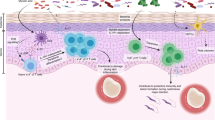
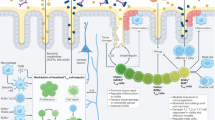
Abstract
γδ T cells are a unique T cell subpopulation that are rare in secondary lymphoid organs but enriched in many peripheral tissues, such as the skin, intestines and lungs. By rapidly producing large amounts of cytokines, γδ T cells make key contributions to immune responses in these tissues. In addition to their immune surveillance activities, recent reports have unravelled exciting new roles for γδ T cells in steady-state tissue physiology, with functions ranging from the regulation of thermogenesis in adipose tissue to the control of neuronal synaptic plasticity in the central nervous system. Here, we review the roles of γδ T cells in tissue homeostasis and in surveillance of infection, aiming to illustrate their major impact on tissue integrity, tissue repair and immune protection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Flajnik, M. F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 18, 438–453 (2018).
Hayday, A. C. gammadelta T cell update: adaptate orchestrators of immune surveillance. J. Immunol. 203, 311–320 (2019).
Haas, J. D. et al. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity 37, 48–59 (2012).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Munoz-Ruiz, M., Sumaria, N., Pennington, D. J. & Silva-Santos, B. Thymic determinants of gammadelta T cell differentiation. Trends Immunol. 38, 336–344 (2017).
Sumaria, N., Martin, S. & Pennington, D. J. Developmental origins of murine gammadelta T-cell subsets. Immunology 156, 299–304 (2019).
Hayday, A. C. & Vantourout, P. The innate biologies of adaptive antigen receptors. Annu. Rev. Immunol. 38, 487–510 (2020).
Afrache, H., Gouret, P., Ainouche, S., Pontarotti, P. & Olive, D. The butyrophilin (BTN) gene family: from milk fat to the regulation of the immune response. Immunogenetics 64, 781–794 (2012).
Boyden, L. M. et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat. Genet. 40, 656–662 (2008).
Lewis, J. M. et al. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat. Immunol. 7, 843–850 (2006). Pioneering work, together with Boyden et al. (2008), on the SKINT1/BTNL family, which identifies SKINT1 as a key stromal determinant of the thymic selection and epidermal homeostasis of Vγ5+ DETCs.
Turchinovich, G. & Hayday, A. C. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity 35, 59–68 (2011).
Di Marco Barros, R. et al. Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell 167, 203–218.e17 (2016). Demonstration that the BTNL paradigm also applies to the selection of intestinal γδ T cell repertoires in both mice and humans.
Melandri, D. et al. The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 19, 1352–1365 (2018).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 120, 2269–2279 (2012).
Sandstrom, A. et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 40, 490–500 (2014).
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, eaay5516 (2020).
Karunakaran, M. M. et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity 52, 487–498.e6 (2020).
Vantourout, P. et al. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing gammadelta T cell biology. Proc. Natl Acad. Sci. USA 115, 1039–1044 (2018).
Willcox, C. R. et al. Butyrophilin-like 3 directly binds a human Vγ4+ T cell receptor using a modality distinct from clonally-restricted antigen. Immunity 51, 813–825.e4 (2019).
Chodaczek, G., Papanna, V., Zal, M. A. & Zal, T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat. Immunol. 13, 272–282 (2012).
Jameson, J. et al. A role for skin γδ T cells in wound repair. Science 296, 747–749 (2002). Seminal article establishing a key role for Vγ5+ DETCs in epidermal wound healing through production of keratinocyte growth factors.
Nielsen, M. M., Witherden, D. A. & Havran, W. L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 17, 733–745 (2017).
Johnson, M. D., Witherden, D. A. & Havran, W. L. The role of tissue-resident T cells in stress surveillance and tissue maintenance. Cells 9, 686 (2020).
Jameson, J. M., Cauvi, G., Sharp, L. L., Witherden, D. A. & Havran, W. L. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J. Exp. Med. 201, 1269–1279 (2005).
