Abstract
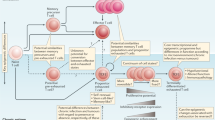
Following their exit from the thymus, T cells are endowed with potent effector functions but must spare host tissue from harm. The fate of these cells is dictated by a series of checkpoints that regulate the quality and magnitude of T cell-mediated immunity, known as tolerance checkpoints. In this Perspective, we discuss the mediators and networks that control the six main peripheral tolerance checkpoints throughout the life of a T cell: quiescence, ignorance, anergy, exhaustion, senescence and death. At the naive T cell stage, two intrinsic checkpoints that actively maintain tolerance are quiescence and ignorance. In the presence of co-stimulation-deficient T cell activation, anergy is a dominant hallmark that mandates T cell unresponsiveness. When T cells are successfully stimulated and reach the effector stage, exhaustion and senescence can limit excessive inflammation and prevent immunopathology. At every stage of the T cell’s journey, cell death exists as a checkpoint to limit clonal expansion and to terminate unrestrained responses. Here, we compare and contrast the T cell tolerance checkpoints and discuss their specific roles, with the aim of providing an integrated view of T cell peripheral tolerance and fate regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Rouse, B. T. & Sehrawat, S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 10, 514–526 (2010).
Skapenko, A., Leipe, J., Lipsky, P. E. & Schulze-Koops, H. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 7, S4–S14 (2005).
Bouneaud, C., Kourilsky, P. & Bousso, P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity 13, 829–840 (2000).
Gallegos, A. M. & Bevan, M. J. Central tolerance: good but imperfect. Immunol. Rev. 209, 290–296 (2006).
Liu, G. Y. et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3, 407–415 (1995).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Saligrama, N. et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572, 481–487 (2019).
Hasegawa, H. & Matsumoto, T. Mechanisms of tolerance induction by dendritic cells in vivo. Front. Immunol. 9, 350 (2018).
Zhang, S. et al. Newly generated CD4+ T cells acquire metabolic quiescence after thymic egress. J. Immunol. 200, 1064–1077 (2018).
Chapman, N. M., Boothby, M. R. & Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70 (2020).
Hamilton, S. E. & Jameson, S. C. CD8 T cell quiescence revisited. Trends Immunol. 33, 224–230 (2012).
Wildey, G. M. & Howe, P. H. Runx1 is a co-activator with FOXO3 to mediate transforming growth factor beta (TGFbeta)-induced Bim transcription in hepatic cells. J. Biol. Chem. 284, 20227–20239 (2009).
Tu, E. et al. T cell receptor-regulated TGF-beta type I receptor expression determines T cell quiescence and activation. Immunity 48, 745–759 (2018).
Tzachanis, D. & Boussiotis, V. A. Tob, a member of the APRO family, regulates immunological quiescence and tumor suppression. Cell Cycle 8, 1019–1025 (2009).
Tzachanis, D. et al. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat. Immunol. 2, 1174–1182 (2001).
Matsuda, S., Rouault, J., Magaud, J. & Berthet, C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 497, 67–72 (2001).
ElTanbouly, M. A. et al. VISTA is a checkpoint regulator for naive T cell quiescence and peripheral tolerance. Science https://doi.org/10.1126/science.aay0524 (2020).
Hwang, S. S. et al. mRNA destabilization by BTG1 and BTG2 maintains T cell quiescence. Science 367, 1255–1260 (2020).
Howden, A. J. M. et al. Quantitative analysis of T cell proteomes and environmental sensors during T cell differentiation. Nat. Immunol. 20, 1542–1554 (2019).
Buckley, A. F., Kuo, C. T. & Leiden, J. M. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat. Immunol. 2, 698–704 (2001).
Haaland, R. E., Yu, W. & Rice, A. P. Identification of LKLF-regulated genes in quiescent CD4+ T lymphocytes. Mol. Immunol. 42, 627–641 (2005).
Kuo, C. T., Veselits, M. L. & Leiden, J. M. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science 277, 1986–1990 (1997).
Wu, J. & Lingrel, J. B. KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1. Oncogene 23, 8088–8096 (2004).
