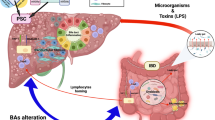
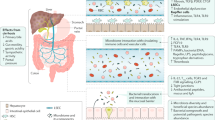
Abstract
Primary sclerosing cholangitis (PSC) offers unique opportunities to explore the gut–liver axis owing to the close association between liver disease and colonic inflammation. It is well established that the gut microbiota in people with PSC differs from that of healthy individuals, but details of the microbial factors that demarcate PSC from inflammatory bowel disease (IBD) without PSC are poorly understood. In this Review, we aim to provide an overview of the latest literature on the gut microbiome in PSC and PSC with IBD, critically examining hypotheses on how microorganisms could contribute to the pathogenesis of PSC. A particular emphasis will be put on pathogenic features of the gut microbiota that might explain the occurrence of bile duct inflammation and liver disease in the context of IBD, and we postulate the potential existence of a specific yet unknown factor related to the gut–liver axis as causative in PSC. Available data are scrutinized in the perspective of therapeutic approaches related to the gut–liver axis.
Key points
-
The close association between primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD) makes PSC a prototype model disease for exploring the gut–liver axis.
-
Clinical data suggest that intestinal inflammation, an intact colon and antibiotics might influence the disease course of sclerosing cholangitis both before and after liver transplantation.
-
The gut microbiota composition in PSC differs from that of healthy individuals as controls and from those with IBD without PSC, but confounding factors and the potential influence of disease stage and IBD activity are so far understudied.
-
Experimental models suggest that the gut microbiota influences sclerosing cholangitis, with both harmful and beneficial effects having been identified.
-
The relative importance of individual microorganisms (‘pathobionts’) versus specific by-products of microbial activity (microbial metabolites) in PSC development is currently unknown.
-
The current sum of evidence provides a strong rationale for clinical trials of therapies that target the gut in PSC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Karlsen, T. H., Folseraas, T., Thorburn, D. & Vesterhus, M. Primary sclerosing cholangitis–a comprehensive review. J. Hepatol. 67, 1298–1323 (2017).
Weismuller, T. J. et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology 152, 1975–1984 (2017).
Boonstra, K. et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 58, 2045–2055 (2013).
Karlsen, T. H., Schrumpf, E. & Boberg, K. M. Update on primary sclerosing cholangitis. Dig. Liver Dis. 42, 390–400 (2010).
Jiang, X. & Karlsen, T. H. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat. Rev. Gastroenterol. Hepatol. 14, 279–295 (2017).
Ji, S. G. et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 49, 269–273 (2017).
Liu, J. Z. et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat. Genet. 45, 670–675 (2013). Key study delineating the autoimmune genetic architecture of PSC.
Peng, X. et al. Immunosuppressive agents for the treatment of primary sclerosing cholangitis: a systematic review and meta-analysis. Dig. Dis. 35, 478–485 (2017).
Albillos, A., de Gottardi, A. & Rescigno, M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72, 558–577 (2020).
Iliev, I. D. & Cadwell, K. Effects of intestinal fungi and viruses on immune responses and inflammatory bowel diseases. Gastroenterology 160, 1050–1066 (2021).
Sokol, H. et al. Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048 (2017).
de Vries, E. et al. Carriers of ABCB4 gene variants show a mild clinical course, but impaired quality of life and limited risk for cholangiocarcinoma. Liver Int. 40, 3042–3050 (2020).
Mouchli, M. A. et al. Natural history of established and de novo inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Inflamm. Bowel Dis. 24, 1074–1081 (2018).
Lunder, A. K. et al. Prevalence of sclerosing cholangitis, detected by magnetic resonance cholangiography, in patients with long-term inflammatory bowel disease. Gastroenterology 151, 660–669 (2016). First study to show that the prevalence of sclerosing cholangitis-like changes on MRC are more common than clinically overt PSC.
Broome, U. & Bergquist, A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin. Liver Dis. 26, 31–41 (2006).
Marelli, L. et al. Does the severity of primary sclerosing cholangitis influence the clinical course of associated ulcerative colitis? Gut 60, 1224–1228 (2011).
Navaneethan, U. et al. Progressive primary sclerosing cholangitis requiring liver transplantation is associated with reduced need for colectomy in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 10, 540–546 (2012).
Boden, R. W., Rankin, J. G., Goulston, S. J. & Morrow, W. The liver in ulcerative colitis: the significance of raised serum-alkaline-phosphatase levels. Lancet 2, 245–248 (1959).
Rankin, J. G., Boden, R. W., Goulston, S. J. & Morrow, W. The liver in ulcerative colitis; treatment of pericholangitis with tetracycline. Lancet 2, 1110–1112 (1959).
Farkkila, M. et al. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology 40, 1379–1386 (2004). First randomized clinical trial of antibiotics in PSC.
Tabibian, J. H. et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis–a pilot study. Aliment. Pharmacol. Ther. 37, 604–612 (2013).
Cangemi, J. R. et al. Effect of proctocolectomy for chronic ulcerative colitis on the natural history of primary sclerosing cholangitis. Gastroenterology 96, 790–794 (1989).
Martin, F. M. et al. Surgical aspects of sclerosing cholangitis. Results in 178 patients. Ann. Surg. 212, 551–556 (1990).
Nordenvall, C. et al. Colectomy prior to diagnosis of primary sclerosing cholangitis is associated with improved prognosis in a nationwide cohort study of 2594 PSC-IBD patients. Aliment. Pharmacol. Ther. 47, 238–245 (2018).
Vera, A. et al. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet 360, 1943–1944 (2002).
Graziadei, I. W. et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology 29, 1050–1056 (1999).
Steenstraten, I. C. et al. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment. Pharmacol. Ther. 49, 636–643 (2019).
Peverelle, M., Paleri, S., Hughes, J., De Cruz, P. & Gow, P. J. Activity of inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis predicts poorer clinical outcomes. Inflamm. Bowel Dis. 26, 1901–1908 (2020).
