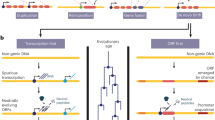
Abstract
Pseudogenes are defined as regions of the genome that contain defective copies of genes. They exist across almost all forms of life, and in mammalian genomes are annotated in similar numbers to recognized protein-coding genes. Although often presumed to lack function, growing numbers of pseudogenes are being found to play important biological roles. In consideration of their evolutionary origins and inherent limitations in genome annotation practices, we posit that pseudogenes have been classified on a scientifically unsubstantiated basis. We reflect that a broad misunderstanding of pseudogenes, perpetuated in part by the pejorative inference of the ‘pseudogene’ label, has led to their frequent dismissal from functional assessment and exclusion from genomic analyses. With the advent of technologies that simplify the study of pseudogenes, we propose that an objective reassessment of these genomic elements will reveal valuable insights into genome function and evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jacq, C., Miller, J. R. & Brownlee, G. G. A pseudogene structure in 5S DNA of Xenopus laevis. Cell 12, 109–120 (1977).
Vierna, J., Wehner, S., Höner zu Siederdissen, C., Martínez-Lage, A. & Marz, M. Systematic analysis and evolution of 5S ribosomal DNA in metazoans. Heredity 111, 410–421 (2013).
Vanin, E. F. Processed pseudogenes: characteristics and evolution. Annu. Rev. Genet. 19, 253–272 (1985).
Esnault, C., Maestre, J. & Heidmann, T. Human LINE retrotransposons generate processed pseudogenes. Nat. Genet. 24, 363–367 (2000).
Pei, B. et al. The GENCODE pseudogene resource. Genome Biol. 13, R51 (2012).
Frankish, A. et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019).
Zhang, Z., Harrison, P. M., Liu, Y. & Gerstein, M. Millions of years of evolution preserved: a comprehensive catalog of the processed pseudogenes in the human genome. Genome Res. 13, 2541–2558 (2003).
Baertsch, R., Diekhans, M., Kent, W. J., Haussler, D. & Brosius, J. Retrocopy contributions to the evolution of the human genome. BMC Genomics 9, 466 (2008).
Navarro, F. C. P. & Galante, P. A. F. RCPedia: a database of retrocopied genes. Bioinformatics 29, 1235–1237 (2013).
Kaessmann, H., Vinckenbosch, N. & Long, M. RNA-based gene duplication: mechanistic and evolutionary insights. Nat. Rev. Genet. 10, 19–31 (2009).
Kaessmann, H. Origins, evolution, and phenotypic impact of new genes. Genome Res. 20, 1313–1326 (2010).
Ewing, A. D. et al. Retrotransposition of gene transcripts leads to structural variation in mammalian genomes. Genome Biol. 14, R22 (2013).
Richardson, S. R., Salvador-Palomeque, C. & Faulkner, G. J. Diversity through duplication: whole-genome sequencing reveals novel gene retrocopies in the human population. Bioessays 36, 475–481 (2014).
Abyzov, A. et al. Analysis of variable retroduplications in human populations suggests coupling of retrotransposition to cell division. Genome Res. 23, 2042–2052 (2013).
Schrider, D. R. et al. Gene copy-number polymorphism caused by retrotransposition in humans. PLOS Genet. 9, e1003242 (2013).
Sisu, C. et al. Comparative analysis of pseudogenes across three phyla. Proc. Natl Acad. Sci. USA 111, 13361–13366 (2014).
Wang, T. et al. Identification and characterization of essential genes in the human genome. Science 350, 1096–1101 (2015).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e16 (2017).
Ghandi, M. et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 569, 503–508 (2019).
Zhang, Z. & Gerstein, M. Large-scale analysis of pseudogenes in the human genome. Curr. Opin. Genet. Dev. 14, 328–335 (2004).
van Baren, M. J. & Brent, M. R. Iterative gene prediction and pseudogene removal improves genome annotation. Genome Res. 16, 678–685 (2006).
Torrents, D., Suyama, M., Zdobnov, E. & Bork, P. A genome-wide survey of human pseudogenes. Genome Res. 13, 2559–2567 (2003).
Zhang, Z. et al. PseudoPipe: an automated pseudogene identification pipeline. Bioinformatics 22, 1437–1439 (2006).
