Abstract
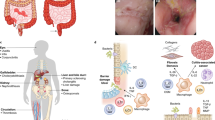
Immune cell trafficking is a complex and tightly regulated process that is indispensable for the body’s fight against pathogens. However, it is also increasingly acknowledged that dysregulation of cell trafficking contributes to the pathogenesis of immune-mediated inflammatory diseases (IMIDs) in gastroenterology and hepatology, such as inflammatory bowel disease and primary sclerosing cholangitis. Moreover, altered cell trafficking has also been implicated as a crucial step in the immunopathogenesis of other IMIDs, such as rheumatoid arthritis and multiple sclerosis. Over the past few years, a central role of the gut in mediating these disorders has progressively emerged, and the partly microbiota-driven imprinting of particular cell trafficking phenotypes in the intestine seems to be crucially involved. Therefore, this Review highlights achievements in understanding immune cell trafficking to, within and from the intestine and delineates its consequences for immune-mediated pathology along the gut–liver, gut–joint and gut–brain axes. We also discuss implications for current and future therapeutic approaches that specifically interfere with homing, retention, egress and recirculation of immune cells.
Key points
-
Immune-mediated inflammatory diseases (IMIDs) are a group of diseases with an important pathogenetic contribution of immune cell infiltration that depends on cell trafficking.
-
Sophisticated organ-specific mechanisms that regulate immune cell trafficking exist in the gut; however, re-routing cannot be precluded owing to pleiotropy and redundancy of the cell trafficking pathways.
-
Clinical and experimental observations established the gut as a central organ for several IMIDs of extra-intestinal organs and demonstrated disease-relevant communication via the gut–liver, gut–joint and gut–brain axes.
-
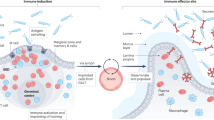
Cell trafficking mechanisms along these axes include the induction of pathogenic immune cells at the interface with the intestinal microbiome and their homing to extra-intestinal sites.
-
Anti-trafficking agents have been established for the treatment of inflammatory bowel disease and multiple sclerosis. The field is expected to grow further.
-
Gut-derived cell trafficking pathways in IMIDs represent an important yet insufficiently explored field for potential future therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Girard, J.-P., Moussion, C. & Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012).
von Andrian, U. H. & Mackay, C. R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Habtezion, A., Nguyen, L. P., Hadeiba, H. & Butcher, E. C. Leukocyte trafficking to the small intestine and colon. Gastroenterology 150, 340–354 (2016).
Schett, G., McInnes, I. B. & Neurath, M. F. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 385, 628–639 (2021).
Ruff, W. E., Greiling, T. M. & Kriegel, M. A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 18, 521–538 (2020).
Tripathi, A. et al. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 15, 397–411 (2018).
Zaiss, M. M., Joyce, Wu,H.-J., Mauro, D., Schett, G. & Ciccia, F. The gut–joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 17, 224–237 (2021).
Cryan, J. F. et al. The microbiota–gut–brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Rantapää-Dahlqvist, S. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48, 2741–2749 (2003).
Agirman, G., Yu, K. B. & Hsiao, E. Y. Signaling inflammation across the gut–brain axis. Science 374, 1087–1092 (2021).
Henriksen, E. K. K. et al. Gut and liver T-cells of common clonal origin in primary sclerosing cholangitis-inflammatory bowel disease. J. Hepatol. 66, 116–122 (2017).
Tajik, N. et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 11, 1995 (2020).
Duc, D. et al. Disrupting myelin-specific Th17 cell gut homing confers protection in an adoptive transfer experimental autoimmune encephalomyelitis. Cell Rep. 29, 378–390.e4 (2019).
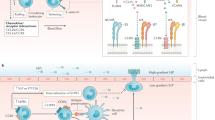
Zundler, S., Becker, E., Schulze, L. L. & Neurath, M. F. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut 68, 1688–1700 (2019).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
McEver, R. P. & Zhu, C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 26, 363–396 (2010).
Briskin, M. et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 151, 97–110 (1997).