Trivedi, P. J. et al. The impact of ileal pouch–anal anastomosis on graft survival following liver transplantation for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 48, 322–332 (2018).
Davies, Y. K. et al. Successful treatment of recurrent primary sclerosing cholangitis after orthotopic liver transplantation with oral vancomycin. Case Rep. Transpl. 2013, 314292 (2013).
Hey, P., Lokan, J., Johnson, P. & Gow, P. Efficacy of oral vancomycin in recurrent primary sclerosing cholangitis following liver transplantation. BMJ Case Rep. 2017, bcr2017221165 (2017).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in IL10−/− mice. Nature 487, 104–108 (2012).
Schwartz, D. J., Langdon, A. E. & Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 12, 82 (2020).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Acharya, C. & Bajaj, J. S. Chronic liver diseases and the microbiome — translating our knowledge of gut microbiota to management of chronic liver disease. Gastroenterology 160, 556–572 (2021).
Wang, J. et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 48, 1396–1406 (2016).
Ruhlemann, M. C. et al. Genome-wide association study in 8,956 German individuals identifies influence of ABO histo-blood groups on gut microbiome. Nat. Genet. 53, 147–155 (2021).
Lopera-Maya, E. A. et al. Effect of host genetics on the gut microbiome in 7738 participants of the Dutch Microbiome Project. Nat. Genet. 54, 143–151 (2022).
Qin, Y. et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 54, 134–142 (2022).
Gevers, D. et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392 (2014).
Xu, Z. & Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 113 (Suppl.), 1–5 (2015).
Vujkovic-Cvijin, I. et al. Host variables confound gut microbiota studies of human disease. Nature 587, 448–454 (2020).
Yap, C. X. et al. Autism-related dietary preferences mediate autism–gut microbiome associations. Cell 184, 5916–5931 (2021). Key study in highlighting the importance of confounding factors in assessing dietary impact on the gut microbiome.
de Vries, E. M. et al. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: an international cohort study. Hepatology 65, 907–919 (2017).
Mazhar, A. & Russo, M. W. Systematic review: non-invasive prognostic tests for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 53, 774–783 (2021).
Fairfield, B. & Schnabl, B. Gut dysbiosis as a driver in alcohol-induced liver injury. JHEP Rep. 3, 100220 (2021).
Aron-Wisnewsky, J. et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 17, 279–297 (2020).
Iwasawa, K. et al. Characterisation of the faecal microbiota in Japanese patients with paediatric-onset primary sclerosing cholangitis. Gut 66, 1344–1346 (2016).
Kummen, M. et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 66, 611–619 (2017). First DNA-based study of the faecal microbiota in people with PSC.
Sabino, J. et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 65, 1681–1689 (2016).
Bajer, L. et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 23, 4548–4558 (2017).
Ruhlemann, M. et al. Consistent alterations in faecal microbiomes of patients with primary sclerosing cholangitis independent of associated colitis. Aliment. Pharmacol. Ther. 50, 580–589 (2019).
Lemoinne, S. et al. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut 69, 92–102 (2019).
Lapidot, Y. et al. Alterations of the salivary and fecal microbiome in patients with primary sclerosing cholangitis. Hepatol. Int. 15, 191–201 (2021).
Liu, Q. et al. Altered faecal microbiome and metabolome in IgG4-related sclerosing cholangitis and primary sclerosing cholangitis. Gut 71, 899–909 (2022).
Kummen, M. et al. Altered gut microbial metabolism of essential nutrients in primary sclerosing cholangitis. Gastroenterology 160, 1784–1798 (2021).
Kevans, D. et al. Characterization of intestinal microbiota in ulcerative colitis patients with and without primary sclerosing cholangitis. J. Crohns Colitis 10, 330–337 (2016).
Torres, J. et al. The features of mucosa-associated microbiota in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 43, 790–801 (2016).
Rossen, N. G. et al. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J. Crohns Colitis 9, 342–348 (2015). First DNA-based study of the mucosal microbiota in people with PSC.
Quraishi, M. N. et al. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut 66, 386–388 (2017).
Iwasawa, K. et al. Dysbiosis of the salivary microbiota in pediatric-onset primary sclerosing cholangitis and its potential as a biomarker. Sci. Rep. 8, 5480 (2018).
Liwinski, T. et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 69, 665–672 (2020).
Pereira, P. et al. Bile microbiota in primary sclerosing cholangitis: impact on disease progression and development of biliary dysplasia. PLoS ONE 12, e0182924 (2017).
Kummen, M. et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J. Am. Coll. Cardiol. 71, 1184–1186 (2018).
Nowak, P. et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS 29, 2409–1418 (2015).
Jorgensen, S. F. et al. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol. 9, 1455–1465 (2016).
Kostic, A. D. et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 17, 260–273 (2015).
Tang, R. et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 67, 534–541 (2018).
Wei, Y. et al. Alterations of gut microbiome in autoimmune hepatitis. Gut 69, 569–577 (2020).
Blaser, M. J. & Falkow, S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 7, 887–894 (2009).
Liwinski, T. et al. A disease-specific decline of the relative abundance of Bifidobacterium in patients with autoimmune hepatitis. Aliment. Pharmacol. Ther. 51, 1417–1428 (2020).
Abe, K. et al. Dysbiosis of oral microbiota and its association with salivary immunological biomarkers in autoimmune liver disease. PLoS ONE 13, e0198757 (2018).
Zhang, Y., Xu, J., Wang, X., Ren, X. & Liu, Y. Changes of intestinal bacterial microbiota in coronary heart disease complicated with nonalcoholic fatty liver disease. BMC Genomics 20, 862 (2019).
Chen, Z. et al. Featured gut microbiomes associated with the progression of chronic hepatitis B disease. Front. Microbiol. 11, 383 (2020).
