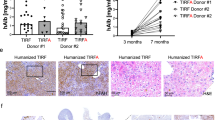
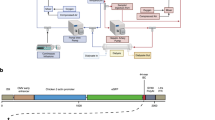
Abstract
Gene therapy is poised to revolutionize modern medicine, with seemingly unlimited potential for treating and curing genetic disorders. For otherwise incurable indications, including most inherited metabolic liver disorders, gene therapy provides a realistic therapeutic option. In this Review, we discuss gene supplementation and gene editing involving the use of recombinant adeno-associated virus (rAAV) vectors for the treatment of inherited liver diseases, including updates on several ongoing clinical trials that are producing promising results. Clinical testing has been essential in highlighting many key translational challenges associated with this transformative therapy. In particular, the interaction of a patient’s immune system with the vector raises issues of safety and the duration of treatment efficacy. Furthermore, several serious adverse events after the administration of high doses of rAAVs suggest greater involvement of innate immune responses and pre-existing hepatic conditions than initially anticipated. Finally, permanent modification of the host genome associated with rAAV genome integration and gene editing raises concerns about the risk of oncogenicity that require careful evaluation. We summarize the main progress, challenges and pathways forward for gene therapy for liver diseases.
Key points
-
Gene therapy mediated by recombinant adeno-associated virus (rAAV) vectors has emerged as a therapeutic option, with inherited liver disorders being prime targets for this strategy.
-
The first gene therapy for haemophilia B has been approved by the FDA, another for haemophilia A is nearing approval, and trials of several other rAAV-based liver-targeted gene therapies have produced promising results.
-
High rAAV doses (>1 × 1014 vg/kg) seem to be associated with severe adverse effects, including hepatotoxicity and immune response-associated sequelae.
-
Due to the primarily non-integrative nature of rAAV genomes, loss of vector genomes during cell turnover is of essential concern for the durability of therapeutic effect, particularly in paediatric patients.
-
Gene-editing strategies offer the most powerful tools for permanently correcting genetic disorders via direct modification of the genome but pose additional safety risks, such as oncogenicity, owing to insertional mutagenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schulze, R. J., Schott, M. B., Casey, C. A., Tuma, P. L. & McNiven, M. A. The cell biology of the hepatocyte: a membrane trafficking machine. J. Cell Biol. 218, 2096–2112 (2019).
Wang, L. et al. AAV gene therapy corrects OTC deficiency and prevents liver fibrosis in aged OTC-knock out heterozygous mice. Mol. Genet. Metab. 120, 299–305 (2017).
Unzu, C. et al. Sustained enzymatic correction by rAAV-mediated liver gene therapy protects against induced motor neuropathy in acute porphyria mice. Mol. Ther. 19, 243–250 (2011).
Kaiser, R. A. et al. Use of an adeno-associated virus serotype Anc80 to provide durable cure of phenylketonuria in a mouse model. J. Inherit. Metab. Dis. 44, 1369–1381 (2021).
Weber, N. D. et al. Gene therapy for progressive familial intrahepatic cholestasis type 3 in a clinically relevant mouse model. Nat. Commun. 10, 5694 (2019).
Wojnarowska, F. The disfigured patient. Practitioner 232, 1022–1025 (1988).
Puzzo, F. et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid alpha-glucosidase. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aam6375 (2017).
Ronzitti, G. et al. A translationally optimized AAV-UGT1A1 vector drives safe and long-lasting correction of Crigler-Najjar syndrome. Mol. Ther. Methods Clin. Dev. 3, 16049 (2016). In this study, in vitro and in vivo testing of non-coding and codon-optimized transgene sequences revealed a great deal about vector construct design and about producing long-lasting disease correction in multiple animal models.
Le Quellec, S. et al. Recombinant adeno-associated viral vectors expressing human coagulation FIX-E456H variant in Hemophilia B mice. Thromb. Haemost. 119, 1956–1967 (2019).
McIntosh, J. et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 121, 3335–3344 (2013).
Friedmann, T. & Roblin, R. Gene therapy for human genetic disease? Science 175, 949–955 (1972).
Jackson, D. A., Symons, R. H. & Berg, P. Biochemical method for inserting new genetic information into DNA of simian virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. 1972. Biotechnology 24, 11–16 (1992).
Mertz, J. E. & Davis, R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc. Natl Acad. Sci. USA 69, 3370–3374 (1972).
Lobban, P. E. & Kaiser, A. D. Enzymatic end-to end joining of DNA molecules. J. Mol. Biol. 78, 453–471 (1973).
Cohen, S. N., Chang, A. C., Boyer, H. W. & Helling, R. B. Construction of biologically functional bacterial plasmids in vitro. Proc. Natl Acad. Sci. USA 70, 3240–3244 (1973).
