kremlin-Bicêtre).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
B.B.F. is a cofounding scientific advisory board member for Vedanta Biosciences. K.H. is a scientific advisory board member of Vedanta Biosciences and 4BIO CAPITAL. J.W. reports compensation and honoraria from Imedex, Dava Oncology, Omniprex, Ilumina, Gilead, PeerView, Physician Education Resource, MedImmune and Bristol-Myers Squibb. J.W. serves as a consultant advisory board member for Roche/Genentech, Novartis, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb, Merck, Biothera Pharmaceuticals and Microbiome DX. J.W. also receives research support from GlaxoSmithKline, Roche/Genentech, Bristol-Myers Squibb and Novartis. J.W. is an adviser and has stock options for Ella Therapeutics. L.Z. is a founder of everImmune, a biotechnology company that develops anticancer probiotics and diagnostic tools to define intestinal dysbiosis in cancer. L.Z. also has an active scientific collaboration (research contract) with Kaleido, Innovate Pharma and Bioaster, which are companies involved in the microbiome space. R.G. and G.T. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Finlay, B.B., Goldszmid, R., Honda, K. et al. Can we harness the microbiota to enhance the efficacy of cancer immunotherapy?. Nat Rev Immunol 20, 522–528 (2020). https://doi.org/10.1038/s41577-020-0374-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-0374-6
This article is cited by
-
Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors
Nature Reviews Clinical Oncology (2023)
-
Immune checkpoint blockade breaches the mucosal firewall to induce gut microbiota translocation
Nature Reviews Immunology (2023)
-
The Microbiome in Advanced Melanoma: Where Are We Now?
Current Oncology Reports (2023)
-
Cancer bio-immunotherapy XVIII annual NIBIT-(Italian network for tumor biotherapy) meeting, October 15–16, 2020
Cancer Immunology, Immunotherapy (2022)
-
The role of the tumor microbe microenvironment in the tumor immune microenvironment: bystander, activator, or inhibitor?
Journal of Experimental & Clinical Cancer Research (2021)