Wei, X. et al. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol. 13, 175 (2013).
Reddel, S. et al. A parallel tracking of salivary and gut microbiota profiles can reveal maturation and interplay of early life microbial communities in healthy infants. Microorganisms 10, 468 (2022).
Chalmers, N. I., Palmer, R. J. Jr, Cisar, J. O. & Kolenbrander, P. E. Characterization of a Streptococcus sp.–Veillonella sp. community micromanipulated from dental plaque. J. Bacteriol. 190, 8145–8154 (2008).
Gibbons, R. J., Socransky, S. S. & Kapsimalis, B. Establishment of human indigenous bacteria in germ-free mice. J. Bacteriol. 88, 1316–1323 (1964).
Vieira-Silva, S. et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 4, 1826–1831 (2019).
Ruhlemann, M. C. et al. Gut mycobiome of primary sclerosing cholangitis patients is characterised by an increase of Trichocladium griseum and Candida species. Gut 69, 1890–1892 (2019).
Chu, H. et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 72, 391–400 (2020).
Rudolph, G. et al. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J. Hepatol. 51, 149–155 (2009).
Bjornsson, E. S., Kilander, A. F. & Olsson, R. G. Bile duct bacterial isolates in primary sclerosing cholangitis and certain other forms of cholestasis — a study of bile cultures from ERCP. Hepatogastroenterology 47, 1504–1508 (2000).
Folseraas, T. et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol. 57, 366–375 (2012).
Zimmer, C. L. et al. A biliary immune landscape map of primary sclerosing cholangitis reveals a dominant network of neutrophils and tissue-resident T cells. Sci. Transl Med. 13, eabb3107 (2021).
Valestrand, L. et al. Bile from patients with primary sclerosing cholangitis contains mucosal-associated invariant T-cell antigens. Am. J. Pathol. 192, 629–641 (2022).
Valestrand, L. et al. Lipid antigens in bile from patients with chronic liver diseases activate natural killer T cells. Clin. Exp. Immunol. 203, 304–314 (2021).
D’Amico, F. et al. Bile microbiota in liver transplantation: proof of concept using gene amplification in a heterogeneous clinical scenario. Front. Surg. 8, 621525 (2021).
Molinero, N. et al. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome 7, 100 (2019).
Acharya, C. & Bajaj, J. S. Altered microbiome in patients with cirrhosis and complications. Clin. Gastroenterol. Hepatol. 17, 307–321 (2019).
Bushyhead, D. & Quigley, E. M. M. Small intestinal bacterial overgrowth pathophysiology and its implications for definition and management. Gastroenterology 163, 593–607 (2022).
Pittayanon, R. et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology 158, 930–946 (2020).
Lichtman, S. N., Keku, J., Clark, R. L., Schwab, J. H. & Sartor, R. B. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology 13, 766–772 (1991). First experimental evidence of an impact of the gut microbiota on development of sclerosing cholangitis.
Lichtman, S. N., Keku, J., Schwab, J. H. & Sartor, R. B. Evidence for peptidoglycan absorption in rats with experimental small bowel bacterial overgrowth. Infect. Immun. 59, 555–562 (1991).
Sakiani, S., Kleiner, D. E., Heller, T. & Koh, C. Hepatic manifestations of cystic fibrosis. Clin. Liver Dis. 23, 263–277 (2019).
Fiorotto, R. et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology 141, 1498–1508 (2011).
Debray, D. et al. Diet-induced dysbiosis and genetic background synergize with cystic fibrosis transmembrane conductance regulator deficiency to promote cholangiopathy in mice. Hepatol. Commun. 2, 1533–1549 (2018).
Henckaerts, L. et al. Cystic fibrosis transmembrane conductance regulator gene polymorphisms in patients with primary sclerosing cholangitis. J. Hepatol. 50, 150–157 (2009).
Schrumpf, E. et al. The gut microbiota contributes to a mouse model of spontaneous bile duct inflammation. J. Hepatol. 66, 382–389 (2017).
Tabibian, J. H. et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis in mice. Hepatology 63, 185–196 (2016).
Schneider, K. M. et al. Gut microbiota depletion exacerbates cholestatic liver injury via loss of FXR signalling. Nat. Metab. 3, 1228–1241 (2021).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Tedesco, D. et al. Alterations in intestinal microbiota lead to production of interleukin 17 by intrahepatic gammadelta T-cell receptor-positive cells and pathogenesis of cholestatic liver disease. Gastroenterology 154, 2178–2193 (2018).
Liao, L. et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut 68, 1477–1492 (2019).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17, 588–606 (2018).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Nakamoto, N. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 4, 492–503 (2019). First study to highlight a potential role for specific pathobionts in pathogenesis of PSC.
Katt, J. et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 58, 1084–1093 (2013).
Kunzmann, L. K. et al. Monocytes as potential mediators of pathogen-induced T-helper 17 differentiation in patients with primary sclerosing cholangitis (PSC). Hepatology 72, 1310–1326 (2020).
Poch, T. et al. Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4+ T cells in primary sclerosing cholangitis. J. Hepatol. 75, 414–423 (2021).
Johansson, M. E. & Hansson, G. C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16, 639–649 (2016).
Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
Wang, L. et al. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 421, 44–53 (2015).
Camilleri, M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 68, 1516–1526 (2019).
Bjornsson, E. et al. Intestinal permeability and bacterial growth of the small bowel in patients with primary sclerosing cholangitis. Scand. J. Gastroenterol. 40, 1090–1094 (2005).
Dhillon, A. K. et al. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 39, 371–381 (2019).
Bellot, P., Frances, R. & Such, J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 33, 31–39 (2013).
Bossen, L. et al. Circulating macrophage activation markers predict transplant-free survival in patients with primary sclerosing cholangitis. Clin. Transl Gastroenterol. 12, e00315 (2021).
Krautkramer, K. A., Fan, J. & Backhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 19, 77–94 (2021).
