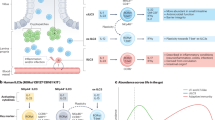
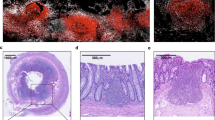
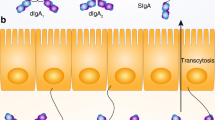
Abstract
The intestinal lumen contains an abundance of bacteria, viruses and fungi alongside ingested material that shape the chronically active intestinal immune system from early life to maintain the integrity of the gut epithelial barrier. In health, the response is intricately balanced to provide active protection against pathogen invasion whilst tolerating food and avoiding inflammation. B cells are central to achieving this protection. Their activation and maturation generates the body’s largest plasma cell population that secretes IgA, and the niches they provide support systemic immune cell specialization. For example, the gut supports the development and maturation of a splenic B cell subset — the marginal zone B cells. In addition, cells such as the T follicular helper cells, which are enriched in many autoinflammatory diseases, are intrinsically associated with the germinal centre microenvironment that is more abundant in the gut than in any other tissue in health. In this Review, we discuss intestinal B cells and their role when a loss of homeostasis results in intestinal and systemic inflammatory diseases.
Key points
-
Gut B cell responses are initiated in organized gut-associated lymphoid tissues (GALT).
-
Antibody-secreting plasma cells and their immediate precursors generated in GALT disseminate widely to diffusely populate the extensive lamina propria.
-
Although memory B cells expressing IgG in GALT are not uncommon in health, IgG-secreting intestinal plasma cells are.
-
Gut IgG plasma cells are major contributors to intestinal inflammation in inflammatory bowel disease.
-
The microbiota shapes many aspects of gut B cell responses, from determining the specificity of IgA responses to driving the functionality of regulatory B cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brandtzaeg, P. et al. The B-cell system of human mucosae and exocrine glands. Immunol. Rev. 171, 45–87 (1999).
Gasaly, N., de Vos, P. & Hermoso, M. A. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 12, 658354 (2021).
Johansson, M. E. & Hansson, G. C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16, 639–649 (2016).
Hasleton, P. S. The internal surface area of the adult human lung. J. Anat. 112, 391–400 (1972).
Combs, M. P. & Dickson, R. P. Turning the lungs inside out: the intersecting microbiomes of the lungs and the built environment. Am. J. Respir. Crit. Care Med. 202, 1618–1620 (2020).
Helander, H. F. & Fandriks, L. Surface area of the digestive tract – revisited. Scand. J. Gastroenterol. 49, 681–689 (2014).
Gallo, R. L. Human skin is the largest epithelial surface for interaction with microbes. J. Invest. Dermatol. 137, 1213–1214 (2017).
Gustafson, C. E. et al. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol. 7, 467–477 (2014).
Cornes, J. S. Number, size, and distribution of Peyer’s patches in the human small intestine: Part I The development of Peyer’s patches. Gut 6, 225–229 (1965).
Spencer, J. & Sollid, L. M. The human intestinal B-cell response. Mucosal Immunol. 9, 1113–1124 (2016).
Reboldi, A. & Cyster, J. G. Peyer’s patches: organizing B-cell responses at the intestinal frontier. Immunol. Rev. 271, 230–245 (2016).
Fagarasan, S. et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 (2002).
Quartier, P. et al. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to activation-induced cytidine deaminase deficiency. Clin. Immunol. 110, 22–29 (2004).
Zhao, Y. et al. Spatiotemporal segregation of human marginal zone and memory B cell populations in lymphoid tissue. Nat. Commun. 9, 3857 (2018).
Farstad, I. N., Carlsen, H., Morton, H. C. & Brandtzaeg, P. Immunoglobulin A cell distribution in the human small intestine: phenotypic and functional characteristics. Immunology 101, 354–363 (2000).
Landsverk, O. J. et al. Antibody-secreting plasma cells persist for decades in human intestine. J. Exp. Med. 214, 309–317 (2017).