Bai, A., Hu, H., Yeung, M. & Chen, J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J. Immunol. 178, 7632–7639 (2007).
Carlson, C. M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Kerdiles, Y. M. et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 10, 176–184 (2009).
Ouyang, W., Beckett, O., Flavell, R. A. & Li, M. O. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 30, 358–371 (2009).
Ouyang, W. & Li, M. O. Foxo: in command of T lymphocyte homeostasis and tolerance. Trends Immunol. 32, 26–33 (2011).
Wong, W. F. et al. The artificial loss of Runx1 reduces the expression of quiescence-associated transcription factors in CD4+ T lymphocytes. Mol. Immunol. 68, 223–233 (2015).
Wong, W. F. et al. Runx1 deficiency in CD4+ T cells causes fatal autoimmune inflammatory lung disease due to spontaneous hyperactivation of cells. J. Immunol. 188, 5408–5420 (2012).
Wu, Q. et al. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J. Immunol. 187, 1106–1112 (2011).
Yang, K., Neale, G., Green, D. R., He, W. & Chi, H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12, 888–897 (2011).
Neama, A. F., Looi, C. Y. & Wong, W. F. in Lymphocyte Updates - Cancer, Autoimmunity and Infection (ed Isvoranu, G.) (InTechOpen, 2017).
Chang, M. et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 12, 1002–1009 (2011).
Gorelik, L. & Flavell, R. A. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 (2000).
Rubtsov, Y. P. & Rudensky, A. Y. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 7, 443–453 (2007).
ElTanbouly, M. A., Schaafsma, E., Noelle, R. J. & Lines, J. L. VISTA: coming of age as a multi-lineage immune checkpoint. Clin. Exp. Immunol. https://doi.org/10.1111/cei.13415 (2020).
ElTanbouly, M. A., Croteau, W., Noelle, R. J. & Lines, J. L. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin. Immunol. 42, 101308 (2019).
ElTanbouly, M. A. et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science 367, eaay0524 (2020).
Wang, L. et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc. Natl Acad. Sci. USA 111, 14846–14851 (2014).
van den Broek, T., Borghans, J. A. M. & van Wijk, F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 18, 363–373 (2018).
Baranzini, S. E. The role of antiproliferative gene Tob1 in the immune system. Clin. Exp. Neuroimmunol. 5, 132–136 (2014).
Bista, P., Mele, D. A., Baez, D. V. & Huber, B. T. Lymphocyte quiescence factor Dpp2 is transcriptionally activated by KLF2 and TOB1. Mol. Immunol. 45, 3618–3623 (2008).
Wolf, T. et al. Dynamics in protein translation sustaining T cell preparedness. Nat. Immunol. https://doi.org/10.1038/s41590-020-0714-5 (2020).
Parish, I. A. & Heath, W. R. Too dangerous to ignore: self-tolerance and the control of ignorant autoreactive T cells. Immunol. Cell Biol. 86, 146–152 (2008).
Janeway C. A. Jr., Travers, P., Walport, M. & Shlomchik, M. J. Immunobiology. the immune system in health and disease 5th edn (Garland Science, 2001).
Legoux, F. P. et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity 43, 896–908 (2015).
Malhotra, D. et al. Tolerance is established in polyclonal CD4+ T cells by distinct mechanisms, according to self-peptide expression patterns. Nat. Immunol. 17, 187–195 (2016).
Kurts, C. et al. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc. Natl Acad. Sci. USA 96, 12703–12707 (1999).
Ohashi, P. S. et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991).
Oldstone, M. B., Nerenberg, M., Southern, P., Price, J. & Lewicki, H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 65, 319–331 (1991).
Cao, Y. et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl Med. 7, 287ra274 (2015).
Danke, N. A., Koelle, D. M., Yee, C., Beheray, S. & Kwok, W. W. Autoreactive T cells in healthy individuals. J. Immunol. 172, 5967–5972 (2004).
Snir, O. et al. Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum. 63, 2873–2883 (2011).
Heath, W. R. et al. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature 359, 547–549 (1992).
Ramanathan, S. et al. Exposure to IL-15 and IL-21 enables autoreactive CD8 T cells to respond to weak antigens and cause disease in a mouse model of autoimmune diabetes. J. Immunol. 186, 5131–5141 (2011).