Blaese, R. M. et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science 270, 475–480 (1995). This study was a first-in-human gene therapy clinical trial, in which a patient with severe immunodeficiency received T cells modified with a retroviral vector carrying the therapeutic gene.
Shahryari, A. et al. Development and clinical translation of approved gene therapy products for genetic disorders. Front. Genet. 10, 868 (2019).
Anguela, X. M. & High, K. A. Entering the modern era of gene therapy. Annu. Rev. Med. 70, 273–288 (2019).
Zabaleta, N., Hommel, M., Salas, D. & Gonzalez-Aseguinolaza, G. Genetic-based approaches to inherited metabolic liver diseases. Hum. Gene Ther. 30, 1190–1203 (2019).
Alkhouri, N. & Gawrieh, S. A perspective on RNA interference-based therapeutics for metabolic liver diseases. Expert. Opin. Investig. Drugs 30, 237–244 (2021).
Doudna, J. A. The promise and challenge of therapeutic genome editing. Nature 578, 229–236 (2020).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
CSL Behring. FDA Accepts CSL Behring’s Biologics License Application for Etranacogene Dezaparvovec for Priority Review https://www.cslbehring.com/newsroom/2022/fda-bla-etranacogene-dezaparvovec (2022).
European Medicines Agency. First Gene Therapy to Treat Severe Haemophilia A https://www.ema.europa.eu/en/news/first-gene-therapy-treat-severe-haemophilia (2022).
Srivastava, A., Lusby, E. W. & Berns, K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 45, 555–564 (1983).
Atchison, R. W., Casto, B. C. & Hammon, W. M. Adenovirus-associated defective virus particles. Science 149, 754–756 (1965).
Gao, G. et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 (2004).
Shirley, J. L., de Jong, Y. P., Terhorst, C. & Herzog, R. W. Immune responses to viral gene therapy vectors. Mol. Ther. 28, 709–722 (2020).
Mendell, J. R. et al. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 29, 464–488 (2021).
Wang, D., Tai, P. W. L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 (2019).
Samulski, R. J. & Muzyczka, N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 1, 427–451 (2014).
Flotte, T. R., Afione, S. A. & Zeitlin, P. L. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am. J. Respir. Cell Mol. Biol. 11, 517–521 (1994).
Vincent-Lacaze, N. et al. Structure of adeno-associated virus vector DNA following transduction of the skeletal muscle. J. Virol. 73, 1949–1955 (1999).
Yang, J. et al. Concatamerization of adeno-associated virus circular genomes occurs through intermolecular recombination. J. Virol. 73, 9468–9477 (1999).
Maestro, S., Weber, N. D., Zabaleta, N., Aldabe, R. & Gonzalez-Aseguinolaza, G. Novel vectors and approaches for gene therapy in liver diseases. JHEP Rep. 3, 100300 (2021).
Gao, G., Vandenberghe, L. H. & Wilson, J. M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5, 285–297 (2005).
Tiegs, G. & Lohse, A. W. Immune tolerance: what is unique about the liver. J. Autoimmun. 34, 1–6 (2010).
Keeler, G. D., Markusic, D. M. & Hoffman, B. E. Liver induced transgene tolerance with AAV vectors. Cell Immunol. 342, 103728 (2019).
Crispe, I. N. Immune tolerance in liver disease. Hepatology 60, 2109–2117 (2014).
Lerut, J. & Sanchez-Fueyo, A. An appraisal of tolerance in liver transplantation. Am. J. Transpl. 6, 1774–1780 (2006).
Protzer, U., Maini, M. K. & Knolle, P. A. Living in the liver: hepatic infections. Nat. Rev. Immunol. 12, 201–213 (2012).
Crispe, I. N. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3, 51–62 (2003).
Zhang, P. et al. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum. Gene Ther. 23, 460–472 (2012).
Wu, Z. et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol. Ther. 16, 280–289 (2008).
Szafranska, K., Kruse, L. D., Holte, C. F., McCourt, P. & Zapotoczny, B. The whole story about fenestrations in LSEC. Front. Physiol. 12, 735573 (2021).
Von Drygalski, A. et al. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3, 3241–3247 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03569891 (2018).
US Food and Drug Administration. BLA approval letter for etranacogene dezaparvovec. FDA https://www.fda.gov/media/163466/download (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03861273 (2019).
Robinson, M. M. et al. Factor IX assay discrepancies in the setting of liver gene therapy using a hyperfunctional variant factor IX-Padua. J. Thromb. Haemost. 19, 1212–1218 (2021).
Konkle, B. A. et al. Updated follow-up of the Alta study, a phase 1/2, open label, adaptive, dose-ranging study to assess the safety and tolerability of SB-525 gene therapy in adult patients with severe hemophilia A. Blood 134, 2060 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04370054 (2020).