Schirmer, M., Garner, A., Vlamakis, H. & Xavier, R. J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 17, 497–511 (2019).
Bell, L. N., Wulff, J., Comerford, M., Vuppalanchi, R. & Chalasani, N. Serum metabolic signatures of primary biliary cirrhosis and primary sclerosing cholangitis. Liver Int. 35, 263–274 (2015).
Tietz-Bogert, P. S. et al. Metabolomic profiling of portal blood and bile reveals metabolic signatures of primary sclerosing cholangitis. Int. J. Mol. Sci. 19, 3188 (2018).
Koh, A. et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 175, 947–961 (2018).
Hassan, M. et al. Paneth cells promote angiogenesis and regulate portal hypertension in response to microbial signals. J. Hepatol. 73, 628–639 (2020).
Reich, M. et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut 65, 487–501 (2016).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Reich, M. et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J. Hepatol. 75, 634–646 (2021).
Khurana, S., Raufman, J. P. & Pallone, T. L. Bile acids regulate cardiovascular function. Clin. Transl Sci. 4, 210–218 (2011).
Fuchs, C. D. & Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 19, 432–450 (2022).
Schaap, F. G., Trauner, M. & Jansen, P. L. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 11, 55–67 (2014).
Jia, W., Xie, G. & Jia, W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15, 111–128 (2018).
Banales, J. M. et al. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 16, 269–281 (2019).
Fischer, S., Beuers, U., Spengler, U., Zwiebel, F. M. & Koebe, H. G. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin. Chim. Acta 251, 173–186 (1996).
Trottier, J. et al. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: a pilot study. Dig. Liver Dis. 44, 303–310 (2012).
Midtvedt, T. Microbial bile acid transformation. Am. J. Clin. Nutr. 27, 1341–1347 (1974).
Heinken, A. et al. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 7, 75 (2019).
Torres, J. et al. The gut microbiota, bile acids and their correlation in primary sclerosing cholangitis associated with inflammatory bowel disease. United European Gastroenterol. J. 6, 112–122 (2018).
Vaughn, B. P. et al. A pilot study of fecal bile acid and microbiota profiles in inflammatory bowel disease and primary sclerosing cholangitis. Clin. Exp. Gastroenterol. 12, 9–19 (2019).
Duboc, H. et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 62, 531–539 (2013).
Franzosa, E. A. et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 4, 293–305 (2019).
Quraishi, M. N. et al. A pilot integrative analysis of colonic gene expression, gut microbiota and immune infiltration in primary sclerosing cholangitis-inflammatory bowel disease: association of disease with bile acid pathways. J. Crohns Colitis 30, 935–947 (2020).
Roediger, W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83, 424–429 (1982).
Marino, E. et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562 (2017).
Imhann, F. et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 67, 108–119 (2018).
Zhuang, X. et al. Systematic review and meta-analysis: short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 25, 1751–1763 (2019).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Dhillon, A. K. et al. Associations of neopterin and kynurenine-tryptophan ratio with survival in primary sclerosing cholangitis. Scand. J. Gastroenterol. 56, 443–452 (2021).
Nikolaus, S. et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 153, 1504–1516 e1502 (2017).
Gallagher, K., Catesson, A., Griffin, J. L., Holmes, E. & Williams, H. R. T. Metabolomic analysis in inflammatory bowel disease: a systematic review. J. Crohns Colitis 15, 813–826 (2020).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Natividad, J. M. et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 28, 737–749 (2018).
D’Onofrio, F. et al. Indole-3-carboxaldehyde restores gut mucosal integrity and protects from liver fibrosis in murine sclerosing cholangitis. Cells 10, 1622 (2021).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
ter Borg, P. C., Fekkes, D., Vrolijk, J. M. & van Buuren, H. R. The relation between plasma tyrosine concentration and fatigue in primary biliary cirrhosis and primary sclerosing cholangitis. BMC Gastroenterol. 5, 11 (2005).
Magnusdottir, S., Ravcheev, D., de Crecy-Lagard, V. & Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 6, 148 (2015).
Henderson, J. M., Codner, M. A., Hollins, B., Kutner, M. H. & Merrill, A. H. The fasting B6 vitamer profile and response to a pyridoxine load in normal and cirrhotic subjects. Hepatology 6, 464–471 (1986).
Selhub, J. et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10−/− model of inflammatory bowel disease. J. Nutr. Biochem. 24, 2138–2143 (2013).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Koeth, R. A. et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Kummen, M. et al. Elevated trimethylamine-N-oxide (TMAO) is associated with poor prognosis in primary sclerosing cholangitis patients with normal liver function. United European Gastroenterol. J. 5, 532–541 (2017).
Lin, J. K. & Ho, Y. S. Hepatotoxicity and hepatocarcinogenicity in rats fed squid with or without exogenous nitrite. Food Chem. Toxicol. 30, 695–702 (1992).
Sehgal, R. et al. Indole-3-propionic acid, a gut-derived tryptophan metabolite, associates with hepatic fibrosis. Nutrients 13, 3509 (2021).
Andrahennadi, S., Sami, A., Manna, M., Pauls, M. & Ahmed, S. Current landscape of targeted therapy in hormone receptor-positive and HER2-negative breast cancer. Curr. Oncol. 28, 1803–1822 (2021).
Wang, Z. et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163, 1585–1595 (2015).
Vesterhus, M. & Karlsen, T. H. Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities. J. Gastroenterol. 55, 588–614 (2020).
Ponsioen, C. Y., Lindor, K. D., Mehta, R. & Dimick-Santos, L. Design and endpoints for clinical trials in primary sclerosing cholangitis. Hepatology 68, 1174–1188 (2018).
Vleggaar, F. P., Monkelbaan, J. F. & van Erpecum, K. J. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur. J. Gastroenterol. Hepatol. 20, 688–692 (2008).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
Rastall, R. A. et al. Structure and function of non-digestible carbohydrates in the gut microbiome. Benef. Microbes 13, 95–168 (2022).