Sun, H. et al. Distinct chemokine signaling regulates integrin ligand specificity to dictate tissue-specific lymphocyte homing. Dev. Cell 30, 61–70 (2014).
Sriramarao, P. et al. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of shear flow. Blood 95, 592–601 (2000).
Sun, H. et al. Distinct integrin activation pathways for effector and regulatory T cell trafficking and function. J. Exp. Med. 218, e20201524 (2021).
Yuan, M. et al. Mucin-like domain of mucosal addressin cell adhesion molecule-1 facilitates integrin α4β7-mediated cell adhesion through electrostatic repulsion. Front. Cell Dev. Biol. 8, 603148 (2020).
Dufour, E. M., Deroche, A., Bae, Y. & Muller, W. A. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun. Adhes. 15, 351–363 (2008).
Park, C. O. & Kupper, T. S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697 (2015).
Rosen, H., Stevens, R. C., Hanson, M., Roberts, E. & Oldstone, M. B. A. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu. Rev. Biochem. 82, 637–662 (2013).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
McNamee, E. N. et al. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn’s-like murine ileitis. J. Leukoc. Biol. 97, 1011–1022 (2015).
Yamada, K. M. & Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752 (2019).
Molenaar, R. et al. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J. Immunol. 186, 1934–1942 (2011).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Belarif, L. et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J. Clin. Invest. 129, 1910–1925 (2019).
Ballet, R. et al. A CD22–Shp1 phosphatase axis controls integrin β7 display and B cell function in mucosal immunity. Nat. Immunol. 22, 381–390 (2021).
Osorio-Barrios, F. et al. The heteromeric complex formed by dopamine receptor D5 and CCR9 leads the gut homing of CD4+ T cells upon inflammation. Cell Mol. Gastroenterol. Hepatol. 12, 489–506 (2021).
Kim, M. H., Taparowsky, E. J. & Kim, C. H. Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity 43, 107–119 (2015).
Schleier, L. et al. Non-classical monocyte homing to the gut via α4β7 integrin mediates macrophage-dependent intestinal wound healing. Gut 69, 252–263 (2020).
Kim, S. V. et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459 (2013).
Suply, T. et al. A natural ligand for the orphan receptor GPR15 modulates lymphocyte recruitment to epithelia. Sci. Signal. 10, eaal0180 (2017).
Xiong, L. et al. Ahr-Foxp3-RORγt axis controls gut homing of CD4+ T cells by regulating GPR15. Sci. Immunol. 5, eaaz7277 (2020).
Sheldon, P. Rheumatoid arthritis and gut related lymphocytes: the iteropathy concept. Ann. Rheum. Dis. 47, 697–700 (1988).
Cattin, A. et al. RALDH activity induced by bacterial/fungal pathogens in CD16+ monocyte-derived dendritic cells boosts HIV infection and outgrowth in CD4+ T cells. J. Immunol. 206, 2638–2651 (2021).
Bhattacharya, N. et al. Normalizing microbiota-induced retinoic acid deficiency stimulates protective CD8+ T cell-mediated immunity in colorectal cancer. Immunity 45, 641–655 (2016).
Grizotte-Lake, M. et al. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity 49, 1103–1115.e6 (2018).
Woo, V. et al. Commensal segmented filamentous bacteria-derived retinoic acid primes host defense to intestinal infection. Cell Host Microbe 29, 1744–1756.e5 (2021).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Powrie, F., Leach, M. W., Mauze, S., Caddle, L. B. & Coffman, R. L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 SCID mice. Int. Immunol. 5, 1461–1471 (1993).
Zhang, H. et al. A mutation that blocks integrin α4β7 activation prevents adaptive immune-mediated colitis without increasing susceptibility to innate colitis. BMC Biol. 18, 64 (2020).
Picarella, D. et al. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J. Immunol. 158, 2099–2106 (1997).
Sydora, B. C. et al. β7 Integrin expression is not required for the localization of T cells to the intestine and colitis pathogenesis. Clin. Exp. Immunol. 129, 35–42 (2002).