Pasi, K. J. et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N. Engl. J. Med. 382, 29–40 (2020). Results from the clinical trial of roctavian, the first approved AAV-based gene therapy drug directed to the liver.
Pasi, K. J. et al. Persistence of haemostatic response following gene therapy with valoctocogene roxaparvovec in severe haemophilia A. Haemophilia 27, 947–956 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04323098 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03370913 (2017).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03392974 (2018).
Kassim, S. H. et al. Gene therapy in a humanized mouse model of familial hypercholesterolemia leads to marked regression of atherosclerosis. PLoS ONE 5, e13424 (2010).
Murillo, O. et al. Liver expression of a MiniATP7B gene results in long-term restoration of copper homeostasis in a Wilson disease model in mice. Hepatology 70, 108–126 (2019).
Lee, Y. M. et al. Long-term safety and efficacy of AAV gene therapy in the canine model of glycogen storage disease type Ia. J. Inherit. Metab. Dis. 41, 977–984 (2018).
Ilyinskii, P. O. et al. ImmTOR nanoparticles enhance AAV transgene expression after initial and repeat dosing in a mouse model of methylmalonic acidemia. Mol. Ther. Methods Clin. Dev. 22, 279–292 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02991144 (2016).
Geberhiwot, T. in ESGCT Collaborative Virtual Congress Vol. A1-A152 (European Society of Gene & Cell Therapy, 2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03952156 (2019).
Bodamer, O. 2021 ACMG Annual Clinical Genetics Meeting. Mol. Gen. Metabol. 132, S1–S382 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03517085 (2018).
Couce Pico, M. L. in ESGCT Collaborative Virtual Congress Vol. 32 A1-A152 (ESGCT, 2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03466463 (2018).
D’Antiga, L. ESGCT Collaborative Virtual Congress 19–22 October 2021 Abstracts. Hum. Gene Ther. 32, A1–A152 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05345171 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05139316 (2021).
Verdera, H. C., Kuranda, K. & Mingozzi, F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 28, 723–746 (2020).
Li, C. et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 19, 288–294 (2012).
Perocheau, D. P. et al. Age-related seroprevalence of antibodies against AAV-LK03 in a UK population cohort. Hum. Gene Ther. 30, 79–87 (2019).
Boutin, S. et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 (2010).
Calcedo, R. & Wilson, J. M. AAV natural infection induces broad cross-neutralizing antibody responses to multiple AAV serotypes in chimpanzees. Hum. Gene Ther. Clin. Dev. 27, 79–82 (2016).
Scallan, C. D. et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107, 1810–1817 (2006).
Mingozzi, F. & High, K. A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122, 23–36 (2013).
Chicoine, L. G. et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol. Ther. 22, 338–347 (2014).
Salas, D. et al. Immunoadsorption enables successful rAAV5-mediated repeated hepatic gene delivery in nonhuman primates. Blood Adv. 3, 2632–2641 (2019).
Mingozzi, F. et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 5, 194ra192 (2013).
Bertin, B. et al. Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci. Rep. 10, 864 (2020).
Leborgne, C. et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 26, 1096–1101 (2020). This study demonstrated that the use of bacterial proteins with capacity to cleave human IgG might open the door for the treatment of patients with antibodies against AAV, currently excluded from gene therapy clinical trials.
von Pawel-Rammingen, U., Johansson, B. P. & Bjorck, L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21, 1607–1615 (2002).
Elmore, Z. C., Oh, D. K., Simon, E. E., Fanous, M. M. & Asokan, A. Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight 5, e139881 (2020).
Winstedt, L. et al. Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme IdeS — a novel therapeutic opportunity. PLoS ONE 10, e0132011 (2015).
Tseng, Y. S. & Agbandje-McKenna, M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front. Immunol. 5, 9 (2014).
Meliani, A. et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun. 9, 4098 (2018). This study showed that the use of lipid nanoparticles containing rapamycin can prevent antibody formation after the first dose of a viral vector, allowing vector re-administration.
Mingozzi, F. et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13, 419–422 (2007).
Li, H. et al. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol. Ther. 19, 2021–2030 (2011).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 (2006). The first AAV-mediated clinical trial targeting the liver for the correction of haemophilia B. This trial highlighted the importance of controlling the cellular immune response against the capsid to achieve sustained transgene expression.
Nathwani, A. C. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 (2011). This study demonstrated long-term factor IX expression after AAV injection in several patients thanks to the use of corticoids to block anti-AAV T cell responses and prevent the disappearance of the transduced cells.
George, L. A. et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 377, 2215–2227 (2017).