Trakman, G. L. et al. Diet and gut microbiome in gastrointestinal disease. J. Gastroenterol. Hepatol. 37, 237–245 (2021).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Andersen, I. M. et al. Effects of coffee consumption, smoking, and hormones on risk for primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 12, 1019–1028 (2014).
Friedrich, K. et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J. Gastroenterol. Hepatol. 31, 1470–1475 (2016).
Lammert, C. et al. Reduced coffee consumption among individuals with primary sclerosing cholangitis but not primary biliary cirrhosis. Clin. Gastroenterol. Hepatol. 12, 1562–1568 (2014).
Fitzpatrick, J. A., Melton, S. L., Yao, C. K., Gibson, P. R. & Halmos, E. P. Dietary management of adults with IBD — the emerging role of dietary therapy. Nat. Rev. Gastroenterol. Hepatol. 19, 652–669 (2022).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Mendes-Soares, H. et al. Model of personalized postprandial glycemic response to food developed for an Israeli cohort predicts responses in Midwestern American individuals. Am. J. Clin. Nutr. 110, 63–75 (2019).
Davies, Y. K. et al. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J. Pediatr. Gastroenterol. Nutr. 47, 61–67 (2008).
Ali, A. H. et al. Open-label prospective therapeutic clinical trials: oral vancomycin in children and adults with primary sclerosing cholangitis. Scand. J. Gastroenterol. 55, 941–950 (2020).
Deneau, M. R. et al. Oral vancomycin, ursodeoxycholic acid, or no therapy for pediatric primary sclerosing cholangitis: a matched analysis. Hepatology 73, 1061–1073 (2021).
Rahimpour, S. et al. A triple blinded, randomized, placebo-controlled clinical trial to evaluate the efficacy and safety of oral vancomycin in primary sclerosing cholangitis: a pilot study. J. Gastrointestin. Liver Dis. 25, 457–464 (2016).
Vrieze, A. et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 60, 824–831 (2014).
Isaac, S. et al. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J. Antimicrob. Chemother. 72, 128–136 (2017).
de Chambrun, G. P. et al. Oral vancomycin induces sustained deep remission in adult patients with ulcerative colitis and primary sclerosing cholangitis. Eur. J. Gastroenterol. Hepatol. 30, 1247–1252 (2018).
Tan, L. Z. et al. Oral vancomycin induces clinical and mucosal remission of colitis in children with primary sclerosing cholangitis-ulcerative colitis. Gut 68, 1533–1535 (2019).
Dao, A. et al. Oral vancomycin induces and maintains remission of ulcerative colitis in the subset of patients with associated primary sclerosing cholangitis. Inflamm. Bowel Dis. 25, e90–e91 (2019).
Abarbanel, D. N. et al. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J. Clin. Immunol. 33, 397–406 (2013).
Silveira, M. G. et al. Minocycline in the treatment of patients with primary sclerosing cholangitis: results of a pilot study. Am. J. Gastroenterol. 104, 83–88 (2009).
Tabibian, J. H. et al. Prospective clinical trial of rifaximin therapy for patients with primary sclerosing cholangitis. Am. J. Ther. 24, e56–e63 (2017).
Hsu, C., Duan, Y., Fouts, D. E. & Schnabl, B. Intestinal virome and therapeutic potential of bacteriophages in liver disease. J. Hepatol. 75, 1465–1475 (2021).
Principi, N., Silvestri, E. & Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 10, 513 (2019).
Kredo-Russo, S. et al. Use of a bacteriophage cocktail for eradication of Klebsiella pneumoniae in primary sclerosing cholangitis. Hepatology 70, 18A (2019).
Duan, Y. et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 575, 505–511 (2019).
Liu, X., Li, Y., Wu, K., Shi, Y. & Chen, M. Fecal microbiota transplantation as therapy for treatment of active ulcerative colitis: a systematic review and meta-analysis. Gastroenterol. Res. Pract. 2021, 6612970 (2021).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109 (2015).
Costello, S. P. et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 321, 156–164 (2019).
Paramsothy, S. et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228 (2017).
Allegretti, J. R. et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am. J. Gastroenterol. 114, 1071–1079 (2019). First clinical trial of FMT in PSC.
Ponsioen, C. Y. et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology 63, 1357–1367 (2016).
Kootte, R. S. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619 e616 (2017).
Fickert, P. et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J. Hepatol. 67, 549–558 (2017).
Konigshofer, P. et al. Nuclear receptors in liver fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166235 (2021).
Gadaleta, R. M. & Moschetta, A. Dark and bright side of targeting fibroblast growth factor receptor 4 in the liver. J. Hepatol. 75, 1440–1451 (2021).
Wahlstrom, A., Kovatcheva-Datchary, P., Stahlman, M., Backhed, F. & Marschall, H. U. Crosstalk between bile acids and gut microbiota and its impact on farnesoid X receptor signalling. Dig. Dis. 35, 246–250 (2017).
Kowdley, K. V. et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J. Hepatol. 73, 94–101 (2020).
Friedman, E. S. et al. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology 155, 1741–1752 (2018).
Pathak, P. et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588 (2018).
Hirschfield, G. M. et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J. Hepatol. 70, 483–493 (2019).
Al-Dury, S. et al. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci. Rep. 8, 6658 (2018).
Hegade, V. S. et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet 389, 1114–1123 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02061540 (2022).
Hruz, P. et al. Adaptive regulation of the ileal apical sodium dependent bile acid transporter (ASBT) in patients with obstructive cholestasis. Gut 55, 395–402 (2006).
Craddock, A. L. et al. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am. J. Physiol. 274, G157–G169 (1998).
Stokkeland, K., Hoijer, J., Bottai, M., Soderberg-Lofdal, K. & Bergquist, A. Statin use is associated with improved outcomes of patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 17, 1860–1866 (2018).