Miller, S. D. et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3, 1133–1136 (1997).
Vezys, V. & Lefrancois, L. Cutting edge: inflammatory signals drive organ-specific autoimmunity to normally cross-tolerizing endogenous antigen. J. Immunol. 169, 6677–6680 (2002).
DeSilva, D. R., Feeser, W. S., Tancula, E. J. & Scherle, P. A. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J. Exp. Med. 183, 2017–2023 (1996).
Fields, P. E., Gajewski, T. F. & Fitch, F. W. Blocked Ras activation in anergic CD4+ T cells. Science 271, 1276–1278 (1996).
Li, W., Whaley, C. D., Mondino, A. & Mueller, D. L. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science 271, 1272–1276 (1996).
Zha, Y. et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat. Immunol. 7, 1166–1173 (2006).
Kang, S. M. et al. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science 257, 1134–1138 (1992).
Macian, F. et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109, 719–731 (2002).
Olenchock, B. A. et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 7, 1174–1181 (2006).
Harris, J. E. et al. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J. Immunol. 173, 7331–7338 (2004).
Safford, M. et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat. Immunol. 6, 472–480 (2005).
Zheng, Y., Zha, Y., Driessens, G., Locke, F. & Gajewski, T. F. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J. Exp. Med. 209, 2157–2163 (2012).
Bandyopadhyay, S. et al. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood 109, 2878–2886 (2007).
Thomas, R. M., Saouaf, S. J. & Wells, A. D. Superantigen-induced CD4+ T cell tolerance is associated with DNA methylation and histone hypo-acetylation at cytokine gene loci. Genes. Immun. 8, 613–618 (2007).
Villarino, A. V. et al. Posttranscriptional silencing of effector cytokine mRNA underlies the anergic phenotype of self-reactive T cells. Immunity 34, 50–60 (2011).
Liu, X. et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 567, 525–529 (2019).
Pape, K. A., Merica, R., Mondino, A., Khoruts, A. & Jenkins, M. K. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 160, 4719–4729 (1998).
Rocha, B., Grandien, A. & Freitas, A. A. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J. Exp. Med. 181, 993–1003 (1995).
Rocha, B., Tanchot, C. & Von Boehmer, H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J. Exp. Med. 177, 1517–1521 (1993).
Brown, I. E., Blank, C., Kline, J., Kacha, A. K. & Gajewski, T. F. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J. Immunol. 177, 4521–4529 (2006).
Martinez, R. J. et al. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J. Immunol. 188, 170–181 (2012).
Kalekar, L. A. et al. CD4+ T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol. 17, 304–314 (2016).
Kalekar, L. A. & Mueller, D. L. Relationship between CD4 regulatory T cells and anergy in vivo. J. Immunol. 198, 2527–2533 (2017).
Wells, A. D. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J. Immunol. 182, 7331–7341 (2009).
Delgoffe, G. M. & Powell, J. D. Feeding an army: the metabolism of T cells in activation, anergy, and exhaustion. Mol. Immunol. 68, 492–496 (2015).
Zheng, Y., Delgoffe, G. M., Meyer, C. F., Chan, W. & Powell, J. D. Anergic T cells are metabolically anergic. J. Immunol. 183, 6095–6101 (2009).
Powell, J. D. & Delgoffe, G. M. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33, 301–311 (2010).
Li, L., Iwamoto, Y., Berezovskaya, A. & Boussiotis, V. A. A pathway regulated by cell cycle inhibitor p27Kip1 and checkpoint inhibitor Smad3 is involved in the induction of T cell tolerance. Nat. Immunol. 7, 1157–1165 (2006).
Williams, J. B. et al. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J. Exp. Med. 214, 381–400 (2017).
Maeda, Y. et al. Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science 346, 1536–1540 (2014).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187, 1383–1393 (1998).
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Fuller, M. J. et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174, 5926–5930 (2005).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Wherry, E. J., Barber, D. L., Kaech, S. M., Blattman, J. N. & Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA 101, 16004–16009 (2004).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37 (2009).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Fuertes Marraco, S. A., Neubert, N. J., Verdeil, G. & Speiser, D. E. Inhibitory receptors beyond T cell exhaustion. Front. Immunol. 6, 310 (2015).