MacLeod, A. S. et al. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J. Clin. Invest. 123, 4364–4374 (2013).
MacLeod, A. S. et al. Skin-resident T cells sense ultraviolet radiation-induced injury and contribute to DNA repair. J. Immunol. 192, 5695–5702 (2014).
Pantelyushin, S. et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122, 2252–2256 (2012).
Cai, Y. et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 35, 596–610 (2011).
Gray, E. E. et al. Deficiency in IL-17-committed Vgamma4+ gammadelta T cells in a spontaneous Sox13-mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat. Immunol. 14, 584–592 (2013).
Cai, Y. et al. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat. Commun. 5, 3986 (2014).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17+ gammadelta T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Spidale, N. A. et al. Neonatal-derived IL-17 producing dermal γδ T cells are required to prevent spontaneous atopic dermatitis. eLife 9, e51188 (2020).
Lee, P. et al. Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of γδT-cells. eLife 6, e2887 (2017).
Gay, D. et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 19, 916–923 (2013). Demonstration that dermal γδ T cells produce FGF9, which activates WNT signalling in fibroblasts, in a self-amplifying loop that promotes hair follicle regeneration on wounding.
Edelblum, K. L. et al. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc. Natl Acad. Sci. USA 109, 7097–7102 (2012).
Boismenu, R. & Havran, W. L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science 266, 1253–1255 (1994).
Komano, H. et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc. Natl Acad. Sci. USA 92, 6147–6151 (1995).
Dalton, J. E. et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology 131, 818–829 (2006).
Inagaki-Ohara, K. et al. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J. Immunol. 173, 1390–1398 (2004).
Ahlfors, H. et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
McMenamin, C., Pimm, C., McKersey, M. & Holt, P. G. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science 265, 1869–1871 (1994).
Lahn, M. et al. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat. Med. 5, 1150–1156 (1999).
Zuany-Amorim, C. et al. Requirement for gammadelta T cells in allergic airway inflammation. Science 280, 1265–1267 (1998).
Seymour, B. W., Gershwin, L. J. & Coffman, R. L. Aerosol-induced immunoglobulin (Ig)-E unresponsiveness to ovalbumin does not require CD8+ or T cell receptor (TCR)-gamma/delta+ T cells or interferon (IFN)-gamma in a murine model of allergen sensitization. J. Exp. Med. 187, 721–731 (1998).
Jin, C. et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013.e16 (2019).
Simonian, P. L. et al. gammadelta T cells protect against lung fibrosis via IL-22. J. Exp. Med. 207, 2239–2253 (2010).
Gollwitzer, E. S. et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 20, 642–647 (2014).
de Kleer, I. M. et al. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 45, 1285–1298 (2016).
Wilharm, A. et al. Mutual interplay between IL-17-producing gammadeltaT cells and microbiota orchestrates oral mucosal homeostasis. Proc. Natl Acad. Sci. USA 116, 2652–2661 (2019).
Dutzan, N. et al. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity 46, 133–147 (2017).
Eskan, M. A. et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 13, 465–473 (2012).
Moutsopoulos, N. M. et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci. Transl. Med. 6, 229ra240 (2014).
Yu, J. J. et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109, 3794–3802 (2007).
Krishnan, S. et al. Amphiregulin-producing gammadelta T cells are vital for safeguarding oral barrier immune homeostasis. Proc. Natl Acad. Sci. USA 115, 10738–10743 (2018). An article showing that γδ T cells produce the wound healing-associated cytokine amphiregulin, which prevents spontaneous development of oral inflammatory diseases such as periodontitis.
Hajishengallis, G. et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506 (2011).
Itohara, S. et al. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature 343, 754–757 (1990).
Carding, S. R. & Egan, P. J. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 (2002).
Pinget, G. V. et al. The majority of murine gammadelta T cells at the maternal-fetal interface in pregnancy produce IL-17. Immunol. Cell Biol. 94, 623–630 (2016).
Song, Z. H. et al. Seminal plasma induces inflammation in the uterus through the gammadelta T/IL-17 pathway. Sci. Rep. 6, 25118 (2016).