Frith, M. C. et al. Pseudo-messenger RNA: phantoms of the transcriptome. PLOS Genet. 2, e23 (2006).
Vinckenbosch, N., Dupanloup, I. & Kaessmann, H. Evolutionary fate of retroposed gene copies in the human genome. Proc. Natl Acad. Sci. USA 103, 3220–3225 (2006).
Jorquera, R. et al. SinEx DB: a database for single exon coding sequences in mammalian genomes. Database (Oxford) 2016, baw095 (2016).
Hurst, L. D. The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet. 18, 486 (2002).
Chiang, J. J. et al. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat. Immunol. 19, 53–62 (2018).
Pink, R. C. et al. Pseudogenes: pseudo-functional or key regulators in health and disease? RNA 17, 792–798 (2011).
Pink, R. C. & Carter, D. R. F. Pseudogenes as regulators of biological function. Essays Biochem. 54, 103–112 (2013).
Kovalenko, T. F. & Patrushev, L. I. Pseudogenes as functionally significant elements of the genome. Biochemistry 83, 1332–1349 (2018).
McCarrey, J. R. & Thomas, K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 326, 501–505 (1987).
McCarrey, J. R. Nucleotide sequence of the promoter region of a tissue-specific human retroposon: comparison with its housekeeping progenitor. Gene 61, 291–298 (1987).
Sayah, D. M., Sokolskaja, E., Berthoux, L. & Luban, J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430, 569–573 (2004).
Burki, F. & Kaessmann, H. Birth and adaptive evolution of a hominoid gene that supports high neurotransmitter flux. Nat. Genet. 36, 1061–1063 (2004).
Hayashi, H. et al. The OCT4 pseudogene POU5F1B is amplified and promotes an aggressive phenotype in gastric cancer. Oncogene 34, 199–208 (2015).
Suzuki, I. K. et al. Human-specific NOTCH2NL genes expand cortical neurogenesis through Delta/Notch regulation. Cell 173, 1370–1384.e16 (2018).
Fiddes, I. T. et al. Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell 173, 1356–1369.e22 (2018).
Dennis, M. Y. et al. Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell 149, 912–922 (2012).
Charrier, C. et al. Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell 149, 923–935 (2012).
Korneev, S. A., Park, J. H. & O’Shea, M. Neuronal expression of neural nitric oxide synthase (nNOS) protein is suppressed by an antisense RNA transcribed from an NOS pseudogene. J. Neurosci. 19, 7711–7720 (1999).
Tam, O. H. et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453, 534–538 (2008).
Watanabe, T. et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453, 539–543 (2008).
Rapicavoli, N. A. et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. eLife 2, e00762 (2013).
Poliseno, L. et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465, 1033–1038 (2010).
Karreth, F. A. et al. The BRAF pseudogene functions as a competitive endogenous RNA and induces lymphoma in vivo. Cell 161, 319–332 (2015).
Salmena, L., Poliseno, L., Tay, Y., Kats, L. & Pandolfi, P. P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146, 353–358 (2011).
Denzler, R., Agarwal, V., Stefano, J., Bartel, D. P. & Stoffel, M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell 54, 766–776 (2014).
Thomson, D. W. & Dinger, M. E. Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 17, 272–283 (2016).
Huang, P. et al. Comparative analysis of three-dimensional chromosomal architecture identifies a novel fetal hemoglobin regulatory element. Genes Dev. 31, 1704–1713 (2017).
Vergés, L. et al. An exploratory study of predisposing genetic factors for DiGeorge/velocardiofacial syndrome. Sci. Rep. 7, 40031 (2017).
Lai, J. et al. A variant of the KLK4 gene is expressed as a cis sense–antisense chimeric transcript in prostate cancer cells. RNA 16, 1156–1166 (2010).
Chakravarthi, B. V. et al. Pseudogene associated recurrent gene fusion in prostate cancer. Neoplasia 21, 989–1002 (2019).
Bischof, J. M. et al. Genome-wide identification of pseudogenes capable of disease-causing gene conversion. Hum. Mutat. 27, 545–552 (2006).