Xu, J. et al. 5-Aminosalicylic acid alters the gut bacterial microbiota in patients with ulcerative colitis. Front. Microbiol. 9, 1274 (2018).
Tada, S., Ebinuma, H., Saito, H. & Hibi, T. Therapeutic benefit of sulfasalazine for patients with primary sclerosing cholangitis. J. Gastroenterol. 41, 388–389 (2006).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03561584 (2022).
Hommes, D. W. et al. A double-blind, placebo-controlled, randomized study of infliximab in primary sclerosing cholangitis. J. Clin. Gastroenterol. 42, 522–526 (2008).
Epstein, M. P. & Kaplan, M. M. A pilot study of etanercept in the treatment of primary sclerosing cholangitis. Dig. Dis. Sci. 49, 1–4 (2004).
Tse, C. S., Loftus, E. V. Jr, Raffals, L. E., Gossard, A. A. & Lightner, A. L. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment. Pharmacol. Ther. 48, 190–195 (2018).
Caron, B. et al. Vedolizumab therapy is ineffective for primary sclerosing cholangitis in patients with inflammatory bowel disease: a GETAID multicentre cohort study. J. Crohns Colitis 13, 1239–1247 (2019).
Christensen, B. et al. Vedolizumab in patients with concurrent primary sclerosing cholangitis and inflammatory bowel disease does not improve liver biochemistry but is safe and effective for the bowel disease. Aliment. Pharmacol. Ther. 47, 753–762 (2018).
Lynch, K. D. et al. Effects of vedolizumab in patients with primary sclerosing cholangitis and inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 18, 179–187 (2020).
Laborda, T. J. et al. Vedolizumab therapy in children with primary sclerosing cholangitis: data from the pediatric primary sclerosing cholangitis consortium. J. Pediatr. Gastroenterol. Nutr. 71, 459–464 (2020).
Kolho, K. L. et al. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am. J. Gastroenterol. 110, 921–930 (2015).
Magnusson, M. K. et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J. Crohns Colitis 10, 943–952 (2016).
Ananthakrishnan, A. N. et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe 21, 603–610 (2017).
Hole, M. J. et al. A shared mucosal gut microbiota signature in primary sclerosing cholangitis before and after liver transplantation. Hepatology https://doi.org/10.1002/hep.32773 (2022).
Furukawa, M. et al. Gut dysbiosis associated with clinical prognosis of patients with primary biliary cholangitis. Hepatol. Res. 50, 840–852 (2020).
Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 60, 940–947 (2014).
Lou, J. et al. Fecal microbiomes distinguish patients with autoimmune hepatitis from healthy individuals. Front. Cell Infect. Microbiol. 10, 342 (2020).
Lv, L. X. et al. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ. Microbiol. 18, 2272–2286 (2016).
Bajaj, J. S. & Khoruts, A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J. Hepatol. 72, 1003–1027 (2020).
Li, B. et al. Alterations in microbiota and their metabolites are associated with beneficial effects of bile acid sequestrant on icteric primary biliary Cholangitis. Gut Microbes 13, 1946366 (2021).
Zhang, H. et al. Bifidobacterium animalis ssp. Lactis 420 mitigates autoimmune hepatitis through regulating intestinal barrier and liver immune cells. Front. Immunol. 11, 569104 (2020).
Chen, W. et al. Comprehensive analysis of serum and fecal bile acid profiles and interaction with gut microbiota in primary biliary cholangitis. Clin. Rev. Allergy Immunol. 58, 25–38 (2020).
Kakiyama, G. et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 58, 949–955 (2013).
Feld, J. J., Meddings, J. & Heathcote, E. J. Abnormal intestinal permeability in primary biliary cirrhosis. Dig. Dis. Sci. 51, 1607–1613 (2006).
Pascual, S. et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology 50, 1482–1486 (2003).
Cariello, R. et al. Intestinal permeability in patients with chronic liver diseases: its relationship with the aetiology and the entity of liver damage. Dig. Liver Dis. 42, 200–204 (2010).
Lin, R., Zhou, L., Zhang, J. & Wang, B. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int. J. Clin. Exp. Pathol. 8, 5153–5160 (2015).
Zhang, H. et al. Leaky gut driven by dysbiosis augments activation and accumulation of liver macrophages via RIP3 signaling pathway in autoimmune hepatitis. Front. Immunol. 12, 624360 (2021).
Zhao, J. et al. Altered biliary epithelial cell and monocyte responses to lipopolysaccharide as a TLR ligand in patients with primary biliary cirrhosis. Scand. J. Gastroenterol. 46, 485–494 (2011).
Umemura, T. et al. Association between serum soluble CD14 and IL-8 levels and clinical outcome in primary biliary cholangitis. Liver Int. 37, 897–905 (2017).
Fukui, H. Gut-liver axis in liver cirrhosis: how to manage leaky gut and endotoxemia. World J. Hepatol. 7, 425–442 (2015).
Raparelli, V. et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology 65, 571–581 (2017).
Jorgensen, K. K. et al. Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm. Bowel Dis. 18, 536–545 (2012).
Manzano, M., Abadia-Molina, A. C., Garcia-Olivares, E., Gil, A. & Rueda, R. Absolute counts and distribution of lymphocyte subsets in small intestine of BALB/c mice change during weaning. J. Nutr. 132, 2757–2762 (2002).
Middendorp, S. & Nieuwenhuis, E. E. NKT cells in mucosal immunity. Mucosal Immunol. 2, 393–402 (2009).
Schmitz, F. et al. The composition and differentiation potential of the duodenal intraepithelial innate lymphocyte compartment is altered in coeliac disease. Gut 65, 1269–1278 (2016).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Freitas-Lopes, M. A., Mafra, K., David, B. A., Carvalho-Gontijo, R. & Menezes, G. B. Differential location and distribution of hepatic immune cells. Cells 6, 48 (2017).