Monin, L. et al. gammadelta T cells compose a developmentally regulated intrauterine population and protect against vaginal candidiasis. Mucosal Immunol. (2020).
Polese, B. et al. Accumulation of IL-17+ Vgamma6+ gammadelta T cells in pregnant mice is not associated with spontaneous abortion. Clin. Transl. Immunol. 7, e1008 (2018).
Gomez-Lopez, N., StLouis, D., Lehr, M. A., Sanchez-Rodriguez, E. N. & Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell Mol. Immunol. 11, 571–581 (2014).
Wilharm, A. et al. Microbiota-dependent expansion of testicular IL-17-producing Vgamma6+ gammadelta T cells upon puberty promotes local tissue immune surveillance. Mucosal Immunol. https://doi.org/10.1038/s41385-020-0330-6 (2020).
Mukasa, A. et al. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J. Immunol. 155, 2047–2056 (1995).
Mukasa, A., Born, W. K. & O’Brien, R. L. Inflammation alone evokes the response of a TCR-invariant mouse gamma delta T cell subset. J. Immunol. 162, 4910–4913 (1999).
Munoz, G., Posnett, D. N. & Witkin, S. S. Enrichment of gamma delta T lymphocytes in human semen: relation between gamma delta T cell concentration and antisperm antibody status. J. Reprod. Immunol. 22, 47–57 (1992).
Fraczek, M. & Kurpisz, M. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 108, 98–104 (2015).
Colburn, N. T., Zaal, K. J., Wang, F. & Tuan, R. S. A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis Rheum. 60, 1694–1703 (2009).
Ono, T. et al. IL-17-producing gammadelta T cells enhance bone regeneration. Nat. Commun. 7, 10928 (2016). Identification of a key role for γδ17 T cells in skeletal tissue regeneration, namely by enhancing the differentiation of mesenchymal progenitors into osteoblasts during bone formation.
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Mehta, P., Nuotio-Antar, A. M. & Smith, C. W. gammadelta T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J. Leukoc. Biol. 97, 121–134 (2015).
Goldberg, E. L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2, 50–61 (2020).
Kohlgruber, A. C. et al. gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19, 464–474 (2018). Characterization of a population of PLZF+ γδ Tcells that reside in the adipose tissue and produce IL-17 and TNF, which stimulate IL-33 production by stromal cells and activate UCP1 in adipocytes, thus regulating thermogenesis.
Hu, B. et al. gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020). Demonstration that thermogenesis regulation by γδ T cells requires production of IL-17F, which induces IL-17RC-expressing adipocytes to produce transforming growth factor-β, which promotes sympathetic innervation of the adipose tissue.
Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771 (2016).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Ribeiro, M. et al. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci. Immunol. 4, eaay5199 (2019). Identification of meningeal-resident γδ T cells making IL-17 that promotes glial BDNF production and neuronal synaptic plasticity in the hippocampus during short-term learning.
Reisel, D. et al. Spatial memory dissociations in mice lacking GluR1. Nat. Neurosci. 5, 868–873 (2002).
Pikor, N. B. et al. Integration of Th17- and lymphotoxin-derived signals initiates meningeal-resident stromal cell remodeling to propagate neuroinflammation. Immunity 43, 1160–1173 (2015).
Benakis, C. et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 22, 516–523 (2016).
Sheridan, B. S. et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 39, 184–195 (2013). Demonstration of a memory-like protective response of intestinal γδ T cells to oral Listeria infection in mice.
Romagnoli, P. A., Sheridan, B. S., Pham, Q. M., Lefrancois, L. & Khanna, K. M. IL-17A-producing resident memory gammadelta T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc. Natl Acad. Sci. USA 113, 8502–8507 (2016).
Hayday, A. C. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026 (2000).
Ramsburg, E., Tigelaar, R., Craft, J. & Hayday, A. Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite. J. Exp. Med. 198, 1403–1414 (2003).