Rygiel, A. M. et al. Gene conversion between cationic trypsinogen (PRSS1) and the pseudogene trypsinogen 6 (PRSS3P2) in patients with chronic pancreatitis. Hum. Mutat. 36, 350–356 (2015).
Concolino, P. & Costella, A. Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency: a comprehensive focus on 233 pathogenic variants of CYP21A2 gene. Mol. Diagn. Ther. 22, 261–280 (2018).
Watnick, T., Gandolph, M. A., Weber, H., Neumann, H. P. & Germino, G. G. Gene conversion is a likely cause of mutation in PKD1. Hum. Mol. Genet. 7, 1239–1243 (1998).
Vanita et al. A unique form of autosomal dominant cataract explained by gene conversion between β-crystallin B2 and its pseudogene. J. Med. Genet. 38, 392–396 (2001).
Habib, A. M. et al. Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity. Br. J. Anaesth. 123, e249–e253 (2019).
Ali, H. et al. PKD1 duplicated regions limit clinical utility of whole exome sequencing for genetic diagnosis of autosomal dominant polycystic kidney disease. Sci. Rep. 9, 4141 (2019).
Gallagher, M. D. & Chen-Plotkin, A. S. The post-GWAS era: from association to function. Am. J. Hum. Genet. 102, 717–730 (2018).
Bartonicek, N. et al. Intergenic disease-associated regions are abundant in novel transcripts. Genome Biol. 18, 241 (2017).
GTEx Consortium et al. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).
Marques, A. C., Dupanloup, I., Vinckenbosch, N., Reymond, A. & Kaessmann, H. Emergence of young human genes after a burst of retroposition in primates. PLOS Biol. 3, e357 (2005).
Kabza, M., Ciomborowska, J. & Makałowska, I. RetrogeneDB—a database of animal retrogenes. Mol. Biol. Evol. 31, 1646–1648 (2014).
van Heesch, S. et al. The translational landscape of the human heart. Cell 178, 242–260.e29 (2019).
Kim, M.-S. et al. A draft map of the human proteome. Nature 509, 575–581 (2014).
Ji, Z., Song, R., Regev, A. & Struhl, K. Many lncRNAs, 5′UTRs, and pseudogenes are translated and some are likely to express functional proteins. eLife 4, e08890 (2015).
Brosch, M. et al. Shotgun proteomics aids discovery of novel protein-coding genes, alternative splicing, and ‘resurrected’ pseudogenes in the mouse genome. Genome Res. 21, 756–767 (2011).
Doolittle, W. F. We simply cannot go on being so vague about ‘function’. Genome Biol. 19, 223 (2018).
Kafri, R., Springer, M. & Pilpel, Y. Genetic redundancy: new tricks for old genes. Cell 136, 389–392 (2009).
Duret, L., Chureau, C., Samain, S., Weissenbach, J. & Avner, P. The Xist RNA gene evolved in eutherians by pseudogenization of a protein-coding gene. Science 312, 1653–1655 (2006).
Hezroni, H. et al. A subset of conserved mammalian long non-coding RNAs are fossils of ancestral protein-coding genes. Genome Biol. 18, 162 (2017).
Liu, W.-H., Tsai, Z. T.-Y. & Tsai, H.-K. Comparative genomic analyses highlight the contribution of pseudogenized protein-coding genes to human lincRNAs. BMC Genomics 18, 786 (2017).
Carninci, P. et al. The transcriptional landscape of the mammalian genome. Science 309, 1559–1563 (2005).
Mattick, J. S. Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Bioessays 25, 930–939 (2003).
Gloss, B. S. & Dinger, M. E. The specificity of long noncoding RNA expression. Biochim. Biophys. Acta 1859, 16–22 (2016).
Clark, M. B. et al. Quantitative gene profiling of long noncoding RNAs with targeted RNA sequencing. Nat. Methods 12, 339–342 (2015).
Mercer, T. R., Dinger, M. E., Sunkin, S. M., Mehler, M. F. & Mattick, J. S. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl Acad. Sci. USA 105, 716–721 (2008).
Dinger, M. E. et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 18, 1433–1445 (2008).
Martianov, I., Ramadass, A., Serra Barros, A., Chow, N. & Akoulitchev, A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 445, 666–670 (2007).
Rinn, J. L. et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 (2007).
