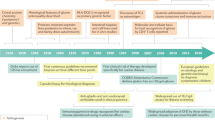
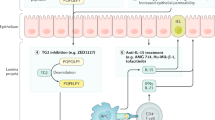
Abstract
Coeliac disease is a systemic disorder characterized by immune-mediated enteropathy, which is caused by gluten ingestion in genetically susceptible individuals. The clinical presentation of coeliac disease is highly variable and ranges from malabsorption through solely extra-intestinal manifestations to asymptomatic. As a result, the majority of patients with coeliac disease remain undiagnosed, misdiagnosed or experience a substantial delay in diagnosis. Coeliac disease is diagnosed by a combination of serological findings of disease-related antibodies and histological evidence of villous abnormalities in duodenal biopsy samples. However, variability in histological grading and in the diagnostic performance of some commercially available serological tests remains unacceptably high and confirmatory assays are not readily available in many parts of the world. Currently, the only effective treatment for coeliac disease is a lifelong, strict, gluten-free diet. However, many barriers impede patients’ adherence to this diet, including lack of widespread availability, high cost, cross-contamination and its overall restrictive nature. Routine follow-up is necessary to ensure adherence to a gluten-free diet but considerable variation is evident in follow-up protocols and the optimal disease management strategy is not clear. However, these challenges in the diagnosis and management of coeliac disease suggest opportunities for future research.
Key points
-
Coeliac disease is a global disease with a worldwide prevalence of around 1%.
-
Most patients with coeliac disease are undiagnosed, misdiagnosed and/or experience a substantial delay in diagnosis, which suggests an inadequate awareness of the spectrum of its clinical manifestations.
-
Despite improvements in the diagnosis of coeliac disease, persistent challenges include high inter-assay and intra-assay variation in serological test performance and high inter-observer variability in the grading of villous abnormalities.
-
The optimal follow-up strategy for coeliac disease is unclear and studies are needed to define the timing and role of serological testing, gluten immunogenic peptide measurement and repeat biopsy.
-
Increased access to dietitians, improved tools to assess adherence to a gluten-free diet, increased availability of gluten-free foods and reduced gluten contamination of gluten-free food products are needed.
-
As maintaining strict adherence to a gluten-free diet is restrictive and challenging, the development of adjunct or alternative non-dietary therapies for coeliac disease is crucial.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fasano, A. & Catassi, C. Clinical practice. Celiac disease. N. Engl. J. Med. 367, 2419–2426 (2012).
Leonard, M. M., Sapone, A., Catassi, C. & Fasano, A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA 318, 647–656 (2017).
Singh, P. et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 16, 823–836 (2018).
[No Authors Listed.] Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch. Dis. Child. 65, 909–911 (1990).
Husby, S. et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 54, 136–160 (2012).
Husby, S. et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for diagnosing coeliac disease 2020. J. Pediatr. Gastroenterol. Nutr. 70, 141–156 (2020).
Ludvigsson, J. F. et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 63, 1210–1228 (2014).
Green, P. A. & Wollaeger, E. E. The clinical behavior of sprue in the United States. Gastroenterology 38, 399–418 (1960).
Swinson, C. M. & Levi, A. J. Is coeliac disease underdiagnosed? Br. Med. J. 281, 1258–1260 (1980).
Beaumont, D. M. & Mian, M. S. Coeliac disease in old age: ‘a catch in the rye’. Age Ageing 27, 535–538 (1998).
Murray, J. A. et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin. Gastroenterol. Hepatol. 1, 19–27 (2003).
Leffler, D. A., Green, P. H. R. & Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 12, 561–571 (2015).
Caio, G. et al. Celiac disease: a comprehensive current review. BMC Med. 17, 142 (2019).
Jamnik, J., Villa, C. R., Dhir, S. B., Jenkins, D. J. A. & El-Sohemy, A. Prevalence of positive coeliac disease serology and HLA risk genotypes in a multiethnic population of adults in Canada: a cross-sectional study. BMJ Open 7, e017678 (2017).
Comba, A., Eren, N. B. & Demir, E. Prevalence of celiac disease among school-age children in Çorum, Turkey. Turk. J. Gastroenterol. 29, 595–600 (2018).