Legat, A., Speiser, D. E., Pircher, H., Zehn, D. & Fuertes Marraco, S. A. Inhibitory receptor expression depends more dominantly on differentiation and activation than “exhaustion” of human CD8 T cells. Front. Immunol. 4, 455 (2013).
Utzschneider, D. T. et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14, 603–610 (2013).
Baitsch, L. et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS ONE 7, e30852 (2012).
Duraiswamy, J. et al. Phenotype, function, and gene expression profiles of programmed death-1hi CD8 T cells in healthy human adults. J. Immunol. 186, 4200–4212 (2011).
Angelosanto, J. M., Blackburn, S. D., Crawford, A. & Wherry, E. J. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Collins, M. H. & Henderson, A. J. Transcriptional regulation and T cell exhaustion. Curr. Opin. HIV. AIDS 9, 459–463 (2014).
Quigley, M. et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 16, 1147–1151 (2010).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature https://doi.org/10.1038/s41586-019-1325-x (2019).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature https://doi.org/10.1038/s41586-019-1324-y (2019).
Kallies, A., Zehn, D. & Utzschneider, D. T. Precursor exhausted T cells: key to successful immunotherapy? Nat. Rev. Immunol. 20, 128–136 (2020).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Kratchmarov, R., Magun, A. M. & Reiner, S. L. TCF1 expression marks self-renewing human CD8+ T cells. Blood Adv. 2, 1685–1690 (2018).
McKinney, E. F., Lee, J. C., Jayne, D. R., Lyons, P. A. & Smith, K. G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Zielinski, M. et al. Impact of donor and recipient human cytomegalovirus status on kidney transplantation. Int. Immunol. 29, 541–549 (2017).
Shahbazi, M., Soltanzadeh-Yamchi, M. & Mohammadnia-Afrouzi, M. T cell exhaustion implications during transplantation. Immunol. Lett. 202, 52–58 (2018).
Steger, U. et al. Exhaustive differentiation of alloreactive CD8+ T cells: critical for determination of graft acceptance or rejection. Transplantation 85, 1339–1347 (2008).
Wang, H. et al. Prevention of allograft rejection in heart transplantation through concurrent gene silencing of TLR and kinase signaling pathways. Sci. Rep. 6, 33869 (2016).
Frebel, H. et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209, 2485–2499 (2012).
Zinselmeyer, B. H. et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 210, 757–774 (2013).
Gil, J. Cellular senescence causes ageing. Nat. Rev. Mol. Cell Biol. 20, 388 (2019).
Reed, J. R. et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J. Exp. Med. 199, 1433–1443 (2004).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018).
Hildeman, D. A., Mitchell, T., Kappler, J. & Marrack, P. T cell apoptosis and reactive oxygen species. J. Clin. Invest. 111, 575–581 (2003).
Hildeman, D. A. et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10, 735–744 (1999).
Yarosz, E. L. & Chang, C. H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 18, e14 (2018).
Akbar, A. N., Henson, S. M. & Lanna, A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 37, 866–876 (2016).
Libri, V. et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process. Immunology 132, 326–339 (2011).
Plunkett, F. J. et al. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 178, 7710–7719 (2007).
Henson, S. M., Macaulay, R., Riddell, N. E., Nunn, C. J. & Akbar, A. N. Blockade of PD-1 or p38 MAP kinase signaling enhances senescent human CD8+ T-cell proliferation by distinct pathways. Eur. J. Immunol. 45, 1441–1451 (2015).
Di Mitri, D. et al. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J. Immunol. 187, 2093–2100 (2011).
Lanna, A., Henson, S. M., Escors, D. & Akbar, A. N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 15, 965–972 (2014).
Chou, J. P. & Effros, R. B. T cell replicative senescence in human aging. Curr. Pharm. Des. 19, 1680–1698 (2013).
Goronzy, J. J. & Weyand, C. M. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res. Ther. 5, 225–234 (2003).
Zhao, Y., Shao, Q. & Peng, G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol. Immunol. 17, 27–35 (2020).