Morris, K. V. & Mattick, J. S. The rise of regulatory RNA. Nat. Rev. Genet. 15, 423–437 (2014).
Cabili, M. N. et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011).
Pang, K. C. et al. Genome-wide identification of long noncoding RNAs in CD8+ T cells. J. Immunol. 182, 7738–7748 (2009).
Sunwoo, H. et al. MEN ε/β nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 19, 347–359 (2009).
Mercer, T. R. et al. Long noncoding RNAs in neuronal–glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 11, 14 (2010).
Lockhart, D. J. et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat. Biotechnol. 14, 1675–1680 (1996).
Millson, A. et al. Processed pseudogene confounding deletion/duplication assays for SMAD4. J. Mol. Diagn. 17, 576–582 (2015).
Cloonan, N. et al. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat. Methods 5, 613–619 (2008).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods 5, 621–628 (2008).
Kalyana-Sundaram, S. et al. Expressed pseudogenes in the transcriptional landscape of human cancers. Cell 149, 1622–1634 (2012).
Oikonomopoulos, S., Wang, Y. C., Djambazian, H., Badescu, D. & Ragoussis, J. Benchmarking of the Oxford Nanopore MinION sequencing for quantitative and qualitative assessment of cDNA populations. Sci. Rep. 6, 31602 (2016).
Au, K. F. et al. Characterization of the human ESC transcriptome by hybrid sequencing. Proc. Natl Acad. Sci. USA 110, E4821–E4830 (2013).
Garalde, D. R. et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 15, 201–206 (2018).
Jinek, M. et al. RNA-programmed genome editing in human cells. eLife 2, e00471 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Anderson, E. M. et al. Systematic analysis of CRISPR–Cas9 mismatch tolerance reveals low levels of off-target activity. J. Biotechnol. 211, 56–65 (2015).
Zhang, X.-H., Tee, L. Y., Wang, X.-G., Huang, Q.-S. & Yang, S.-H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 4, e264 (2015).
Kim, D. et al. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 34, 863–868 (2016).
Kleinstiver, B. P. et al. Genome-wide specificities of CRISPR–Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 34, 869–874 (2016).
Kocak, D. D. et al. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat. Biotechnol. 37, 657–666 (2019).
Groff, A. F. et al. In vivo characterization of Linc-p21 reveals functional cis-regulatory DNA elements. Cell Rep. 16, 2178–2186 (2016).
Bassett, A. R. et al. Considerations when investigating lncRNA function in vivo. eLife 3, e03058 (2014).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Yeo, N. C. et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods 15, 611–616 (2018).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Cheng, A. W. et al. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 23, 1163–1171 (2013).
Endrizzi, K. et al. Discriminative quantification of cytochrome P4502D6 and 2D7/8 pseudogene expression by TaqMan real-time reverse transcriptase polymerase chain reaction. Anal. Biochem. 300, 121–131 (2002).
Simon, M. D. et al. The genomic binding sites of a noncoding RNA. Proc. Natl Acad. Sci. USA 108, 20497–20502 (2011).
Chu, C., Qu, K., Zhong, F. L., Artandi, S. E. & Chang, H. Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA–chromatin interactions. Mol. Cell 44, 667–678 (2011).
Cheetham, S. W. & Brand, A. H. RNA-DamID reveals cell-type-specific binding of roX RNAs at chromatin-entry sites. Nat. Struct. Mol. Biol. 25, 109–114 (2018).
Li, X. et al. GRID-seq reveals the global RNA–chromatin interactome. Nat. Biotechnol. 35, 940–950 (2017).
Bell, J. C. et al. Chromatin-associated RNA sequencing (ChAR-seq) maps genome-wide RNA-to-DNA contacts. eLife 7, e27024 (2018).
Bonetti, A. et al. RADICL-seq identifies general and cell type-specific principles of genome-wide RNA–chromatin interactions. Preprint at bioRxiv https://doi.org/10.1101/681924 (2019).
Lu, Z. et al. RNA duplex map in living cells reveals higher-order transcriptome structure. Cell 165, 1267–1279 (2016).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009).
Kuhn, T. S. The Structure of Scientific Revolutions (Univ. Chicago Press, 1962).
Stanier, R. Y. & van Niel, C. B. The concept of a bacterium. Arch. Mikrobiol. 42, 17–35 (1962).