Gibbons, D. L. et al. Neonates harbour highly active gammadelta T cells with selective impairments in preterm infants. Eur. J. Immunol. 39, 1794–1806 (2009).
Dimova, T. et al. Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc. Natl Acad. Sci. USA 112, E556–E565 (2015).
Molne, L., Corthay, A., Holmdahl, R. & Tarkowski, A. Role of gamma/delta T cell receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus. Clin. Exp. Immunol. 132, 209–215 (2003).
Cho, J. S. et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 (2010).
Murphy, A. G. et al. Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J. Immunol. 192, 3697–3708 (2014).
Dillen, C. A. et al. Clonally expanded gammadelta T cells protect against Staphylococcus aureus skin reinfection. J. Clin. Invest. 128, 1026–1042 (2018).
Lefrancois, L. & Goodman, T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science 243, 1716–1718 (1989).
Shires, J., Theodoridis, E. & Hayday, A. C. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 15, 419–434 (2001).
Fahrer, A. M. et al. Attributes of gammadelta intraepithelial lymphocytes as suggested by their transcriptional profile. Proc. Natl Acad. Sci. USA 98, 10261–10266 (2001).
Ismail, A. S. et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc. Natl Acad. Sci. USA 108, 8743–8748 (2011). Characterization of a rapid production of innate antimicrobial factors on activation of γδ IELs by resident bacteria that penetrate the intestinal epithelium.
Swamy, M. et al. Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat. Commun. 6, 7090 (2015).
Hatano, S., Murakami, T., Noguchi, N., Yamada, H. & Yoshikai, Y. CD5− NK1.1+ gammadelta T cells that develop in a Bcl11b-independent manner participate in early protection against infection. Cell Rep. 21, 1191–1202 (2017).
Schmolka, N. et al. microRNA-146a controls functional plasticity in γδ T cells by targeting NOD1. Sci. Immunol. 3, eaao1392 (2018).
Xu, S. et al. IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J. Immunol. 185, 5879–5887 (2010).
Chen, Y. S. et al. IL-17-producing γδ T cells protect against Clostridium difficile infection. J. Clin. Invest. 130, 2377–2390 (2020). Establishment of a key role for γδ17 T cells in neonatal defence against C. difficile infection, providing a mechanistic explanation for the clinical resistance of infants to C. difficile-induced colitis.
Muzaki, A. et al. Long-lived innate IL-17-producing gamma/delta T cells modulate antimicrobial epithelial host defense in the colon. J. Immunol. 199, 3691–3699 (2017).
Nakasone, C. et al. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 9, 251–258 (2007).
Kirby, A. C., Newton, D. J., Carding, S. R. & Kaye, P. M. Evidence for the involvement of lung-specific gammadelta T cell subsets in local responses to Streptococcus pneumoniae infection. Eur. J. Immunol. 37, 3404–3413 (2007).
Hassane, M. et al. Interleukin-7 protects against bacterial respiratory infection by promoting IL-17A-producing innate T-cell response. Mucosal Immunol. 13, 128–139 (2020).
Kirby, A. C., Newton, D. J., Carding, S. R. & Kaye, P. M. Pulmonary dendritic cells and alveolar macrophages are regulated by gammadelta T cells during the resolution of S. pneumoniae-induced inflammation. J. Pathol. 212, 29–37 (2007).
Lockhart, E., Green, A. M. & Flynn, J. L. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177, 4662–4669 (2006).
Umemura, M. et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 178, 3786–3796 (2007).
Peng, M. Y. et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol. Immunol. 5, 203–208 (2008).
Shen, H. et al. Th17-related cytokines contribute to recall-like expansion/effector function of HMBPP-specific Vgamma2Vdelta2 T cells after Mycobacterium tuberculosis infection or vaccination. Eur. J. Immunol. 45, 442–451 (2015).
Shen, Y. et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science 295, 2255–2258 (2002).
Shen, L. et al. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl Acad. Sci. USA 116, 6371–6378 (2019). Demonstration, together with Shen et al. (2002), of the major role and therapeutic potential of γδ T cells in non-human primate models of tuberculosis.