Zanella, S. et al. Cross-sectional study of coeliac autoimmunity in a population of Vietnamese children. BMJ Open 6, e011173 (2016).
Zhou, C. et al. Prevalence of coeliac disease in Northwest China: heterogeneity across Northern Silk road ethnic populations. Aliment. Pharmacol. Ther. 51, 1116–1129 (2020).
Al-Hussaini, A. et al. Mass screening for celiac disease among school-aged children: toward exploring celiac iceberg in Saudi Arabia. J. Pediatr. Gastroenterol. Nutr. 65, 646–651 (2017).
Gatti, S. et al. Increased prevalence of celiac disease in school-age children in Italy. Clin. Gastroenterol. Hepatol. 18, 596–603 (2020).
Ivarsson, A. et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 131, e687–e694 (2013).
Catassi, C. et al. Why is coeliac disease endemic in the people of the Sahara? Lancet 354, 647–648 (1999).
Singh, P., Arora, S., Singh, A., Strand, T. A. & Makharia, G. K. Prevalence of celiac disease in Asia: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 31, 1095–1101 (2016).
Poddighe, D., Rakhimzhanova, M., Marchenko, Y. & Catassi, C. Pediatric celiac disease in Central and East Asia: current knowledge and prevalence. Medicina 55, 11 (2019).
Fukunaga, M. et al. Serological screening for celiac disease in adults in Japan: Shimane CoHRE study. JGH Open 4, 558–560 (2020).
Ramakrishna, B. S. et al. Prevalence of adult celiac disease in India: regional variations and associations. Am. J. Gastroenterol. 111, 115–123 (2016).
Yuan, J. et al. Prevalence of celiac disease autoimmunity among adolescents and young adults in China. Clin. Gastroenterol. Hepatol. 15, 1572–1579 (2017).
Lionetti, E. & Catassi, C. Co-localization of gluten consumption and HLA-DQ2 and -DQ8 genotypes, a clue to the history of celiac disease. Dig. Liver Dis. 46, 1057–1063 (2014).
Catassi, C. et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann. Med. 42, 530–538 (2010).
Rubio-Tapia, A. et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 137, 88–93 (2009).
Rubio-Tapia, A., Ludvigsson, J. F., Brantner, T. L., Murray, J. A. & Everhart, J. E. The prevalence of celiac disease in the United States. Am. J. Gastroenterol. 107, 1538–1544 (2012).
Choung, R. S. et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am. J. Gastroenterol. 110, 455–461 (2015).
King, J. A. et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am. J. Gastroenterol. 115, 507–525 (2020).
Holmes, G. K. T. & Muirhead, A. Epidemiology of coeliac disease in a single centre in Southern Derbyshire 1958–2014. BMJ Open Gastroenterol. 4, e000137 (2017).
Ludvigsson, J. F. et al. Increasing incidence of celiac disease in a North American population. Am. J. Gastroenterol. 108, 818–824 (2013).
Al-Toma, A. et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol. J. 7, 583–613 (2019).
Rubio-Tapia, A., Hill, I. D., Kelly, C. P., Calderwood, A. H. & Murray, J. A. ACG clinical guidelines: diagnosis and management of celiac disease. Am. J. Gastroenterol. 108, 656–676 (2013).
Popp, A., Kivelä, L., Fuchs, V. & Kurppa, K. Diagnosing celiac disease: towards wide-scale screening and serology-based criteria? Gastroenterol. Res. Pract. 2019, 2916024 (2019).
van den Broeck, H. C. et al. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: wheat breeding may have contributed to increased prevalence of celiac disease. Theor. Appl. Genet. 121, 1527–1539 (2010).
Dubcovsky, J. & Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316, 1862–1866 (2007).
Catassi, C. Where is celiac disease coming from and why? J. Pediatr. Gastroenterol. Nutr. 40, 279–282 (2005).
Unalp-Arida, A., Ruhl, C. E., Choung, R. S., Brantner, T. L. & Murray, J. A. Lower prevalence of celiac disease and gluten-related disorders in persons living in southern vs northern latitudes of the United States. Gastroenterology 152, 1922–1932 (2017).
Ivarsson, A., Hernell, O., Stenlund, H. & Persson, L. A. Breast-feeding protects against celiac disease. Am. J. Clin. Nutr. 75, 914–921 (2002).