Mottet, C., Uhlig, H. H. & Powrie, F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170, 3939–3943 (2003).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Chang, J. T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664 (2020).
Watanabe, C. et al. Spatial heterogeneity of TNF-alpha-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G1379–G1387 (2002).
Hegazy, A. N. et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 153, 1320–1337.e16 (2017).
Zundler, S. et al. Hobit- and Blimp-1-driven CD4+tissue-resident memory T cells control chronic intestinal inflammation. Nat. Immunol. 20, 288–300 (2019).
Wiendl, M. et al. Targeting immune cell trafficking–insights from research models and implications for future IBD therapy. Front. Immunol. 12, 656452 (2021).
Sandborn, W. J. et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 353, 1912–1925 (2005).
Sandborn, W. J. et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 369, 711–721 (2013).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369, 699–710 (2013).
Sandborn, W. J. et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 385, 1280–1291 (2021).
Na, Y. R., Stakenborg, M., Seok, S. H. & Matteoli, G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16, 531–543 (2019).
Ott, C. & Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 10, 585–595 (2013).
Adams, D. H. & Eksteen, B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat. Rev. Immunol. 6, 244–251 (2006).
Hedin, C. R. H. et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J. Crohn’s Colitis 13, 541–554 (2019).
Albillos, A., de Gottardi, A. & Rescigno, M. The gut–liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72, 558–577 (2020).
Gao, W. et al. Impaired CCR9/CCL25 signalling induced by inefficient dendritic cells contributes to intestinal immune imbalance in nonalcoholic steatohepatitis. Biochem. Biophys. Res. Commun. 534, 34–40 (2021).
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479 (2020).
Barberio, B. et al. Prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease: a systematic review and meta-analysis. Gastroenterology 161, 1865–1877 (2021).
Di Ciaula, A. et al. Liver steatosis, gut–liver axis, microbiome and environmental factors. a never-ending bidirectional cross-talk. J. Clin. Med. 9, E2648 (2020).
Lunder, A. K. et al. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology 151, 660–669.e4 (2016).
Ong, J., Bath, M. F., Swift, C. & Al-Naeeb, Y. Does colectomy affect the progression of primary sclerosing cholangitis? A systematic review and meta-analysis. Gastroenterol. Hepatol. Bed Bench 11, 277–283 (2018).
Steenstraten, I. C. et al. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment. Pharmacol. Ther. 49, 636–643 (2019).
Ji, S.-G. et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 49, 269–273 (2017).
Zimmer, C. L. et al. A biliary immune landscape map of primary sclerosing cholangitis reveals a dominant network of neutrophils and tissue-resident T cells. Sci. Transl. Med. 13, eabb3107 (2021).
de Krijger, M. et al. Characterization of gut-homing molecules in non-endstage livers of patients with primary sclerosing cholangitis and inflammatory bowel disease. J. Transl. Autoimmun. 3, 100054 (2020).
Schippers, A. et al. MAdCAM-1/α4β7 integrin-mediated lymphocyte/endothelium interactions exacerbate acute immune-mediated hepatitis in mice. Cell Mol. Gastroenterol. Hepatol. 11, 1227–1250.e1 (2021).
Grant, A. J., Lalor, P. F., Hübscher, S. G., Briskin, M. & Adams, D. H. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology 33, 1065–1072 (2001).
Eksteen, B. et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology 137, 320–329 (2009).
Wiggins, B. G. et al. The human liver microenvironment shapes the homing and function of CD4+ T-cell populations. Gut 71, 1399–1411 (2022).
Graham, J. J. et al. Aberrant hepatic trafficking of gut-derived T cells is not specific to primary sclerosing cholangitis. Hepatology 75, 518–530 (2022).
Seidel, D. et al. CD8 T cells primed in the gut-associated lymphoid tissue induce immune-mediated cholangitis in mice. Hepatology 59, 601–611 (2014).
Rai, R. P. et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J. Hepatol. 73, 1013–1022 (2020).