Barone, F. et al. IgA-producing plasma cells originate from germinal centers that are induced by B-cell receptor engagement in humans. Gastroenterology 140, 947–956 (2011).
Bergqvist, P. et al. Re-utilization of germinal centers in multiple Peyer’s patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal Immunol. 6, 122–135 (2013).
Young, C. & Brink, R. The unique biology of germinal center B cells. Immunity 54, 1652–1664 (2021).
Spencer, J., Finn, T., Pulford, K. A., Mason, D. Y. & Isaacson, P. G. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin. Exp. Immunol. 62, 607–612 (1985).
Lindner, C. et al. Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat. Immunol. 16, 880–888 (2015).
Stavnezer, J. & Kang, J. The surprising discovery that TGFβ specifically induces the IgA class switch. J. Immunol. 182, 5–7 (2009).
Bergqvist, P., Stensson, A., Lycke, N. Y. & Bemark, M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J. Immunol. 184, 3545–3553 (2010).
Roco, J. A. et al. Class-switch recombination occurs infrequently in germinal centers. Immunity 51, 337–350.e7 (2019).
Magri, G. et al. Human secretory IgM emerges from plasma cells clonally related to gut memory B cells and targets highly diverse commensals. Immunity 47, 118–134.e8 (2017).
Boursier, L., Dunn-Walters, D. K. & Spencer, J. Characteristics of IgVH genes used by human intestinal plasma cells from childhood. Immunology 97, 558–564 (1999).
Catanzaro, J. R. et al. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci. Rep. 9, 13574 (2019).
Nowosad, C. R. et al. Tunable dynamics of B cell selection in gut germinal centres. Nature 588, 321–326 (2020).
Brandtzaeg, P. & Baklien, K. Immunohistochemical studies of the immunoglobulin-producing cell systems of the human intestinal mucosa. Acta Histochem. Suppl. 21, 105–119 (1980).
Mei, H. E. et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 113, 2461–2469 (2009).
O’Leary, A. D. & Sweeney, E. C. Lymphoglandular complexes of the colon: structure and distribution. Histopathology 10, 267–283 (1986).
Shikuwa, S. et al. Magnifying videoendoscopic findings of Peyer’s patches in the terminal ileum of Crohn’s disease. Gut 56, 894–895 (2007).
MacDonald, T. T., Spencer, J., Viney, J. L., Williams, C. B. & Walker-Smith, J. A. Selective biopsy of human Peyer’s patches during ileal endoscopy. Gastroenterology 93, 1356–1362 (1987).
Siu, J. H. Y. et al. Two subsets of human marginal zone B cells resolved by global analysis of lymphoid tissues and blood. Sci. Immunol. 7, eabm9060 (2022).
Fenton, T. M. et al. Immune profiling of human gut-associated lymphoid tissue identifies a role for isolated lymphoid follicles in priming of region-specific immunity. Immunity 52, 557–570.e6 (2020).
Jorgensen, P. B. et al. Identification, isolation and analysis of human gut-associated lymphoid tissues. Nat. Protoc. 16, 2051–2067 (2021).
Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & Honjo, T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413, 639–643 (2001).
Shlomchik, M. J. & Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 247, 52–63 (2012).
Toboso-Navasa, A. et al. Restriction of memory B cell differentiation at the germinal center B cell positive selection stage. J. Exp. Med. https://doi.org/10.1084/jem.20191933 (2020).
Mora, J. R. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm. Bowel Dis. 14, 275–289 (2008).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Meng, W. et al. An atlas of B-cell clonal distribution in the human body. Nat. Biotechnol. 35, 879–884 (2017).
Zundler, S. et al. The α4β1 homing pathway is essential for ileal homing of Crohn’s disease effector T cells in vivo. Inflamm. Bowel Dis. 23, 379–391 (2017).