Rogler, G. Resolution of inflammation in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2, 521–530 (2017).
Simoni, Y. & Newell, E. W. Dissecting human ILC heterogeneity: more than just three subsets. Immunology 153, 297–303 (2018).
Agace, W. W. & McCoy, K. D. Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity 46, 532–548 (2017).
Geremia, A. & Arancibia-Carcamo, C. V. Innate lymphoid cells in intestinal inflammation. Front. Immunol. 8, 1296 (2017).
Faria, A. M. C., Reis, B. S. & Mucida, D. Tissue adaptation: implications for gut immunity and tolerance. J. Exp. Med. 214, 1211–1226 (2017).
Van Acker, A. et al. A murine intestinal intraepithelial NKp46-negative innate lymphoid cell population characterized by group 1 properties. Cell Rep. 19, 1431–1443 (2017).
Bain, C. C. & Schridde, A. Origin, differentiation, and function of intestinal macrophages. Front. Immunol. 9, 2733 (2018).
Mayassi, T. & Jabri, B. Human intraepithelial lymphocytes. Mucosal Immunol. 11, 1281–1289 (2018).
Qiu, Z. & Sheridan, B. S. Isolating lymphocytes from the mouse small intestinal immune system. J. Vis. Exp. 132, 57281 (2018).
Sagebiel, A. F. et al. Tissue-resident Eomes+ NK cells are the major innate lymphoid cell population in human infant intestine. Nat. Commun. 10, 975 (2019).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Dillon, S. M., Thompson, T. A., Christians, A. J., McCarter, M. D. & Wilson, C. C. Reduced immune-regulatory molecule expression on human colonic memory CD4 T cells in older adults. Immun. Ageing 18, 6 (2021).
Febbraio, M. A. & Karin, M. “Sweet death”: fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 33, 2316–2328 (2021).
Xue, R. et al. Bile acid receptors and the gut-liver axis in nonalcoholic fatty liver disease. Cells 10, 2806 (2021).
De Muynck, K., Vanderborght, B., Van Vlierberghe, H. & Devisscher, L. The gut-liver axis in chronic liver disease: a macrophage perspective. Cells 10, 2959 (2021).
Chen, Y. & Tian, Z. Innate lymphocytes: pathogenesis and therapeutic targets of liver diseases and cancer. Cell Mol. Immunol. 18, 57–72 (2021).
Bozward, A. G., Ronca, V., Osei-Bordom, D. & Oo, Y. H. Gut-liver immune traffic: deciphering immune-pathogenesis to underpin translational therapy. Front. Immunol. 12, 711217 (2021).
Kronsten, V. T., Tranah, T. H., Pariante, C. & Shawcross, D. L. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J. Hepatol. 76, 665–680 (2021).
Nel, I., Bertrand, L., Toubal, A. & Lehuen, A. MAIT cells, guardians of skin and mucosa? Mucosal Immunol. 14, 803–814 (2021).
Schreurs, R. et al. Intestinal CD8+ T cell responses are abundantly induced early in human development but show impaired cytotoxic effector capacities. Mucosal Immunol. 14, 605–614 (2021).
Brescia, P. & Rescigno, M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol. Med. 27, 844–855 (2021).
Rebelos, E., Iozzo, P., Guzzardi, M. A., Brunetto, M. R. & Bonino, F. Brain-gut-liver interactions across the spectrum of insulin resistance in metabolic fatty liver disease. World J. Gastroenterol. 27, 4999–5018 (2021).
Stamataki, Z. & Swadling, L. The liver as an immunological barrier redefined by single-cell analysis. Immunology 160, 157–170 (2020).
Takikawa, H. & Manabe, T. Primary sclerosing cholangitis in Japan — analysis of 192 cases. J. Gastroenterol. 32, 134–137 (1997).
Tanaka, A. et al. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J. Hepato-Biliary-Pancreat. Sci. 21, 43–50 (2014).
Ang, T. L. et al. Clinical profile of primary sclerosing cholangitis in Singapore. J. Gastroenterol. Hepatol. 17, 908–913 (2002).
Escorsell, A. et al. Epidemiology of primary sclerosing cholangitis in Spain. Spanish Association for the Study of the Liver. J. Hepatol. 21, 787–791 (1994).
Franceschet, I. et al. Primary sclerosing cholangitis associated with inflammatory bowel disease: an observational study in a Southern Europe population focusing on new therapeutic options. Eur. J. Gastroenterol. Hepatol. 28, 508–513 (2016).
Nardelli, M. J. et al. Clinical features and outcomes of primary sclerosing cholangitis in the highly admixed Brazilian population. Can. J. Gastroenterol. Hepatol. 2021, 7746401 (2021).
Wiesner, R. H. et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology 10, 430–436 (1989).
Gudnason, H. O. et al. Primary sclerosing cholangitis in Iceland 1992–2012 [Icelandic]. Laeknabladid 105, 371–376 (2019).
Bergquist, A. et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J. Hepatol. 36, 321–327 (2002).
Lindkvist, B., Benito de Valle, M., Gullberg, B. & Bjornsson, E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology 52, 571–577 (2010).
Culver, E. L. et al. Prevalence and long-term outcome of sub-clinical primary sclerosing cholangitis in patients with ulcerative colitis. Liver Int. 40, 2744–2757 (2020).
Fausa, O., Schrumpf, E. & Elgjo, K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin. Liver Dis. 11, 31–39 (1991).
Loftus, E. V. Jr. et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54, 91–96 (2005).
Krugliak Cleveland, N. et al. Patients with ulcerative colitis and primary sclerosing cholangitis frequently have subclinical inflammation in the proximal colon. Clin. Gastroenterol. Hepatol. 16, 68–74 (2018).
Sorensen, J. O. et al. Inflammatory bowel disease with primary sclerosing cholangitis: a Danish population-based cohort study 1977–2011. Liver Int. 38, 532–541 (2018).