Neumann, K. et al. Connecting liver and gut: murine liver sinusoidal endothelium induces gut tropism of CD4+ T cells via retinoic acid. Hepatology 55, 1976–1984 (2012).
Harms, M. H. et al. Number needed to treat with ursodeoxycholic acid therapy to prevent liver transplantation or death in primary biliary cholangitis. Gut 69, 1502–1509 (2020).
Abbas, N., Quraishi, M. N. & Trivedi, P. Emerging drugs for the treatment of primary sclerosing cholangitis. Curr. Opin. Pharmacol. 62, 23–35 (2021).
Deneau, M. R. et al. Oral vancomycin, ursodeoxycholic acid, or no therapy for pediatric primary sclerosing cholangitis: a matched analysis. Hepatology 73, 1061–1073 (2021).
Christensen, B. et al. Vedolizumab in patients with concurrent primary sclerosing cholangitis and inflammatory bowel disease does not improve liver biochemistry but is safe and effective for the bowel disease. Aliment. Pharmacol. Ther. 47, 753–762 (2018).
Lynch, K. D. et al. Effects of vedolizumab in patients with primary sclerosing cholangitis and inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 18, 179–187.e6 (2020).
Tse, C. S., Loftus, E. V., Raffals, L. E., Gossard, A. A. & Lightner, A. L. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment. Pharmacol. Ther. 48, 190–195 (2018).
Laborda, T. J. et al. Vedolizumab therapy in children with primary sclerosing cholangitis: data from the pediatric primary sclerosing cholangitis consortium. J. Pediatr. Gastroenterol. Nutr. 71, 459–464 (2020).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Baeten, D. et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann. Rheum. Dis. 59, 945–953 (2000).
McInnes, I. B. & Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389, 2328–2337 (2017).
Abbot, S. E., Whish, W. J., Jennison, C., Blake, D. R. & Stevens, C. R. Tumour necrosis factor alpha stimulated rheumatoid synovial microvascular endothelial cells exhibit increased shear rate dependent leucocyte adhesion in vitro. Ann. Rheum. Dis. 58, 573–581 (1999).
Salmi, M., Rajala, P. & Jalkanen, S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J. Clin. Invest. 99, 2165–2172 (1997).
Lally, F. et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 52, 3460–3469 (2005).
McGettrick, H. M. et al. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur. J. Immunol. 39, 113–125 (2009).
Söderström, K. et al. High expression of V gamma 8 is a shared feature of human gamma delta T cells in the epithelium of the gut and in the inflamed synovial tissue. J. Immunol. 152, 6017–6027 (1994).
Trollmo, C., Nilsson, I. M., Sollerman, C. & Tarkowski, A. Expression of the mucosal lymphocyte integrin alpha E beta 7 and its ligand E-cadherin in the synovium of patients with rheumatoid arthritis. Scand. J. Immunol. 44, 293–298 (1996).
Salmi, M., Andrew, D. P., Butcher, E. C. & Jalkanen, S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J. Exp. Med. 181, 137–149 (1995).
May, E. et al. Identical T-cell expansions in the colon mucosa and the synovium of a patient with enterogenic spondyloarthropathy. Gastroenterology 119, 1745–1755 (2000).
Cho, Y.-N. et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J. Immunol. 193, 3891–3901 (2014).
Kim, M. et al. TNFα and IL-1β in the synovial fluid facilitate mucosal-associated invariant T (MAIT) cell migration. Cytokine 99, 91–98 (2017).
Kurioka, A., Walker, L. J., Klenerman, P. & Willberg, C. B. MAIT cells: new guardians of the liver. Clin. Transl. Immunol. 5, e98 (2016).
Teng, F. et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity 44, 875–888 (2016).
Tomura, M. et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl Acad. Sci. USA 105, 10871–10876 (2008).
Flak, M. B. et al. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight 4, e125191 (2019).
Jubair, W. K. et al. Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheumatol. 70, 1220–1233 (2018).
Matei, D. E. et al. Intestinal barrier dysfunction plays an integral role in arthritis pathology and can be targeted to ameliorate disease. Med 2, 864–883.e9 (2021).