Camponeschi, A. et al. Dissecting integrin expression and function on memory B cells in mice and humans in autoimmunity. Front. Immunol. 10, 534 (2019).
Jovani, M. & Danese, S. Vedolizumab for the treatment of IBD: a selective therapeutic approach targeting pathogenic a4b7 cells. Curr. Drug Targets 14, 1433–1443 (2013).
Uzzan, M. et al. Anti-α4β7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1-infected individuals. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aau4711 (2018).
Tyler, C. J. et al. Antibody secreting cells are critically dependent on integrin α4β7/MAdCAM-1 for intestinal recruitment and control of the microbiota during chronic colitis. Mucosal Immunol. 15, 109–119 (2022).
Berteloot, L. et al. Alternative pathways for the development of lymphoid structures in humans. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2108082118 (2021).
Stebegg, M. et al. Regulation of the germinal center response. Front. Immunol. 9, 2469 (2018).
Gribonika, I. et al. Peyer’s patch TH17 cells are dispensable for gut IgA responses to oral immunization. Sci. Immunol. 7, eabc5500 (2022).
Wing, J. B. et al. A distinct subpopulation of CD25− T-follicular regulatory cells localizes in the germinal centers. Proc. Natl Acad. Sci. USA 114, E6400–E6409 (2017).
Wing, J. B., Tekguc, M. & Sakaguchi, S. Control of germinal center responses by T-follicular regulatory cells. Front. Immunol. 9, 1910 (2018).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Heit, A. et al. Vaccination establishes clonal relatives of germinal center T cells in the blood of humans. J. Exp. Med. 214, 2139–2152 (2017).
Hill, D. L. et al. The adjuvant GLA-SE promotes human Tfh cell expansion and emergence of public TCRβ clonotypes. J. Exp. Med. 216, 1857–1873 (2019).
Hiyama, S. et al. Endoscopic alterations in Peyer’s patches in patients with ulcerative colitis: a prospective, multicenter study. J. Gastroenterol. Hepatol. 35, 1143–1149 (2020).
Morson, B. C. The early histological lesion of Crohn’s disease. Proc. R. Soc. Med. 65, 71–72 (1972).
Fujimura, Y., Kamoi, R. & Iida, M. Pathogenesis of aphthoid ulcers in Crohn’s disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut 38, 724–732 (1996).
Russel, M. G. et al. Appendectomy and the risk of developing ulcerative colitis or Crohn’s disease: results of a large case-control study. South Limburg Inflammatory Bowel Disease Study Group. Gastroenterology 113, 377–382 (1997).
Andersson, R. E., Olaison, G., Tysk, C. & Ekbom, A. Appendectomy and protection against ulcerative colitis. N. Engl. J. Med. 344, 808–814 (2001).
Parian, A. et al. Appendectomy does not decrease the risk of future colectomy in UC: results from a large cohort and meta-analysis. Gut https://doi.org/10.1136/gutjnl-2016-311550 (2016).
Reijntjes, M. A. et al. Clinical relevance of endoscopic peri-appendiceal red patch in ulcerative colitis patients. Ther. Adv. Gastroenterol. 15, 17562848221098849 (2022).
Di Sabatino, A. et al. Splenic hypofunction and the spectrum of autoimmune and malignant complications in celiac disease. Clin. Gastroenterol. Hepatol. 4, 179–186 (2006).
Pararasa, C. et al. Reduced CD27−IgD− B cells in blood and raised CD27−IgD− B cells in gut-associated lymphoid tissue in inflammatory bowel disease. Front. Immunol. 10, 361 (2019).
Kosoy, R. et al. Deep analysis of the peripheral immune system in IBD reveals new insight in disease subtyping and response to monotherapy or combination therapy. Cell Mol. Gastroenterol. Hepatol. 12, 599–632 (2021).
Glass, D. R. et al. An integrated multi-omic single-cell atlas of human B cell identity. Immunity 53, 217–232.e5 (2020).