Misiak, A., Wilk, M. M., Raverdeau, M. & Mills, K. H. IL-17-producing innate and pathogen-specific tissue resident memory gammadelta T cells expand in the lungs of Bordetella pertussis-infected mice. J. Immunol. 198, 363–374 (2017).
Guo, X. J. et al. Lung γδ T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immununity. Immunity 49, 531–544.e6 (2018).
Khairallah, C., Dechanet-Merville, J. & Capone, M. gammadelta T cell-mediated immunity to cytomegalovirus infection. Front. Immunol. 8, 105 (2017).
Pamplona, A. & Silva-Santos, B. gammadelta T cells in malaria: a double-edged sword. FEBS J. https://doi.org/10.1111/febs.15494 (2020).
Dechanet, J. et al. Major expansion of gammadelta T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J. Infect. Dis. 179, 1–8 (1999).
Ravens, S. et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 18, 393–401 (2017).
Prinz, I. et al. Donor Vdelta1+ gammadelta T cells expand after allogeneic hematopoietic stem cell transplantation and show reactivity against CMV-infected cells but not against progressing B-CLL. Exp. Hematol. Oncol. 2, 14 (2013).
Knight, A. et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 116, 2164–2172 (2010).
Vermijlen, D. et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J. Exp. Med. 207, 807–821 (2010).
Lafarge, X. et al. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J. Infect. Dis. 184, 533–541 (2001).
Couzi, L. et al. Common features of gammadelta T cells and CD8+ alphabeta T cells responding to human cytomegalovirus infection in kidney transplant recipients. J. Infect. Dis. 200, 1415–1424 (2009).
Dechanet, J. et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J. Clin. Invest. 103, 1437–1449 (1999).
Halary, F. et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201, 1567–1578 (2005).
Ninomiya, T. et al. Vgamma1+gammadelta T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-gamma. Immunology 99, 187–194 (2000).
Khairallah, C. et al. gammadelta T cells confer protection against murine cytomegalovirus (MCMV). PLoS Pathog. 11, e1004702 (2015).
Sell, S. et al. Control of murine cytomegalovirus infection by γδ T cells. PLoS Pathog. 11, e1004481 (2015). Together with the article by Khairallah et al. (2015), this article provides mechanistic evidence in mouse models for the implication of γδ T cells in CMV infection, as documented in the previous references from studies in humans.
Tsuji, M. et al. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc. Natl Acad. Sci. USA 91, 345–349 (1994).
McKenna, K. C. et al. gammadelta T cells are a component of early immunity against preerythrocytic malaria parasites. Infect. Immun. 68, 2224–2230 (2000).
Mamedov, M. R. et al. A macrophage colony-stimulating-factor-producing γδ T cell subset prevents malarial parasitemic recurrence. Immunity 48, 350–363.e7 (2018).
Teirlinck, A. C. et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 7, e1002389 (2011).
Ho, M. et al. Polyclonal expansion of peripheral gamma delta T cells in human Plasmodium falciparum malaria. Infect. Immun. 62, 855–862 (1994).
Ribot, J. C. et al. gammadelta-T cells promote IFN-gamma-dependent Plasmodium pathogenesis upon liver-stage infection. Proc. Natl Acad. Sci. USA 116, 9979–9988 (2019).
Jagannathan, P. et al. Loss and dysfunction of Vdelta2+ gammadelta T cells are associated with clinical tolerance to malaria. Sci. Transl. Med. 6, 251ra117 (2014).
Willcox, B. E. & Willcox, C. R. Publisher correction: gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat. Immunol. 20, 516 (2019).
Li, Y. et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 (2011).
Mielke, L. A. et al. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 210, 1117–1124 (2013).
Ridaura, V. K. et al. Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med. 215, 785–799 (2018).
St Leger, A. J. et al. An ocular commensal protects against corneal infection by driving an interleukin-17 response from mucosal γδ T cells. Immunity 47, 148–158.e5 (2017).
Naik, S. et al. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 (2012).