Takaki-Kuwahara, A. et al. CCR6+ group 3 innate lymphoid cells accumulate in inflamed joints in rheumatoid arthritis and produce Th17 cytokines. Arthritis Res. Ther. 21, 198 (2019).
Ren, J., Feng, Z., Lv, Z., Chen, X. & Li, J. Natural Killer-22 cells in the synovial fluid of patients with rheumatoid arthritis are an innate source of interleukin 22 and tumor necrosis factor-α. J. Rheumatol. 38, 2112–2118 (2011).
Venken, K. et al. RORγt inhibition selectively targets IL-17 producing iNKT and γδ-T cells enriched in spondyloarthritis patients. Nat. Commun. 10, 9 (2019).
Gracey, E. et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann. Rheum. Dis. 75, 2124–2132 (2016).
Shen, H., Goodall, J. C. & Hill Gaston, J. S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 (2009).
Kenna, T. J. et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64, 1420–1429 (2012).
Ciccia, F. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 74, 1739–1747 (2015).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Wekerle, H. Experimental autoimmune encephalomyelitis as a model of immune-mediated CNS disease. Curr. Opin. Neurobiol. 3, 779–784 (1993).
Hemmer, B., Kerschensteiner, M. & Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 14, 406–419 (2015).
Miller, I. The gut–brain axis: historical reflections. Microb. Ecol. Health Dis. 29, 1542921 (2018).
Parodi, B. & Kerlero de Rosbo, N. The gut–brain axis in multiple sclerosis. is its dysfunction a pathological trigger or a consequence of the disease? Front. Immunol. 12, 3911 (2021).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Ochoa-Repáraz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108, 4615–4622 (2011).
Lee, Y. K. & Mazmanian, S. K. Microbial learning lessons: SFB educate the immune system. Immunity 40, 457–459 (2014).
Kent, S. J. et al. A monoclonal antibody to α4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J. Neuroimmunol. 58, 1–10 (1995).
Kuhbandner, K. et al. MAdCAM-1-mediated intestinal lymphocyte homing is critical for the development of active experimental autoimmune encephalomyelitis. Front. Immunol. 10, 903 (2019).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Mindur, J. E. et al. Surface layer protein A expressed in Clostridioides difficile DJNS06-36 possesses an encephalitogenic mimotope of myelin basic protein. Microorganisms 9, 34 (2021).
Erturk-Hasdemir, D., Ochoa-Repáraz, J., Kasper, D. L. & Kasper, L. H. Exploring the gut–brain axis for the control of CNS inflammatory demyelination: immunomodulation by Bacteroides fragilis’ polysaccharide A. Front. Immunol. 12, 1561 (2021).
Ochoa-Repáraz, J. et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3, 487–495 (2010).
Wang, Y. et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun. 5, 4432 (2014).
Haase, S. et al. The role of the gut microbiota and microbial metabolites in neuroinflammation. Eur. J. Immunol. 50, 1863–1870 (2020).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Kadowaki, A., Saga, R., Lin, Y., Sato, W. & Yamamura, T. Gut microbiota-dependent CCR9+CD4+ T cells are altered in secondary progressive multiple sclerosis. Brain 142, 916–931 (2019).
Zhang, Y. et al. Toll-like receptor 4 promotes Th17 lymphocyte infiltration via CCL25/CCR9 in pathogenesis of experimental autoimmune encephalomyelitis. J. Neuroimmune Pharmacol. 14, 493–502 (2019).
Kadowaki, A. et al. Gut environment-induced intraepithelial autoreactive CD4+ T cells suppress central nervous system autoimmunity via LAG-3. Nat. Commun. 7, 11639 (2016).
Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016).
Miyake, S. et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 10, e0137429 (2015).
Choileáin, S. N. et al. CXCR3+ T cells in multiple sclerosis correlate with reduced diversity of the gut microbiome. J. Transl. Autoimmun. 3, 100032 (2020).