Konkle, B. A. et al. BAX 335 hemophilia B gene therapy clinical trial results: potential impact of CpG sequences on gene expression. Blood 137, 763–774 (2021).
Chai, Z. et al. Optimization of dexamethasone administration for maintaining global transduction efficacy of adeno-associated virus serotype 9. Hum. Gene Ther. 30, 829–840 (2019).
Schroeder, H. 25th Annual Meeting of the American Society of Gene & Cell Therapy. Mol. Ther. 30 (Suppl. 1), 1–592 (2022).
Hosel, M. et al. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology 55, 287–297 (2012).
Zhu, J., Huang, X. & Yang, Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 119, 2388–2398 (2009).
Rogers, G. L. et al. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J. Innate Immun. 7, 302–314 (2015).
Earley, L. F. et al. Adeno-associated virus serotype-specific inverted terminal repeat sequence role in vector transgene expression. Hum. Gene Ther. 31, 151–162 (2020).
Flotte, T. R. et al. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 7, 349–356 (1992).
Shao, W. et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight 3, e120474 (2018).
Faust, S. M. et al. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Invest. 123, 2994–3001 (2013).
Xiang, Z. et al. The effect of CpG sequences on capsid-specific CD8+ T cell responses to AAV vector gene transfer. Mol. Ther. 28, 771–783 (2020).
Flotte, T. R. Revisiting the “new” inflammatory toxicities of adeno-associated virus vectors. Hum. Gene Ther. 31, 398–399 (2020).
Grieger, J. C., Soltys, S. M. & Samulski, R. J. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol. Ther. 24, 287–297 (2016).
Schnodt, M. & Buning, H. Improving the quality of adeno-associated viral vector preparations: the challenge of product-related impurities. Hum. Gene Ther. Methods 28, 101–108 (2017).
Pierson, E. E., Keifer, D. Z., Asokan, A. & Jarrold, M. F. Resolving adeno-associated viral particle diversity with charge detection mass spectrometry. Anal. Chem. 88, 6718–6725 (2016).
Mullard, A. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat. Rev. Drug Discov. 20, 804–805 (2021).
Chand, D. et al. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J. Hepatol. 74, 560–566 (2021). In this study, systemic administration of high doses of recombinant AAV vector to treat a neuromuscular disease resulted in severe liver damage.
Feldman, A. G. et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J. Pediatr. 225, 252–258.e1 (2020).
Morales, L., Gambhir, Y., Bennett, J. & Stedman, H. H. Broader implications of progressive liver dysfunction and lethal sepsis in two boys following systemic high-dose AAV. Mol. Ther. 28, 1753–1755 (2020).
Hinderer, C. et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29, 285–298 (2018).
Palazzi, X. et al. Biodistribution and tolerability of AAV-PHP.B-CBh-SMN1 in Wistar Han rats and cynomolgus macaques reveal different toxicologic profiles. Hum. Gene Ther. 33, 175–187 (2022).
Chand, D. H. et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J. Pediatr. 231, 265–268 (2021).
Muhuri, M. et al. Overcoming innate immune barriers that impede AAV gene therapy vectors. J. Clin. Invest. https://doi.org/10.1172/JCI143780 (2021).
Zaiss, A. K. et al. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 82, 2727–2740 (2008).
Smith, C. J. et al. Pre-existing humoral immunity and complement pathway contribute to immunogenicity of adeno-associated virus (AAV) vector in human blood. Front. Immunol. 13, 999021 (2022).
Agrawal, P. et al. The imitation game: a viral strategy to subvert the complement system. FEBS Lett. 594, 2518–2542 (2020).
Guillou, J. et al. Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv. https://doi.org/10.1182/bloodadvances.2021006419 (2022).
PR Newswire. LogicBio Therapeutics Provides Update on LB-001 Clinical Development Program https://www.prnewswire.com/news-releases/logicbio-therapeutics-provides-update-on-lb-001-clinical-development-program-301473512.html (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04581785 (2020).
FDA. Cellular, Tissue, and Gene Therapies Advisory Committee September 2, 2021 Meeting Presentation- Toxicity Risks of Adeno-associated Virus (AAV) Vectors for Gene Therapy (GT) www.fda.gov (2021).
Hordeaux, J. et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 31, 808–818 (2020).
Hordeaux, J. et al. MicroRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci. Transl. Med. 12, eaba9188 (2020).
Zolotukhin, S. & Vandenberghe, L. H. AAV capsid design: a Goldilocks challenge. Trends Mol. Med. 28, 183–193 (2022).
Barzel, A. et al. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature 517, 360–364 (2015).
Heinke, P. et al. Diploid hepatocytes drive physiological liver renewal in adult humans. Cell Syst. 13, 499–507.e12 (2022).