Peters, U., Schneeweiss, S., Trautwein, E. A. & Erbersdobler, H. F. A case-control study of the effect of infant feeding on celiac disease. Ann. Nutr. Metab. 45, 135–142 (2001).
Pinto-Sánchez, M. I. et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J. Pediatr. 168, 132–143 (2016).
Silano, M., Agostoni, C., Sanz, Y. & Guandalini, S. Infant feeding and risk of developing celiac disease: a systematic review. BMJ Open 6, e009163 (2016).
Güngör, D. et al. Infant milk-feeding practices and diagnosed celiac disease and inflammatory bowel disease in offspring: a systematic review. Am. J. Clin. Nutr. 109, 838S–851S (2019).
Meijer, C., Shamir, R., Szajewska, H. & Mearin, L. Celiac disease prevention. Front. Pediatr. 6, 368 (2018).
Vriezinga, S. L. et al. Randomized feeding intervention in infants at high risk for celiac disease. N. Engl. J. Med. 371, 1304–1315 (2014).
Logan, K. et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 174, 1041–1047 (2020).
Lionetti, E. et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N. Engl. J. Med. 371, 1295–1303 (2014).
Roberts, S. E., Williams, J. G., Meddings, D., Davidson, R. & Goldacre, M. J. Perinatal risk factors and coeliac disease in children and young adults: a record linkage study. Aliment. Pharmacol. Ther. 29, 222–231 (2009).
Lebwohl, B., Green, P. H. R., Murray, J. A. & Ludvigsson, J. F. Season of birth in a nationwide cohort of coeliac disease patients. Arch. Dis. Child. 98, 48–51 (2013).
Tanpowpong, P. et al. Multicenter study on season of birth and celiac disease: evidence for a new theoretical model of pathogenesis. J. Pediatr. 162, 501–504 (2013).
Mårild, K., Stephansson, O., Montgomery, S., Murray, J. A. & Ludvigsson, J. F. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology 142, 39–45 (2012).
Dydensborg Sander, S. et al. Mode of delivery is not associated with celiac disease. Clin. Epidemiol. 10, 323–332 (2018).
Lionetti, E. et al. Mode of delivery and risk of celiac disease: risk of celiac disease and age at gluten introduction cohort study. J. Pediatr. 184, 81–86 (2017).
Koletzko, S. et al. Cesarean section on the risk of celiac disease in the offspring: the Teddy study. J. Pediatr. Gastroenterol. Nutr. 66, 417–424 (2018).
Stroud, C. et al. Evolving patterns in the presentation of coeliac disease over the last 25 years. Frontline Gastroenterol. 11, 98–103 (2020).
Stene, L. C. et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am. J. Gastroenterol. 101, 2333–2340 (2006).
Tjernberg, A. R. & Ludvigsson, J. F. Children with celiac disease are more likely to have attended hospital for prior respiratory syncytial virus infection. Dig. Dis. Sci. 59, 1502–1508 (2014).
Kahrs, C. R. et al. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort. BMJ 364, l231 (2019).
Welander, A., Tjernberg, A. R., Montgomery, S. M., Ludvigsson, J. & Ludvigsson, J. F. Infectious disease and risk of later celiac disease in childhood. Pediatrics 125, e530–e536 (2010).
Lindfors, K. et al. Metagenomics of the faecal virome indicate a cumulative effect of enterovirus and gluten amount on the risk of coeliac disease autoimmunity in genetically at risk children: the TEDDY study. Gut 69, 1416–1422 (2020).
Green, P. H. R. et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am. J. Gastroenterol. 96, 126–131 (2001).
Zipser, R. D., Patel, S., Yahya, K. Z., Baisch, D. W. & Monarch, E. Presentations of adult celiac disease in a nationwide patient support group. Dig. Dis. Sci. 48, 761–764 (2003).
Norström, F., Lindholm, L., Sandström, O., Nordyke, K. & Ivarsson, A. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol. 11, 118 (2011).
Cranney, A. et al. The Canadian celiac health survey. Dig. Dis. Sci. 52, 1087–1095 (2007).
Gray, A. M. & Papanicolas, I. N. Impact of symptoms on quality of life before and after diagnosis of coeliac disease: results from a UK population survey. BMC Health Serv. Res. 10, 105 (2010).