Gordon, K. B. et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 290, 3073–3080 (2003).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Berger, J. R. Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf. 33, 969–983 (2010).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
McGinley, M. P. & Cohen, J. A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 398, 1184–1194 (2021).
Zundler, S. et al. Three-dimensional cross-sectional light-sheet microscopy imaging of the inflamed mouse gut. Gastroenterology 153, 898–900 (2017).
Zeissig, S. et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 68, 25–39 (2019).
Uzzan, M. et al. Anti-α4β7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1–infected individuals. Sci. Transl. Med. 10, eaau4711 (2018).
Becker, E. et al. Residual homing of α4β7-expressing β1+PI16+ regulatory T cells with potent suppressive activity correlates with exposure-efficacy of vedolizumab. Gut 71, 1551–1566 (2022).
Vitali, F., Simon, D., Neurath, M. F., Schett, G. & Zundler, S. Vedolizumab-associated enthesitis: correlation or causality? Rheumatology 60, 5491–5492 (2021).
Zundler, S. et al. Blockade of αEβ7 integrin suppresses accumulation of CD8+and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut 66, 1936–1948 (2017).
Dai, B. et al. Dual targeting of lymphocyte homing and retention through α4β7 and αEβ7 inhibition in inflammatory bowel disease. Cell Rep. Med. 2, 100381 (2021).
Vermeire, S. et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 384, 309–318 (2014).
Agrawal, M. & Verstockt, B. Etrolizumab for ulcerative colitis: beyond what meets the eye. Lancet Gastroenterol. Hepatol. 7, 2–4 (2022).
Rubin, D. T. et al. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): two phase 3 randomised, controlled trials. Lancet Gastroenterol. Hepatol. 7, 17–27 (2022).
Peyrin-Biroulet, L. et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): a phase 3, randomised, controlled trial. Lancet Gastroenterol. Hepatol. 7, 128–140 (2022).
Vermeire, S. et al. Etrolizumab for maintenance therapy in patients with moderately to severely active ulcerative colitis (LAUREL): a randomised, placebo-controlled, double-blind, phase 3 study. Lancet Gastroenterol. Hepatol. 7, 28–37 (2022).
Danese, S. et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): a randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol. Hepatol. 7, 118–127 (2022).
Binder, M.-T. et al. Similar inhibition of dynamic adhesion of lymphocytes from IBD patients to MAdCAM-1 by vedolizumab and etrolizumab-s. Inflamm. Bowel Dis. 24, 1237–1250 (2018).
Smids, C., Horjus Talabur Horje, C. S., van Wijk, F. & van Lochem, E. G. The complexity of alpha E beta 7 blockade in inflammatory bowel diseases. J. Crohns Colitis 11, 500–508 (2017).
Lamb, C. A. et al. αEβ7 integrin identifies subsets of pro-inflammatory colonic CD4+ T lymphocytes in ulcerative colitis. J. Crohns Colitis 11, 610–620 (2017).
Tew, G. W. et al. Association between response to etrolizumab and expression of integrin αE and granzyme A in colon biopsies of patients with ulcerative colitis. Gastroenterology 150, 477–487.e9 (2016).
Sandborn, W. J. et al. Efficacy and safety of abrilumab in a randomized, placebo-controlled trial for moderate-to-severe ulcerative colitis. Gastroenterology 156, 946–957.e18 (2019).
Vermeire, S. et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 390, 135–144 (2017).
Sandborn, W. J. et al. Eldelumab [anti-interferon-γ-inducible protein-10 antibody] induction therapy for active Crohn’s disease: a randomised, double-blind, placebo-controlled phase IIa study. J. Crohns Colitis 11, 811–819 (2017).
Keshav, S. et al. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS ONE 8, e60094 (2013).
Feagan, B. G. et al. Randomised clinical trial: vercirnon, an oral CCR9 antagonist, vs. placebo as induction therapy in active Crohn’s disease. Aliment. Pharmacol. Ther. 42, 1170–1181 (2015).