Descatoire, M. et al. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties. J. Exp. Med. 211, 987–1000 (2014).
Berkowska, M. A. et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 118, 2150–2158 (2011).
Rubio, C. A. et al. Lymphoid aggregates in Crohn’s colitis and mucosal immunity. Virchows Arch. 463, 637–642 (2013).
Fell, J. M., Walker-Smith, J. A., Spencer, J. & MacDonald, T. T. The distribution of dividing T cells throughout the intestinal wall in inflammatory bowel disease (IBD). Clin. Exp. Immunol. 104, 280–285 (1996).
Dohi, T. et al. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J. Exp. Med. 189, 1169–1180 (1999).
Spahn, T. W. et al. Induction of colitis in mice deficient of Peyer’s patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am. J. Pathol. 161, 2273–2282 (2002).
Uzzan, M. et al. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat. Med. 28, 766–779 (2022).
Dey, A. et al. Human circulating antibody-producing B cell as a predictive measure of mucosal immunity to poliovirus. PLoS ONE 11, e0146010 (2016).
Bemark, M. et al. Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization. Nat. Commun. 7, 12698 (2016).
Sterlin, D., Fadlallah, J., Slack, E. & Gorochov, G. The antibody/microbiota interface in health and disease. Mucosal Immunol. 13, 3–11 (2020).
Dunn-Walters, D. K., Isaacson, P. G. & Spencer, J. Sequence analysis of human IgVH genes indicates that ileal lamina propria plasma cells are derived from Peyer’s patches. Eur. J. Immunol. 27, 463–467 (1997).
James, K. R. et al. Distinct microbial and immune niches of the human colon. Nat. Immunol. 21, 343–353 (2020).
Kabbert, J. et al. High microbiota reactivity of adult human intestinal IgA requires somatic mutations. J. Exp. Med. https://doi.org/10.1084/jem.20200275 (2020).
Kraj, M. Immunoglobulin heavy chain/light chain pairs (HLC, Hevylite) assays for diagnosing and monitoring monoclonal gammopathies. Adv. Clin. Exp. Med. 23, 127–133 (2014).
Su, W. et al. Lambda light chain revision in the human intestinal IgA response. J. Immunol. 181, 1264–1271 (2008).
Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021).
Thoree, V. C. et al. Related IgA1 and IgG producing cells in blood and diseased mucosa in ulcerative colitis. Gut 51, 44–50 (2002).
Weisel, N. M. et al. Comprehensive analyses of B-cell compartments across the human body reveal novel subsets and a gut-resident memory phenotype. Blood 136, 2774–2785 (2020).
Lindner, C. et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J. Exp. Med. 209, 365–377 (2012).
Dunn-Walters, D. K., Boursier, L. & Spencer, J. Hypermutation, diversity and dissemination of human intestinal lamina propria plasma cells. Eur. J. Immunol. 27, 2959–2964 (1997).
Brandtzaeg, P., Carlsen, H. S. & Halstensen, T. S. The B-cell system in inflammatory bowel disease. Adv. Exp. Med. Biol. 579, 149–167 (2006).
Baklien, K. & Brandtzaeg, P. Immunohistochemical characterization of local immunoglobulin formation in Crohn’s disease of the ileum. Scand. J. Gastroenterol. 11, 447–457 (1976).
Brandtzaeg, P., Baklien, K., Fausa, O. & Hoel, P. S. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology 66, 1123–1136 (1974).
Martin, J. C. et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell 178, 1493–1508.e20 (2019).
Dunn-Walters, D. K., Boursier, L., Hackett, M. & Spencer, J. Biased JH usage in plasma cell immunoglobulin gene sequences from colonic mucosa in ulcerative colitis but not in Crohn’s disease. Gut 44, 382–386 (1999).
Boland, B. S. et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abb4432 (2020).
Roozendaal, C. & Kallenberg, C. G. Are anti-neutrophil cytoplasmic antibodies (ANCA) clinically useful in inflammatory bowel disease (IBD)? Clin. Exp. Immunol. 116, 206–213 (1999).