Ukkola, A. et al. Use of health care services and pharmaceutical agents in coeliac disease: a prospective nationwide study. BMC Gastroenterol. 12, 136 (2012).
Zipser, R. D., Farid, M., Baisch, D., Patel, B. & Patel, D. Physician awareness of celiac disease: a need for further education. J. Gen. Intern. Med. 20, 644–646 (2005).
Smukalla, S., Lebwohl, B., Mears, J. G., Leslie, L. A. & Green, P. H. How often do hematologists consider celiac disease in iron-deficiency anemia? Results of a national survey. Clin. Adv. Hematol. Oncol. 12, 100–105 (2014).
Biagi, F., Bianchi, P. I., Campanella, J., Zanellati, G. & Corazza, G. R. The impact of misdiagnosing celiac disease at a referral centre. Can. J. Gastroenterol. 23, 543–545 (2009).
Ianiro, G. et al. Prior misdiagnosis of celiac disease is common among patients referred to a tertiary care center: a prospective cohort study. Clin. Transl. Gastroenterol. 7, e139 (2016).
Pinto Sánchez, M. I. et al. Very high rate of misdiagnosis of celiac disease in clinical practice. Acta Gastroenterol. Latinoam. 39, 250–253 (2009).
Franceschini, E. et al. Misuse of serological screening tests for celiac disease in children: a prospective study in Italy. Dig. Liver Dis. 51, 1547–1550 (2019).
Singh, P. et al. Titers of anti-tissue transglutaminase antibody correlate well with severity of villous abnormalities in celiac disease. J. Clin. Gastroenterol. 49, 212–217 (2015).
Melini, V. & Melini, F. Gluten-free diet: gaps and needs for a healthier diet. Nutrients 11, 170 (2019).
Pallav, K. et al. Clinical utility of celiac disease-associated HLA testing. Dig. Dis. Sci. 59, 2199–2206 (2014).
Wolf, J. et al. Validation of antibody-based strategies for diagnosis of pediatric celiac disease without biopsy. Gastroenterology 153, 410–419 (2017).
Werkstetter, K. J. et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology 153, 924–935 (2017).
Fuchs, V. et al. Serology-based criteria for adult coeliac disease have excellent accuracy across the range of pre-test probabilities. Aliment. Pharmacol. Ther. 49, 277–284 (2019).
Penny, H. A. et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut 70, 876–883 (2021).
Husby, S., Murray, J. A. & Katzka, D. A. AGA clinical practice update on diagnosis and monitoring of celiac disease-changing utility of serology and histologic measures: expert review. Gastroenterology 156, 885–889 (2019).
Smarrazzo, A. et al. Variability of anti-human transglutaminase testing in celiac disease across Mediterranean countries. World J. Gastroenterol. 23, 4437–4443 (2017).
Singh, P. et al. Inter- and intra-assay variation in the diagnostic performance of assays for anti-tissue transglutaminase in 2 populations. Clin. Gastroenterol. Hepatol. 18, 2628–2630 (2020).
Naiyer, A. J. et al. Comparison of commercially available serologic kits for the detection of celiac disease. J. Clin. Gastroenterol. 43, 225–232 (2009).
Wong, R. C. W., Wilson, R. J., Steele, R. H., Radford-Smith, G. & Adelstein, S. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J. Clin. Pathol. 55, 488–494 (2002).
Choung, R. S. et al. Community-based study of celiac disease autoimmunity progression in adults. Gastroenterology 158, 151–159 (2020).
Tye-Din, J. Interpreting tests for coeliac disease: tips, pitfalls and updates. Aust. J. Gen. Pract. 47, 28–33 (2018).
Sugai, E. et al. Dynamics of celiac disease-specific serology after initiation of a gluten-free diet and use in the assessment of compliance with treatment. Dig. Liver Dis. 42, 352–358 (2010).
Lewis, N. R. & Scott, B. B. Meta-analysis: deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment. Pharmacol. Ther. 31, 73–81 (2010).
Hoerter, N. A. et al. Diagnostic yield of isolated deamidated gliadin peptide antibody elevation for celiac disease. Dig. Dis. Sci. 62, 1272–1276 (2017).