Hagedorn, C. et al. S/MAR element facilitates episomal long-term persistence of adeno-associated virus vector genomes in proliferating cells. Hum. Gene Ther. 28, 1169–1179 (2017).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Follenzi, A., Sabatino, G., Lombardo, A., Boccaccio, C. & Naldini, L. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum. Gene Ther. 13, 243–260 (2002).
Milani, M. et al. Liver-directed lentiviral gene therapy corrects hemophilia A mice and achieves normal-range factor VIII activity in non-human primates. Nat. Commun. 13, 2454 (2022).
Gillmore, J. D. et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021). The first clinical trial in which CRISPR was used to target the liver for treatment of an inherited disorder.
Ohmori, T. et al. CRISPR/Cas9-mediated genome editing via postnatal administration of AAV vector cures haemophilia B mice. Sci. Rep. 7, 4159 (2017).
Yang, Y. et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 34, 334–338 (2016).
Richards, D. Y. et al. AAV-Mediated CRISPR/Cas9 gene editing in murine phenylketonuria. Mol. Ther. Methods Clin. Dev. 17, 234–245 (2020).
Suzuki, K. et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 540, 144–149 (2016).
Levy, J. M. et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 4, 97–110 (2020).
Awan, M. J. A., Ali, Z., Amin, I. & Mansoor, S. Twin prime editor: seamless repair without damage. Trends Biotechnol. 40, 374–376 (2022).
Yang, L. & Chen, J. A tale of two moieties: rapidly evolving CRISPR/Cas-based genome editing. Trends Biochem. Sci. 45, 874–888 (2020).
Yin, H. et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol. 34, 328–333 (2016).
Krooss, S. A. et al. Ex vivo/in vivo gene editing in hepatocytes using “All-in-One” CRISPR-adeno-associated virus vectors with a self-linearizing repair template. iScience 23, 100764 (2020).
Villiger, L. et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 24, 1519–1525 (2018).
Liu, P. et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. Nat. Commun. 12, 2121 (2021).
Suzuki, K. & Izpisua Belmonte, J. C. In vivo genome editing via the HITI method as a tool for gene therapy. J. Hum. Genet. 63, 157–164 (2018).
Intellia. Intellia Therapeutics Achieves Normal Human Alpha-1 Antitrypsin Protein Levels in Non-Human Primates Through Targeted Gene Insertion for the Treatment of AAT Deficiency https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-achieves-normal-human-alpha-1-antitrypsin (2020).
Porro, F. et al. Promoterless gene targeting without nucleases rescues lethality of a Crigler-Najjar syndrome mouse model. EMBO Mol. Med. 9, 1346–1355 (2017).
Borel, F. et al. Survival advantage of both human hepatocyte xenografts and genome-edited hepatocytes for treatment of alpha-1 antitrypsin deficiency. Mol. Ther. 25, 2477–2489 (2017).
Chandler, R. J. et al. Promoterless, nuclease-free genome editing confers a growth advantage for corrected hepatocytes in mice with methylmalonic acidemia. Hepatology 73, 2223–2237 (2021).
Cantore, A. et al. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci. Transl. Med. 7, 277ra228 (2015).
Cozmescu, A. C., Counsell, J. & Gissen, P. Gene therapies targeting the liver. J. Hepatol. 74, 235–236 (2021).
Paneda, A. et al. Safety and liver transduction efficacy of rAAV5-cohPBGD in nonhuman primates: a potential therapy for acute intermittent porphyria. Hum. Gene Ther. 24, 1007–1017 (2013).
Gao, G. et al. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol. Ther. 13, 77–87 (2006).
Greig, J. A. et al. Determining the minimally effective dose of a clinical candidate AAV vector in a mouse model of Crigler-Najjar syndrome. Mol. Ther. Methods Clin. Dev. 10, 237–244 (2018).
Wang, L. et al. Prednisolone reduces the interferon response to AAV in cynomolgus macaques and may increase liver gene expression. Mol. Ther. Methods Clin. Dev. 24, 292–305 (2022).
D’Avola, D. et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J. Hepatol. 65, 776–783 (2016). The first clinical trial of AAV for the treatment of an inherited metabolic liver disorder.
Baruteau, J. et al. Safety and efficacy of an engineered hepatotropic AAV gene therapy for ornithine transcarbamylase deficiency in cynomolgus monkeys. Mol. Ther. Methods Clin. Dev. 23, 135–146 (2021).
Bell, P. et al. Inverse zonation of hepatocyte transduction with AAV vectors between mice and non-human primates. Mol. Genet. Metab. 104, 395–403 (2011).