Pereira, B. I. et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. https://doi.org/10.1038/s41590-020-0643-3 (2020).
Akbar, A. N., Beverley, P. C. & Salmon, M. Will telomere erosion lead to a loss of T-cell memory? Nat. Rev. Immunol. 4, 737–743 (2004).
Aspinall, R., Del Giudice, G., Effros, R. B., Grubeck-Loebenstein, B. & Sambhara, S. Challenges for vaccination in the elderly. Immun. Ageing 4, 9 (2007).
Grubeck-Loebenstein, B. et al. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 21, 201–209 (2009).
Beltran-Sanchez, H., Soneji, S. & Crimmins, E. M. Past, present, and future of healthy life expectancy. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a025957 (2015).
Yoshikawa, T. T. Perspective: aging and infectious diseases: past, present, and future. J. Infect. Dis. 176, 1053–1057 (1997).
Berger, R., Florent, G. & Just, M. Decrease of the lymphoproliferative response to varicella-zoster virus antigen in the aged. Infect. Immun. 32, 24–27 (1981).
Fulop, T., Larbi, A. & Pawelec, G. Human T cell aging and the impact of persistent viral infections. Front. Immunol. 4, 271 (2013).
Ouyang, Q. et al. An age-related increase in the number of CD8+ T cells carrying receptors for an immunodominant Epstein-Barr virus (EBV) epitope is counteracted by a decreased frequency of their antigen-specific responsiveness. Mech. Ageing Dev. 124, 477–485 (2003).
Kurts, C., Kosaka, H., Carbone, F. R., Miller, J. F. & Heath, W. R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186, 239–245 (1997).
Kyburz, D. et al. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur. J. Immunol. 23, 1956–1962 (1993).
Liblau, R. S. et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc. Natl Acad. Sci. USA 93, 3031–3036 (1996).
Davey, G. M. et al. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J. Exp. Med. 196, 947–955 (2002).
Hildeman, D. A., Zhu, Y., Mitchell, T. C., Kappler, J. & Marrack, P. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 14, 354–359 (2002).
Boise, L. H. et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 3, 87–98 (1995).
Burr, J. S. et al. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J. Immunol. 166, 5331–5335 (2001).
Okkenhaug, K. et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2, 325–332 (2001).
Rathmell, J. C., Farkash, E. A., Gao, W. & Thompson, C. B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167, 6869–6876 (2001).
Silva-Filho, J. L., Caruso-Neves, C. & Pinheiro, A. A. S. IL-4: an important cytokine in determining the fate of T cells. Biophys. Rev. 6, 111–118 (2014).
Parish, I. A. et al. The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood 113, 4575–4585 (2009).
Hochweller, K. & Anderton, S. M. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur. J. Immunol. 35, 1086–1096 (2005).
Rajpal, A. et al. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 22, 6526–6536 (2003).
Barron, L., Knoechel, B., Lohr, J. & Abbas, A. K. Cutting edge: contributions of apoptosis and anergy to systemic T cell tolerance. J. Immunol. 180, 2762–2766 (2008).
Green, D. R., Droin, N. & Pinkoski, M. Activation-induced cell death in T cells. Immunol. Rev. 193, 70–81 (2003).
Kawabe, Y. & Ochi, A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature 349, 245–248 (1991).
Snow, A. L., Pandiyan, P., Zheng, L., Krummey, S. M. & Lenardo, M. J. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol. Rev. 236, 68–82 (2010).
Dhein, J., Walczak, H., Baumler, C., Debatin, K. M. & Krammer, P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373, 438–441 (1995).
Krammer, P. H. CD95’s deadly mission in the immune system. Nature 407, 789–795 (2000).
Krammer, P. H., Arnold, R. & Lavrik, I. N. Life and death in peripheral T cells. Nat. Rev. Immunol. 7, 532–542 (2007).
Suzuki, I. & Fink, P. J. The dual functions of fas ligand in the regulation of peripheral CD8+ and CD4+ T cells. Proc. Natl Acad. Sci. USA 97, 1707–1712 (2000).
Tham, E. L. & Mescher, M. F. The poststimulation program of CD4 versus CD8 T cells (death versus activation-induced nonresponsiveness). J. Immunol. 169, 1822–1828 (2002).