Onuma, E. K., Amenta, P. S., Ramaswamy, K., Lin, J. J. & Das, K. M. Autoimmunity in ulcerative colitis (UC): a predominant colonic mucosal B cell response against human tropomyosin isoform 5. Clin. Exp. Immunol. 121, 466–471 (2000).
Ebert, E. C., Geng, X., Lin, J. & Das, K. M. Autoantibodies against human tropomyosin isoform 5 in ulcerative colitis destroys colonic epithelial cells through antibody and complement-mediated lysis. Cell Immunol. 244, 43–49 (2006).
Kuwada, T. et al. Identification of an anti-integrin αvβ6 autoantibody in patients with ulcerative colitis. Gastroenterology 160, 2383–2394.e21 (2021).
Baklien, K. & Brandtzaeg, P. Letter: Immunohistochemical localization of complement in intestinal mucosa. Lancet 2, 1087–1088 (1974).
Castro-Dopico, T. & Clatworthy, M. R. Mucosal IgG in inflammatory bowel disease–a question of (sub)class? Gut Microbes 12, 1–9 (2020).
Castro-Dopico, T., Colombel, J. F. & Mehandru, S. Targeting B cells for inflammatory bowel disease treatment: back to the future. Curr. Opin. Pharmacol. 55, 90–98 (2020).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Castro-Dopico, T. et al. Anti-commensal IgG drives intestinal inflammation and type 17 immunity in ulcerative colitis. Immunity 50, 1099–1114.e10 (2019).
Edwards, J. C. & Cambridge, G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat. Rev. Immunol. 6, 394–403 (2006).
Leiper, K. et al. Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut 60, 1520–1526 (2011).
Ben-Horin, S. Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut 61, 327 (2012).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Stamnaes, J. & Sollid, L. M. Celiac disease: autoimmunity in response to food antigen. Semin. Immunol. 27, 343–352 (2015).
Di Niro, R. et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat. Med. 18, 441–445 (2012).
Iversen, R. et al. Evidence that pathogenic transglutaminase 2 in celiac disease derives from enterocytes. Gastroenterology 159, 788–790 (2020).
Jabri, B. & Sollid, L. M. T cells in celiac disease. J. Immunol. 198, 3005–3014 (2017).
Xu, H. et al. The dynamic interplay between the gut microbiota and autoimmune diseases. J. Immunol. Res. 2019, 7546047 (2019).
Tull, T. J. et al. Human marginal zone B cell development from early T2 progenitors. J. Exp. Med. https://doi.org/10.1084/jem.20202001 (2021).
Weller, S. et al. IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients. Blood 120, 4992–5001 (2012).
Maglione, P. J. et al. IRAK-4 and MyD88 deficiencies impair IgM responses against T-independent bacterial antigens. Blood 124, 3561–3571 (2014).
Greenblatt, H. K., Kim, H. A., Bettner, L. F. & Deane, K. D. Preclinical rheumatoid arthritis and rheumatoid arthritis prevention. Curr. Opin. Rheumatol. 32, 289–296 (2020).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2, e01202 (2013).
Erttmann, S. F. et al. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 55, 847–861.e10 (2022).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Menon, M., Rosser, E. C. & Mauri, C. Identification and isolation of regulatory B cells in mouse and human. Methods Mol. Biol. 1899, 55–66 (2019).
Rosser, E. C. et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat. Med. 20, 1334–1339 (2014).
Piper, C. J. M. et al. Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-producing regulatory B cells. Cell Rep. 29, 1878–1892.e7 (2019).
Rosser, E. C. et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 31, 837–851.e10 (2020).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Liang, L. et al. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 136, 291–307 (2022).
Long, Y., Zhao, X., Liu, C., Xia, C. & Liu, C. Activated inducible co-stimulator-positive programmed cell death 1-positive follicular helper T cells indicate disease activity and severity in ulcerative colitis patients. Clin. Exp. Immunol. 202, 106–118 (2020).