Ramachandran, P., Matchett, K. P., Dobie, R., Wilson-Kanamori, J. R. & Henderson, N. C. Single-cell technologies in hepatology: new insights into liver biology and disease pathogenesis. Nat. Rev. Gastroenterol. Hepatol. 17, 457–472 (2020).
Kang, Y. B. A., Eo, J., Mert, S., Yarmush, M. L. & Usta, O. B. Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci. Rep. 8, 8951 (2018).
Lisowski, L. et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506, 382–386 (2014).
Grompe, M. & Strom, S. Mice with human livers. Gastroenterology 145, 1209–1214 (2013).
Cabanes-Creus, M. et al. Preclinical evaluation of AAV vectors in an ex vivo human whole liver explant confirms the potential of bioengineered AAVs as clinically relevant hepatotropic vectors. Mol. Ther. 30, 387 (2022).
George, L. A. et al. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N. Engl. J. Med. 385, 1961–1973 (2021).
Schutgens, R. E. G. Gene therapy for hemophilia A: how long will it last? Hemasphere 6, e720 (2022).
Takeda. TAK-754 and TAK-748 Clinical Studies https://www.hemophiliafed.org/uploads/Takeda-Letter-GT-Trials-Update-August-2020-HFA.pdf (2022).
Cheung, A. K., Hoggan, M. D., Hauswirth, W. W. & Berns, K. I. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J. Virol. 33, 739–748 (1980).
Kotin, R. M. et al. Site-specific integration by adeno-associated virus. Proc. Natl Acad. Sci. USA 87, 2211–2215 (1990).
Chiorini, J. A. et al. Biologically active Rep proteins of adeno-associated virus type 2 produced as fusion proteins in Escherichia coli. J. Virol. 68, 797–804 (1994).
Weitzman, M. D., Kyostio, S. R., Kotin, R. M. & Owens, R. A. Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA. Proc. Natl Acad. Sci. USA 91, 5808–5812 (1994).
Janovitz, T. et al. High-throughput sequencing reveals principles of adeno-associated virus serotype 2 integration. J. Virol. 87, 8559–8568 (2013).
Nault, J. C. et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet. 47, 1187–1193 (2015).
Smith, J. R. et al. Robust, persistent transgene expression in human embryonic stem cells is achieved with AAVS1-targeted integration. Stem Cell 26, 496–504 (2008).
Logan, G. J. et al. Identification of liver-specific enhancer-promoter activity in the 3’ untranslated region of the wild-type AAV2 genome. Nat. Genet. 49, 1267–1273 (2017).
La Bella, T. et al. Adeno-associated virus in the liver: natural history and consequences in tumour development. Gut 69, 737–747 (2020).
Tatsuno, K. et al. Impact of AAV2 and hepatitis B virus integration into genome on development of hepatocellular carcinoma in patients with prior hepatitis B virus infection. Clin. Cancer Res. 25, 6217–6227 (2019).
Park, K. J. et al. Adeno-associated virus 2-mediated hepatocellular carcinoma is very rare in Korean patients. Ann. Lab. Med. 36, 469–474 (2016).
Nault, J. C. et al. Wild-type AAV insertions in hepatocellular carcinoma do not inform debate over genotoxicity risk of vectorized AAV. Mol. Ther. 24, 660–661 (2016).
Schmidt, M., Gil-Farina, I. & Buning, H. Reply to “Wild-type AAV insertions in hepatocellular carcinoma do not inform debate over genotoxicity risk of vectorized AAV”. Mol. Ther. 24, 661–662 (2016).
Schaffer, A. A. et al. Integration of adeno-associated virus (AAV) into the genomes of most Thai and Mongolian liver cancer patients does not induce oncogenesis. BMC Genomics 22, 814 (2021).
Bayard, Q. et al. Cyclin A2/E1 activation defines a hepatocellular carcinoma subclass with a rearrangement signature of replication stress. Nat. Commun. 9, 5235 (2018).
Cantalupo, P. G., Katz, J. P. & Pipas, J. M. Viral sequences in human cancer. Virology 513, 208–216 (2018).
Zapatka, M. et al. The landscape of viral associations in human cancers. Nat. Genet. 52, 320–330 (2020).
Srivastava, A. & Carter, B. J. AAV infection: protection from cancer. Hum. Gene Ther. 28, 323–327 (2017).
Ostrove, J. M., Duckworth, D. H. & Berns, K. I. Inhibition of adenovirus-transformed cell oncogenicity by adeno-associated virus. Virology 113, 521–533 (1981).
Raj, K., Ogston, P. & Beard, P. Virus-mediated killing of cells that lack p53 activity. Nature 412, 914–917 (2001).
Mayor, H. D., Drake, S., Stahmann, J. & Mumford, D. M. Antibodies to adeno-associated satellite virus and herpes simplex in sera from cancer patients and normal adults. Am. J. Obstet. Gynecol. 126, 100–104 (1976).