Zheng, L. et al. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 377, 348–351 (1995).
Kirchhoff, S., Muller, W. W., Krueger, A., Schmitz, I. & Krammer, P. H. TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity. J. Immunol. 165, 6293–6300 (2000).
Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003).
Kalia, V. et al. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Lenardo, M. J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature 353, 858–861 (1991).
Refaeli, Y., Parijs, L. V., London, C. A., Tschopp, J. & Abbas, A. K. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8, 615–623 (1998).
Marrack, P. & Kappler, J. Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 (2004).
Ch’en, I. L., Tsau, J. S., Molkentin, J. D., Komatsu, M. & Hedrick, S. M. Mechanisms of necroptosis in T cells. J. Exp. Med. 208, 633–641 (2011).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Faliti, C. E. et al. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J. Exp. Med. 216, 317–336 (2019).
Kunzli, M. et al. Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aay5552 (2020).
Andreas Linder, S. B. et al. CARD8 inflammasome activation triggers pyroptosis in human T cells. EMBO J. https://doi.org/10.15252/embj.2020105071 (2020).
Johnson, D. C. et al. DPP8/9 inhibitors activate the CARD8 inflammasome in resting lymphocytes. Cell Death Dis. 11, 628 (2020).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Johnston, R. J. et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 26, 923–937 (2014).
Yang, Z. Z. et al. Expression of LAG-3 defines exhaustion of intratumoral PD-1+ T cells and correlates with poor outcome in follicular lymphoma. Oncotarget 8, 61425–61439 (2017).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Otano, I. et al. Human CD8 T cells are susceptible to TNF-mediated activation-induced cell death. Theranostics 10, 4481–4489 (2020).
Janssen, E. M. et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005).
Martinez-Lorenzo, M. J. et al. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur. J. Immunol. 28, 2714–2725 (1998).
Whiting, C. C., Su, L. L., Lin, J. T. & Fathman, C. G. GRAIL: a unique mediator of CD4 T-lymphocyte unresponsiveness. FEBS J. 278, 47–58 (2011).
Fang, D. et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 3, 281–287 (2002).
Venuprasad, K. Cbl-b and itch: key regulators of peripheral T-cell tolerance. Cancer Res. 70, 3009–3012 (2010).
Chemnitz, J. M., Parry, R. V., Nichols, K. E., June, C. H. & Riley, J. L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 173, 945–954 (2004).
Yokosuka, T. et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 209, 1201–1217 (2012).
LaFleur, M. W. et al. PTPN2 regulates the generation of exhausted CD8+ T cell subpopulations and restrains tumor immunity. Nat. Immunol. 20, 1335–1347 (2019).
Masson, F., Kupresanin, F., Mount, A., Strasser, A. & Belz, G. T. Bid and Bim collaborate during induction of T cell death in persistent infection. J. Immunol. 186, 4059–4066 (2011).
Garaud, S. et al. FOXP1 is a regulator of quiescence in healthy human CD4+ T cells and is constitutively repressed in T cells from patients with lymphoproliferative disorders. Eur. J. Immunol. 47, 168–179 (2017).
Wei, H. et al. Cutting edge: Foxp1 controls naive CD8+ T cell quiescence by simultaneously repressing key pathways in cellular metabolism and cell cycle progression. J. Immunol. 196, 3537–3541 (2016).
Man, K. et al. Transcription factor IRF4 promotes CD8+ T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity 47, 1129–1141 (2017).
Singer, M. et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell 166, 1500–1511 (2016).
Shin, H. et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Li, J., He, Y., Hao, J., Ni, L. & Dong, C. High levels of Eomes promote exhaustion of anti-tumor CD8+ T cells. Front. Immunol. 9, 2981 (2018).
Sprent, J. & Surh, C. D. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 12, 478–484 (2011).
Allam, A. et al. The CD8+ memory T-cell state of readiness is actively maintained and reversible. Blood 114, 2121–2130 (2009).
Latner, D. R., Kaech, S. M. & Ahmed, R. Enhanced expression of cell cycle regulatory genes in virus-specific memory CD8+ T cells. J. Virol. 78, 10953–10959 (2004).