Woese, C. R. A new biology for a new century. Microbiol. Mol. Biol. Rev. 68, 173–186 (2004).
Woese, C. R., Kandler, O. & Wheelis, M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl Acad. Sci. USA 87, 4576–4579 (1990).
Woese, C. R. & Goldenfeld, N. How the microbial world saved evolution from the scylla of molecular biology and the charybdis of the modern synthesis. Microbiol. Mol. Biol. Rev. 73, 14–21 (2009).
Mercer, T. R., Dinger, M. E. & Mattick, J. S. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 10, 155–159 (2009).
Brosius, J. & Gould, S. J. On ‘genomenclature’: a comprehensive (and respectful) taxonomy for pseudogenes and other ‘junk DNA’. Proc. Natl Acad. Sci. USA 89, 10706–10710 (1992).
Zhang, J. et al. NANOGP8 is a retrogene expressed in cancers. FEBS J. 273, 1723–1730 (2006).
Kandouz, M., Bier, A., Carystinos, G. D., Alaoui-Jamali, M. A. & Batist, G. Connexin43 pseudogene is expressed in tumor cells and inhibits growth. Oncogene 23, 4763–4770 (2004).
Chiefari, E. et al. Pseudogene-mediated posttranscriptional silencing of HMGA1 can result in insulin resistance and type 2 diabetes. Nat. Commun. 1, 40 (2010).
Hawkins, P. G. & Morris, K. V. Transcriptional regulation of Oct4 by a long non-coding RNA antisense to Oct4-pseudogene 5. Transcription 1, 165–175 (2010).
Reynaud, C. A., Anquez, V., Grimal, H. & Weill, J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell 48, 379–388 (1987).
Reynaud, C. A., Dahan, A., Anquez, V. & Weill, J. C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell 59, 171–183 (1989).
Wang, J., Pitarque, M. & Ingelman-Sundberg, M. 3′-UTR polymorphism in the human CYP2A6 gene affects mRNA stability and enzyme expression. Biochem. Biophys. Res. Commun. 340, 491–497 (2006).
Acknowledgements
The authors thank J. Mattick for feedback on the manuscript and A. Ewing for helpful discussion. S.W.C. acknowledges support from a National Health and Medical Research Council (NHMRC) Early Career Fellowship (GNT1161832) and the Mater Foundation. G.J.F. acknowledges support from a CSL Centenary Fellowship and the Mater Foundation.
Author information
Authors and Affiliations
Contributions
S.W.C. and M.E.D. contributed to all aspects of the article. G.J.F. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Genetics thanks P. Carninci and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Expressed sequence tags
-
(ESTs). Short fragmented sequences of cDNAs. Mapping ESTs identifies transcribed genes.
- Non-synonymous substitutions
-
Nucleotide substitutions that change the encoded amino acid sequence.
- Positive selection
-
Selection for alleles that increase fitness. Positive selection results in shifts of the allele frequency.
- Purifying selection
-
Selection against alleles that are deleterious to fitness. Purifying selection maintains the amino acid sequence.
- Retrotransposition
-
Insertion of a sequence into the genome via the reverse transcription and integration of an RNA intermediate.
- Synonymous substitutions
-
Nucleotide substitutions that do not change the encoded amino acid sequence.
Rights and permissions
About this article
Cite this article
Cheetham, S.W., Faulkner, G.J. & Dinger, M.E. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat Rev Genet 21, 191–201 (2020). https://doi.org/10.1038/s41576-019-0196-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-019-0196-1
This article is cited by
-
Convergent genomic signatures associated with vertebrate viviparity
BMC Biology (2024)
-
Long-insert sequence capture detects high copy numbers in a defence-related beta-glucosidase gene βglu-1 with large variations in white spruce but not Norway spruce
BMC Genomics (2024)
-
A human stomach cell type transcriptome atlas
BMC Biology (2024)
-
Machine learning-based extrachromosomal DNA identification in large-scale cohorts reveals its clinical implications in cancer
Nature Communications (2024)
-
Isolation, heterologous expression, and functional determination of an iron regulated transporter (IRT) gene involved in Fe2+ transport and tolerance to Fe2+ deficiency in Vitis vinifera
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)