Duan, J., Chung, H., Troy, E. & Kasper, D. L. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 7, 140–150 (2010).
Jouan, Y. et al. Thymic program directing the functional development of gammadeltaT17 cells. Front. Immunol. 9, 981 (2018).
Hunter, S. et al. Human liver infiltrating gammadelta T cells are composed of clonally expanded circulating and tissue-resident populations. J. Hepatol. 69, 654–665 (2018).
Davey, M. S. et al. The human Vdelta2+ T-cell compartment comprises distinct innate-like Vgamma9+ and adaptive Vgamma9- subsets. Nat. Commun. 9, 1760 (2018).
Mikulak, J. et al. NKp46-expressing human gut-resident intraepithelial Vδ1 T cell subpopulation exhibits high antitumor activity against colorectal cancer. JCI Insight 4, e125884 (2019).
Silva-Santos, B., Mensurado, S. & Coffelt, S. B. gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 19, 392–404 (2019).
Sebestyen, Z., Prinz, I., Dechanet-Merville, J., Silva-Santos, B. & Kuball, J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 19, 169–184 (2020).
Seder, R. A. et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013).
Zaidi, I. et al. gammadelta T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J. Immunol. 199, 3781–3788 (2017).
Reuling, I. J. et al. An open-label phase 1/2a trial of a genetically modified rodent malaria parasite for immunization against Plasmodium falciparum malaria. Sci. Transl Med. 12, eaay2578 (2020).
Papotto, P. H., Reinhardt, A., Prinz, I. & Silva-Santos, B. Innately versatile: gammadelta17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 87, 26–37 (2018).
Komori, H. K. et al. Cutting edge: dendritic epidermal gammadelta T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J. Immunol. 188, 2972–2976 (2012).
Bandeira, A. et al. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J. Exp. Med. 172, 239–244 (1990).
Li, F. et al. The microbiota maintain homeostasis of liver-resident gammadeltaT-17 cells in a lipid antigen/CD1d-dependent manner. Nat. Commun. 7, 13839 (2017).
Heilig, J. S. & Tonegawa, S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322, 836–840 (1986).
Acknowledgements
This Review is dedicated to the loving memory of the authors’ colleague and γδ T cell pioneer, W. Havran (1955–2020). The authors thank A. Hayday for inspiring discussions on these topics. Their work is supported by La Caixa Banking Foundation (project HR18-00069). J.C.R received a junior investigator contract from Fundação para a Ciência e Tecnologia (IF/00013/2014) and N.L. received an EMBO long-term fellowship (ALTF 752-2018).
Author information
Authors and Affiliations
Contributions
J.C.R., N.L. and B.S.-S. conceived and wrote the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks P. Vantourout, T. Zal and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Dendritic epidermal T cells
-
(DETCs). A common designation for the monoclonal Vγ5+ γδ T cell subset that resides in the murine epidermis and contributes to environmental sensing, wound healing and antitumour immunity.
- Adaptate
-
A term initially proposed by Adrian Hayday to describe the blend of innate and adaptive immune features show by γδ T cells.
- Ketogenic diet
-
A low-carbohydrate, high-fat diet that induces a metabolic state called ‘ketosis’.
Rights and permissions
About this article
Cite this article
Ribot, J.C., Lopes, N. & Silva-Santos, B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol 21, 221–232 (2021). https://doi.org/10.1038/s41577-020-00452-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-00452-4
This article is cited by
-
Activation of mucosal insulin receptor exacerbates intestinal inflammation by promoting tissue resident memory T cells differentiation through EZH2
Journal of Translational Medicine (2024)
-
Roles of exosomes in immunotherapy for solid cancers
Cell Death & Disease (2024)
-
Evolution of T cells in the cancer-resistant naked mole-rat
Nature Communications (2024)
-
Multimodal profiling reveals site-specific adaptation and tissue residency hallmarks of γδ T cells across organs in mice
Nature Immunology (2024)
-
Adaptive immune receptor repertoire analysis
Nature Reviews Methods Primers (2024)