Sabatino, D. E. et al. Evaluating the state of the science for adeno-associated virus (AAV) integration: an integrated perspective. Mol. Ther. https://doi.org/10.1016/j.ymthe.2022.06.004 (2022). This article provides the perspectives of leading experts in the field of AAV integration about the safety of AAVs and their potential genotoxicity.
Rutledge, E. A. & Russell, D. W. Adeno-associated virus vector integration junctions. J. Virol. 71, 8429–8436 (1997).
Miller, D. G., Petek, L. M. & Russell, D. W. Adeno-associated virus vectors integrate at chromosome breakage sites. Nat. Genet. 36, 767–773 (2004). In this study, an elegant system was developed for testing the hypothesis that AAV vectors integrate at existing DNA double-strand breaks without causing breaks themselves.
Philpott, N. J., Gomos, J. & Falck-Pedersen, E. Transgene expression after rep-mediated site-specific integration into chromosome 19. Hum. Gene Ther. 15, 47–61 (2004).
Chen, W. Y. & Townes, T. M. Molecular mechanism for silencing virally transduced genes involves histone deacetylation and chromatin condensation. Proc. Natl Acad. Sci. USA 97, 377–382 (2000).
Nakai, H. et al. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 75, 6969–6976 (2001).
Duan, D. et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol. 72, 8568–8577 (1998). This study showed the episomal nature of recombinant AAV genomes responsible for long-term transgene expression.
Nakai, H. et al. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat. Genet. 34, 297–302 (2003). The first study to establish the techniques to characterize the integration pattern of AAV vectors.
Nakai, H. et al. Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J. Virol. 79, 3606–3614 (2005).
Inagaki, K. et al. DNA palindromes with a modest arm length of greater, similar 20 base pairs are a significant target for recombinant adeno-associated virus vector integration in the liver, muscles, and heart in mice. J. Virol. 81, 11290–11303 (2007).
Donsante, A. et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther. 8, 1343–1346 (2001).
Bell, P. et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol. Ther. 14, 34–44 (2006).
Donsante, A. et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 317, 477 (2007).
Chandler, R. J. et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Invest. 125, 870–880 (2015). The most comprehensive preclinical study of the risk of genotoxicity with liver-directed AAV therapies, in which some of the important factors influencing genotoxicity are defined.
Dalwadi, D. A. et al. Liver injury increases the incidence of HCC following AAV gene therapy in mice. Mol. Ther. 29, 680–690 (2021).
Dalwadi, D. A. et al. AAV integration in human hepatocytes. Mol. Ther. 29, 2898–2909 (2021).
Inagaki, K., Piao, C., Kotchey, N. M., Wu, X. & Nakai, H. Frequency and spectrum of genomic integration of recombinant adeno-associated virus serotype 8 vector in neonatal mouse liver. J. Virol. 82, 9513–9524 (2008).
Zhong, L. et al. Recombinant adeno-associated virus integration sites in murine liver after ornithine transcarbamylase gene correction. Hum. Gene Ther. 24, 520–525 (2013).
Rosas, L. E. et al. Patterns of scAAV vector insertion associated with oncogenic events in a mouse model for genotoxicity. Mol. Ther. 20, 2098–2110 (2012).
Bell, P. et al. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol. Ther. 12, 299–306 (2005).
Li, H. et al. Assessing the potential for AAV vector genotoxicity in a murine model. Blood 117, 3311–3319 (2011).
BioMarin. U.S. FDA Placed a Clinical Hold on BMN 307 Phearless Phase ½ Gene Therapy Study in Adults with PKU Based on Interim Pre-clinical Study Findings https://investors.biomarin.com/2021-09-06-U-S-FDA-Placed-a-Clinical-Hold-on-BMN-307-Phearless-Phase-1-2-Gene-Therapy-Study-in-Adults-with-PKU-Based-on-Interim-Pre-clinical-Study-Findings (2021).
Nguyen, G. N. et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 39, 47–55 (2021). A 10-year follow-up of AAV gene therapy in dogs that provided long-term safety and expression data and a thorough analysis of vector integration characteristics.
Nowrouzi, A. et al. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol. Ther. 20, 1177–1186 (2012).
Gil-Farina, I. et al. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol. Ther. 24, 1100–1105 (2016).
Kaeppel, C. et al. A largely random AAV integration profile after LPLD gene therapy. Nat. Med. 19, 889–891 (2013).
Euroland. uniQure Announces Clinical Update on Hemophilia B Gene Therapy Program. uniQure https://uniqure.gcs-web.com/node/10231/pdf (2021).