Trivedi, P. J. et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology 159, 915–928 (2020).
Altwegg, R. et al. Effectiveness and safety of anti-TNF therapy for inflammatory bowel disease in liver transplant recipients for primary sclerosing cholangitis: a nationwide case series. Dig. Liver Dis. 50, 668–674 (2018).
Hedin, C. R. H. et al. Effects of tumor necrosis factor antagonists in patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 18, 2295–2304 (2020).
Wang, M. H. et al. Unique phenotypic characteristics and clinical course in patients with ulcerative colitis and primary sclerosing cholangitis: a multicenter US experience. Inflamm. Bowel Dis. 26, 774–779 (2019).
Soetikno, R. M., Lin, O. S., Heidenreich, P. A., Young, H. S. & Blackstone, M. O. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest. Endosc. 56, 48–54 (2002).
Shah, S. C. et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 16, 1106–1113 (2018).
Karlsen, T. H. et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 138, 1102–1111 (2010).
Hov, J. R., Boberg, K. M. & Karlsen, T. H. Autoantibodies in primary sclerosing cholangitis. World J. Gastroenterol. 14, 3781–3791 (2008).
Grant, A. J., Lalor, P. F., Salmi, M., Jalkanen, S. & Adams, D. H. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 359, 150–157 (2002).
Graham, J. J. et al. Aberrant hepatic trafficking of gut-derived T cells is not specific to primary sclerosing cholangitis. Hepatology 75, 518–530 (2022).
Trivedi, P. J. et al. Intestinal CCL25 expression is increased in colitis and correlates with inflammatory activity. J. Autoimmun. 68, 98–104 (2016).
Trivedi, P. J. et al. Vascular adhesion protein-1 is elevated in primary sclerosing cholangitis, is predictive of clinical outcome and facilitates recruitment of gut-tropic lymphocytes to liver in a substrate-dependent manner. Gut 67, 1135–1145 (2017).
Henriksen, E. K. et al. Gut and liver T-cells of common clonal origin in primary sclerosing cholangitis-inflammatory bowel disease. J. Hepatol. 66, 116–122 (2017).
Chung, B. K. et al. Gut and liver B cells of common clonal origin in primary sclerosing cholangitis-inflammatory bowel disease. Hepatol. Commun. 2, 956–967 (2018).
Puig, M. et al. Alterations in the HLA-B*57:01 immunopeptidome by flucloxacillin and immunogenicity of drug-haptenated peptides. Front. Immunol. 11, 629399 (2020).
Molberg, O. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 (1998).
Jahnsen, F. L., Baekkevold, E. S., Hov, J. R. & Landsverk, O. J. Do long-lived plasma cells maintain a healthy microbiota in the gut? Trends Immunol. 39, 196–208 (2018).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Terjung, B. et al. p-ANCA in autoimmune liver disorders recognize human beta-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut 59, 808–816 (2010).
Op De Beeck, K. et al. Immune reactivity to beta-tubulin isotype 5 and vesicular integral-membrane protein 36 in patients with autoimmune gastrointestinal disorders. Gut 60, 1601–1602 (2011).
Hov, J. R. et al. Antineutrophil antibodies define clinical and genetic subgroups in primary sclerosing cholangitis. Liver Int. 37, 458–465 (2017).
Sowa, M. et al. Mucosal autoimmunity to cell-bound GP2 isoforms is a sensitive marker in PSC and associated with the clinical phenotype. Front. Immunol. 9, 1959 (2018).
Jendrek, S. T. et al. Anti-GP2 IgA autoantibodies are associated with poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Gut 66, 137–144 (2017).
Tornai, T. et al. Loss of tolerance to gut immunity protein, glycoprotein 2 (GP2) is associated with progressive disease course in primary sclerosing cholangitis. Sci. Rep. 8, 399 (2018).
Wunsch, E. et al. Anti-glycoprotein 2 (anti-GP2) IgA and anti-neutrophil cytoplasmic antibodies to serine proteinase 3 (PR3-ANCA): antibodies to predict severe disease, poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 53, 302–313 (2021).
Hase, K. et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature 462, 226–230 (2009).
von Seth, E. et al. Primary sclerosing cholangitis leads to dysfunction and loss of MAIT cells. Eur. J. Immunol. 48, 1997–2004 (2018).
Olszak, T. et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 509, 497–502 (2014).
Berntsen, N. L. et al. Natural killer T cells mediate inflammation in the bile ducts. Mucosal Immunol. 11, 1582–1590 (2018).
Schrumpf, E. et al. The biliary epithelium presents antigens to and activates natural killer T cells. Hepatology 62, 1249–1259 (2015).
Mehta, H., Lett, M. J., Klenerman, P. & Filipowicz Sinnreich, M. MAIT cells in liver inflammation and fibrosis. Semin. Immunopathol. 44, 429–444 (2022).
Acknowledgements
The authors thank K. Toverud (http://www.karitoverud.com/), P. Braadland (Institute of Clinical Medicine, University of Oslo) and X. Jiang (Norwegian PSC Research Center, Oslo University Hospital) for help in developing the original drafts of Fig. 1, Fig. 4 and Fig. 5, respectively.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.R.H. reports speaker fees from Roche, Novartis and Amgen, consultancy fees from Novartis and Orkla Health, and research funding from Biogen, all unrelated to the present work. T.H.K. reports speaker fees from AlfaSigma, and consultancy fees from Intercept, Gilead and Albireo, all unrelated to the present work.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Pietro Invernizzi, Sara Lemoinne and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hov, J.R., Karlsen, T.H. The microbiota and the gut–liver axis in primary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol 20, 135–154 (2023). https://doi.org/10.1038/s41575-022-00690-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00690-y
This article is cited by
-
Primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD): a condition exemplifying the crosstalk of the gut–liver axis
Experimental & Molecular Medicine (2023)