Sugai, E. et al. Celiac disease serology in patients with different pretest probabilities: is biopsy avoidable? World J. Gastroenterol. 16, 3144–3152 (2010).
Oyaert, M. et al. Combining antibody tests and taking into account antibody levels improves serologic diagnosis of celiac disease. Clin. Chem. Lab. Med. 53, 1537–1546 (2015).
Abdulrahim, A. et al. Deamidated gliadin antibodies: do they add to tissue transglutaminase-IgA assay in screening for celiac disease? J. Pediatr. Gastroenterol. Nutr. 72, e112–e118 (2021).
Gould, M. J., Brill, H., Marcon, M. A., Munn, N. J. & Walsh, C. M. In screening for celiac disease, deamidated gliadin rarely predicts disease when tissue transglutaminase is normal. J. Pediatr. Gastroenterol. Nutr. 68, 20–25 (2019).
Cummins, A. & Thompson, F. Sensitivity of anti-endomysial antibody in detecting celiac disease. Gastroenterology 122, 246–247 (2002).
Chou, R. et al. Screening for celiac disease: evidence report and systematic review for the US preventive services task force. JAMA 317, 1258–1268 (2017).
Singh, P. et al. Diagnostic accuracy of point of care tests for diagnosing celiac disease: a systematic review and meta-analysis. J. Clin. Gastroenterol. 53, 535–542 (2019).
Marsh, M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 102, 330–354 (1992).
Oberhuber, G., Granditsch, G. & Vogelsang, H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 11, 1185–1194 (1999).
Corazza, G. R. et al. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin. Gastroenterol. Hepatol. 5, 838–843 (2007).
Ensari, A. Gluten-sensitive enteropathy (celiac disease): controversies in diagnosis and classification. Arch. Pathol. Lab. Med. 134, 826–836 (2010).
Weile, B., Hansen, B. F., Hägerstrand, I., Hansen, J. P. & Krasilnikoff, P. A. Interobserver variation in diagnosing coeliac disease. A joint study by Danish and Swedish pathologists. APMIS 108, 380–384 (2000).
Lebwohl, B., Kapel, R. C., Neugut, A. I., Green, P. H. R. & Genta, R. M. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest. Endosc. 74, 103–109 (2011).
Lebwohl, B. et al. Prior endoscopy in patients with newly diagnosed celiac disease: a missed opportunity? Dig. Dis. Sci. 58, 1293–1298 (2013).
Mooney, P. D. et al. Clinical and immunologic features of ultra-short celiac disease. Gastroenterology 150, 1125–1134 (2016).
Taavela, J. et al. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One 8, e76163 (2013).
Latorre, M. et al. Endoscopic biopsy technique in the diagnosis of celiac disease: one bite or two? Gastrointest. Endosc. 81, 1228–1233 (2015).
Das, P. et al. Quantitative histology-based classification system for assessment of the intestinal mucosal histological changes in patients with celiac disease. Intest. Res. 17, 387–397 (2019).
Cummins, A. G. et al. Morphometric evaluation of duodenal biopsies in celiac disease. Am. J. Gastroenterol. 106, 145–150 (2011).
Syed, S. et al. Assessment of machine learning detection of environmental enteropathy and celiac disease in children. JAMA Netw. Open 2, e195822 (2019).
Crenn, P. et al. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 124, 1210–1219 (2003).
Pelsers, M. M. A. L., Hermens, W. T. & Glatz, J. F. C. Fatty acid-binding proteins as plasma markers of tissue injury. Clin. Chim. Acta 352, 15–35 (2005).
Morón, B. et al. CYP3A4-catalyzed simvastatin metabolism as a non-invasive marker of small intestinal health in celiac disease. Am. J. Gastroenterol. 108, 1344–1351 (2013).
Singh, A. et al. Non-immunological biomarkers for assessment of villous abnormalities in patients with celiac disease. J. Gastroenterol. Hepatol. 35, 438–445 (2020).
Fragkos, K. C. & Forbes, A. Citrulline as a marker of intestinal function and absorption in clinical settings: a systematic review and meta-analysis. U. Eur. Gastroenterol. J. 6, 181–191 (2018).
Adriaanse, M. P. M. et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment. Pharmacol. Ther. 37, 482–490 (2013).