Hordeaux, J. et al. Safe and sustained expression of human iduronidase after intrathecal administration of adeno-associated virus serotype 9 in infant Rhesus monkeys. Hum. Gene Ther. 30, 957–966 (2019).
Kang, H. R. et al. Pathogenesis of hepatic tumors following gene therapy in murine and canine models of glycogen storage disease. Mol. Ther. Methods Clin. Dev. 15, 383–391 (2019).
Chen, S., Yao, Y., Zhang, Y. & Fan, G. CRISPR system: discovery, development and off-target detection. Cell Signal. 70, 109577 (2020).
Musunuru, K. et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 593, 429–434 (2021).
Rothgangl, T. et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 39, 949–957 (2021).
Muller, H. P. & Schaffner, W. Transcriptional enhancers can act in trans. Trends Genet. 6, 300–304 (1990).
Kadauke, S. & Blobel, G. A. Chromatin loops in gene regulation. Biochim. Biophys. Acta 1789, 17–25 (2009).
Schmidt, F., Kern, F. & Schulz, M. H. Integrative prediction of gene expression with chromatin accessibility and conformation data. Epigenetics Chromatin 13, 4 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT00076557 (2004).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT00979238 (2009).
Nathwani, A. C. et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 371, 1994–2004 (2014).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02396342 (2015).
Miesbach, W. et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 131, 1022–1031 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT01687608 (2012).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03489291 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03369444 (2017).
Chowdary, P. et al. Phase 1-2 trial of AAVS3 gene therapy in patients with hemophilia B. N. Engl. J. Med. 387, 237–247 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02618915 (2018).
Pipe, S. in AHS Annual Meeting & Exposition Vol. 130, 3331 (AHS, 2017).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02576795 (2015).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03003533 (2016).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03061201 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03588299 (2018).
Pipe, S. W. in ASH Annual Meeting & Exposition Vol. 136, 44–45 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03370172 (2017).
GlobeNewswire. Ultragenyx Completes Successful End-of-Phase 2 Meeting with FDA and Finalizes Phase 3 Study Design for DTX301 Ornithine Transcarbamylase (OTC) Gene Therapy Program https://www.globenewswire.com/news-release/2021/04/22/2215092/20739/en/Ultragenyx-Completes-Successful-End-of-Phase-2-Meeting-with-FDA-and-Finalizes-Phase-3-Study-Design-for-DTX301-Ornithine-Transcarbamylase-OTC-Gene-Therapy-Program.html (2021).
GlobeNewswire. Homology Medicines Announces Presentation of Positive Data from the Dose-Escalation Phase of the pheNIX Gene Therapy Trial for Adults with PKU https://www.globenewswire.com/news-release/2020/11/06/2122133/0/en/Homology-Medicines-Announces-Presentation-of-Positive-Data-from-the-Dose-Escalation-Phase-of-the-pheNIX-Gene-Therapy-Trial-for-Adults-with-PKU.html (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04480567 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04452513 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05222178 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02082860 (2014).
PR Newswire. LogicBio Therapeutics Announces FDA Lifts Clinical Hold on SUNRISE Trial in Pediatric Patients with Methylmalonic Acidemia https://www.prnewswire.com/news-releases/logicbio-therapeutics-announces-fda-lifts-clinical-hold-on-sunrise-trial-in-pediatric-patients-with-methylmalonic-acidemia-301542452.html (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02651675 (2016).
Ultragenix. Ultragenix announces positive data from Phase 1/2 study of DTX401 gene therapy in Glycogen Storage Disease type Ia https://ir.ultragenyx.com/news-releases/news-release-details/ultragenyx-announces-positive-data-phase-12-study-dtx401-gene (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04537377 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04884815 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04601051 (2020).
Bonnemann, C. G. A Collaborative Analysis by Clinical Trial Sponsors and Academic Experts of Anti-transgene SAEs in Studies of Gene Therapy for DMD in 25th Annual Meeting of the American Society of Gene & Cell Therapy. Mol. Ther. 30, S1–S592 (2022).
Author information
Authors and Affiliations
Contributions
N.Z., C.U. and G.G.-A. researched data for the article. All authors made substantia contributions to the discussion of content, contributed to writing, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.D.W. and G.G-A. are employees and shareholders of Vivet Therapeutics. The views expressed in this Review belong to the authors alone and do not reflect the opinions of Vivet Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks M. Grompe, G. Ronzitti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zabaleta, N., Unzu, C., Weber, N.D. et al. Gene therapy for liver diseases — progress and challenges. Nat Rev Gastroenterol Hepatol 20, 288–305 (2023). https://doi.org/10.1038/s41575-022-00729-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00729-0
This article is cited by
-
Advances in basic research on glucagon and alpha cells
Diabetology International (2024)