Long, Y. et al. The imbalance of circulating follicular helper T cells and follicular regulatory T cells is associated with disease activity in patients with ulcerative colitis. Front. Immunol. 11, 104 (2020).
Stolfi, C. et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J. Exp. Med. 208, 2279–2290 (2011).
Monteleone, G. et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-γ production in Crohn’s disease. Gastroenterology 128, 687–694 (2005).
Sarra, M. et al. Interferon-γ-expressing cells are a major source of interleukin-21 in inflammatory bowel diseases. Inflamm. Bowel Dis. 16, 1332–1339 (2010).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Long, Y. et al. Increased circulating PD-1hiCXCR5− peripheral helper T cells are associated with disease severity of active ulcerative colitis patients. Immunol. Lett. 233, 2–10 (2021).
Voskens, C. et al. Autologous regulatory T-cell transfer in refractory ulcerative colitis with concomitant primary sclerosing cholangitis. Gut 72, 49–53 (2023).
Barnes, M. J. & Powrie, F. Regulatory T cells reinforce intestinal homeostasis. Immunity 31, 401–411 (2009).
Maloy, K. J. & Powrie, F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 6, 1071–1072 (2005).
Sun, H. et al. β7 Integrin inhibition can increase intestinal inflammation by impairing homing of CD25hiFoxP3+ regulatory T cells. Cell Mol. Gastroenterol. Hepatol. 9, 369–385 (2020).
Sharonov, G. V., Serebrovskaya, E. O., Yuzhakova, D. V., Britanova, O. V. & Chudakov, D. M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 20, 294–307 (2020).
Chiaruttini, G. et al. B cells and the humoral response in melanoma: the overlooked players of the tumor microenvironment. Oncoimmunology 6, e1294296 (2017).
Hoch, T. et al. Multiplexed imaging mass cytometry of the chemokine milieus in melanoma characterizes features of the response to immunotherapy. Sci. Immunol. 7, eabk1692 (2022).
Fridman, W. H. et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 19, 441–457 (2022).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541.e5 (2022).
Mazor, R. D. et al. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell 185, 1208–1222.e21 (2022).
Noel, G. et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Invest. https://doi.org/10.1172/JCI139905 (2021).
Goc, J. et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 74, 705–715 (2014).
Wotherspoon, A. C., Ortiz-Hidalgo, C., Falzon, M. R. & Isaacson, P. G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338, 1175–1176 (1991).
Small, S., Barnea Slonim, L., Williams, C. & Karmali, R. B cell lymphomas of the GI tract. Curr. Gastroenterol. Rep. 23, 9 (2021).
Matysiak-Budnik, T. et al. Primary gastrointestinal follicular lymphomas: a prospective study of 31 patients with long-term follow-up registered in the French Gastrointestinal Lymphoma Study Group (GELD) of the French Federation of Digestive Oncology (FFCD). Gut Liver 16, 207–215 (2022).
Kuppers, R. & Stevenson, F. K. Critical influences on the pathogenesis of follicular lymphoma. Blood 131, 2297–2306 (2018).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 40, 413–442 (2022).
Liu, Y. J. et al. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur. J. Immunol. 21, 1905–1910 (1991).
Su, W., Spencer, J. & Wotherspoon, A. C. Relative distribution of tumour cells and reactive cells in follicular lymphoma. J. Pathol. 193, 498–504 (2001).
Ravetch, J. V. Fc receptors. Curr. Opin. Immunol. 9, 121–125 (1997).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Britta Siegmund, Kathryn Knoop and Hiroshi Ohno for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spencer, J., Bemark, M. Human intestinal B cells in inflammatory diseases. Nat Rev Gastroenterol Hepatol 20, 254–265 (2023). https://doi.org/10.1038/s41575-023-00755-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00755-6