Youngblood, B. et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552, 404–409 (2017).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Milner, J. J. et al. Heterogenous populations of tissue-resident CD8+ T cells are generated in response to infection and malignancy. Immunity 52, 808–824 (2020).
Carbone, F. R. Immovable memories: the journey to permanent residency. Nat. Immunol. 21, 698–699 (2020).
Driessens, G., Kline, J. & Gajewski, T. F. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol. Rev. 229, 126–144 (2009).
Tirapu, I. et al. Low surface expression of B7-1 (CD80) is an immunoescape mechanism of colon carcinoma. Cancer Res. 66, 2442–2450 (2006).
Wu, T. C., Huang, A. Y., Jaffee, E. M., Levitsky, H. I. & Pardoll, D. M. A reassessment of the role of B7-1 expression in tumor rejection. J. Exp. Med. 182, 1415–1421 (1995).
Zou, W. & Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 8, 467–477 (2008).
Cuenca, A. et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 63, 9007–9015 (2003).
Mescher, M. F., Popescu, F. E., Gerner, M., Hammerbeck, C. D. & Curtsinger, J. M. Activation-induced non-responsiveness (anergy) limits CD8 T cell responses to tumors. Semin. Cancer Biol. 17, 299–308 (2007).
Staveley-O’Carroll, K. et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc. Natl Acad. Sci. USA 95, 1178–1183 (1998).
Abe, B. T., Shin, D. S., Mocholi, E. & Macian, F. NFAT1 supports tumor-induced anergy of CD4+ T cells. Cancer Res. 72, 4642–4651 (2012).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 20, 1231–1243 (2019).
Greenwald, R. J., Boussiotis, V. A., Lorsbach, R. B., Abbas, A. K. & Sharpe, A. H. CTLA-4 regulates induction of anergy in vivo. Immunity 14, 145–155 (2001).
Liu, Y. & Zheng, P. How does an anti-CTLA-4 antibody promote cancer immunity? Trends Immunol. 39, 953–956 (2018).
Redmond, W. L., Gough, M. J. & Weinberg, A. D. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur. J. Immunol. 39, 2184–2194 (2009).
Alonso, R. et al. Induction of anergic or regulatory tumor-specific CD4+ T cells in the tumor-draining lymph node. Nat. Commun. 9, 2113 (2018).
Magen, A. et al. Single-cell profiling defines transcriptomic signatures specific to tumor-reactive versus virus-responsive CD4+ T cells. Cell Rep. 29, 3019–3032 e3016 (2019).
Speiser, D. E., Ho, P. C. & Verdeil, G. Regulatory circuits of T cell function in cancer. Nat. Rev. Immunol. 16, 599–611 (2016).
Kahan, S. M., Wherry, E. J. & Zajac, A. J. T cell exhaustion during persistent viral infections. Virology 479–480, 180–193 (2015).
Schietinger, A. et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 45, 389–401 (2016).
Willimsky, G. & Blankenstein, T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 437, 141–146 (2005).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019).
Acknowledgements
The authors thank S. Hedrick and C. Burns for their valuable suggestions and discussion.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.J.N. is an inventor on patent applications (10035857, 9631018, 9217035, 8501915, 8465740, 8236304 and 8231872) submitted by Dartmouth College, and patent applications (9890215 and 9381244) submitted by Kings College London and Dartmouth College and is a co-founder of ImmuNext, a company involved in the development of VISTA-related assets. These applications cover the use of VISTA targeting for modulation of the immune response. M.A.E. declares no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks Daniel Utzschneider, Vassiliki Boussiotis and Pamela Ohashi for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
ElTanbouly, M.A., Noelle, R.J. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat Rev Immunol 21, 257–267 (2021). https://doi.org/10.1038/s41577-020-00454-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-00454-2
This article is cited by
-
Macrophage barrier in the tumor microenvironment and potential clinical applications
Cell Communication and Signaling (2024)
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Non-mutational neoantigens in disease
Nature Immunology (2024)
-
Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment
Molecular Cancer (2023)
-
Pathogenesis of autoimmune disease
Nature Reviews Nephrology (2023)