Matsuoka, K. et al. AJM300 (carotegrast methyl), an oral antagonist of α4-integrin, as induction therapy for patients with moderately active ulcerative colitis: a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol. Hepatol. 7, 648–657 (2022).
Cohen, J. A. et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 18, 1021–1033 (2019).
Sandborn, W. J. et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with Ulcerative colitis. Gastroenterology 158, 550–561 (2020).
Silverberg, J. I. et al. Results from ADVISE: a randomized, double-blind, placebo-controlled phase 2 study of etrasimod, an oral, selective, sphingosine 1-phosphate receptor modulator, in adults with moderate-to-severe atopic dermatitis. In Revolutionizing Atopic Dermatitis Conference. Abstr. #390 (2020).
D’Haens, G., Danese, S., Davies, M., Watanabe, M. & Hibi, T. A phase II, multicentre, randomised, double-blind, placebo-controlled study to evaluate safety, tolerability and efficacy of amiselimod in patients with moderate to severe active Crohn’s disease. J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjab201 (2021).
Yacyshyn, B. R. et al. Double blind, placebo controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent Crohn’s disease. Gut 51, 30–36 (2002).
Atlantic Healthcare. Atlantic healthcare announces results from phase 3 trial of alicaforsen enema for orphan-designated pouchitis. https://www.atlantichc.com/atlantic-healthcare-announces-results-from-phase-3-trial-of-alicaforsen-enema-for-orphan-designated-pouchitis/ (2019).
Ghosh, S. et al. Natalizumab for active Crohn’s disease. N. Engl. J. Med. 348, 24–32 (2003).
James, D. G., Seo, D. H., Chen, J., Vemulapalli, C. & Stone, C. D. Efalizumab, a human monoclonal anti-CD11a antibody, in the treatment of moderate to severe Crohn’s disease: an open-label pilot study. Dig. Dis. Sci. 56, 1806–1810 (2011).
Sandborn, W. J. et al. OP035 Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. J. Crohns Colitis 11, S22–S23 (2017).
Sandborn, W. J. et al. Eldelumab [anti-IP-10] induction therapy for ulcerative colitis: a randomised, placebo-controlled, phase 2b study. J. Crohns Colitis 10, 418–428 (2016).
Sandborn, W. J. et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut 67, 1824–1835 (2018).
Acknowledgements
The authors thank the German Research Foundation for their funding (DFG, ZU 377/4-1, TRR241 A02, B08 and C04).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.F.N. has served as an adviser for Pentax, Giuliani, MSD, AbbVie, Janssen, Takeda and Boehringer. S.Z. received speaker’s fees from Takeda, Roche, Galapagos, Ferring, Lilly and Janssen. M.F.N. and S.Z. received research support from Takeda, Shire (a part of Takeda) and Roche. C.G., M.M.Z, V.R. and A.E.K. declare no conflicts of interest.
Peer review
Peer review information
Nature Reviews Reviews Gastroenterology & Hepatology thanks Giovanni Monteleone and Angela Schippers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NCT02394028: https://clinicaltrials.gov/ct2/show/NCT02394028
NCT03996369: https://clinicaltrials.gov/ct2/show/NCT03996369
NCT04857112: https://clinicaltrials.gov/ct2/show/NCT04857112
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zundler, S., Günther, C., Kremer, A.E. et al. Gut immune cell trafficking: inter-organ communication and immune-mediated inflammation. Nat Rev Gastroenterol Hepatol 20, 50–64 (2023). https://doi.org/10.1038/s41575-022-00663-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00663-1
This article is cited by
-
Impaired immune tolerance mediated by reduced Tfr cells in rheumatoid arthritis linked to gut microbiota dysbiosis and altered metabolites
Arthritis Research & Therapy (2024)
-
Parkinson’s disease and gut microbiota: from clinical to mechanistic and therapeutic studies
Translational Neurodegeneration (2023)
-
Gut–liver axis: barriers and functional circuits
Nature Reviews Gastroenterology & Hepatology (2023)
-
New insights into muscularis macrophages in the gut: from their origin to therapeutic targeting
Immunologic Research (2023)