Choung, R. S. et al. Synthetic neoepitopes of the transglutaminase-deamidated gliadin complex as biomarkers for diagnosing and monitoring celiac disease. Gastroenterology 156, 582–591 (2019).
Kelly, C. P., Bai, J. C., Liu, E. & Leffler, D. A. Advances in diagnosis and management of celiac disease. Gastroenterology 148, 1175–1186 (2015).
Ciccocioppo, R. et al. The spectrum of differences between childhood and adulthood celiac disease. Nutrients 7, 8733–8751 (2015).
Lebwohl, B., Murray, J. A., Rubio-Tapia, A., Green, P. H. R. & Ludvigsson, J. F. Predictors of persistent villous atrophy in coeliac disease: a population-based study. Aliment. Pharmacol. Ther. 39, 488–495 (2014).
Rubio-Tapia, A. et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am. J. Gastroenterol. 105, 1412–1420 (2010).
Ghazzawi, Y., Rubio-Tapia, A., Murray, J. A. & Absah, I. Mucosal healing in children with treated celiac disease. J. Pediatr. Gastroenterol. Nutr. 59, 229–231 (2014).
Lanzini, A. et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 29, 1299–1308 (2009).
Daveson, A. J. M. et al. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten-free diet. GastroHep 2, 22–30 (2020).
Adelman, D. C. et al. Measuring change in small intestinal histology in patients with celiac disease. Am. J. Gastroenterol. 113, 339–347 (2018).
Lebwohl, B. et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann. Intern. Med. 159, 169–175 (2013).
Lebwohl, B., Michaëlsson, K., Green, P. H. R. & Ludvigsson, J. F. Persistent mucosal damage and risk of fracture in celiac disease. J. Clin. Endocrinol. Metab. 99, 609–616 (2014).
Lebwohl, B. et al. Psychiatric disorders in patients with a diagnosis of celiac disease during childhood from 1973 to 2016. Clin. Gastroenterol. Hepatol. 19, 2093–2101 (2021).
Cadenhead, J. W. et al. Diminished quality of life among adolescents with coeliac disease using maladaptive eating behaviours to manage a gluten-free diet: a cross-sectional, mixed-methods study. J. Hum. Nutr. Diet. 32, 311–320 (2019).
Bai, J. C. & Ciacci, C. World Gastroenterology Organisation global guidelines: celiac disease February 2017. J. Clin. Gastroenterol. 51, 755–768 (2017).
Leffler, D. A. et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin. Gastroenterol. Hepatol. 7, 530–536 (2009).
Herman, M. L. et al. Patients with celiac disease are not followed up adequately. Clin. Gastroenterol. Hepatol. 10, 893–899 (2012).
Silvester, J. A. et al. Tests for serum transglutaminase and endomysial antibodies do not detect most patients with celiac disease and persistent villous atrophy on gluten-free diets: a meta-analysis. Gastroenterology 153, 689–701 (2017).
Midhagen, G. et al. Antibody levels in adult patients with coeliac disease during gluten-free diet: a rapid initial decrease of clinical importance. J. Intern. Med. 256, 519–524 (2004).
Stefanolo, J. P. et al. Real-world gluten exposure in patients with celiac disease on gluten-free diets, determined from gliadin immunogenic peptides in urine and fecal samples. Clin. Gastroenterol. Hepatol. 19, 484–491 (2021).
Comino, I. et al. Fecal gluten peptides reveal limitations of serological tests and food questionnaires for monitoring gluten-free diet in celiac disease patients. Am. J. Gastroenterol. 111, 1456–1465 (2016).
Leonard, M. M. et al. Evaluating responses to gluten challenge: a randomized, double-blind, 2-dose gluten challenge trial. Gastroenterology 160, 720–733 (2021).
Moreno, M., de, L., Rodríguez-Herrera, A., Sousa, C. & Comino, I. Biomarkers to monitor gluten-free diet compliance in celiac patients. Nutrients 9, 46 (2017).
Ruiz-Carnicer, Á. et al. Negative predictive value of the repeated absence of gluten immunogenic peptides in the urine of treated celiac patients in predicting mucosal healing: new proposals for follow-up in celiac disease. Am. J. Clin. Nutr. 112, 1240–1251 (2020).
Moreno, M. et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 66, 250–257 (2017).
Zammit, C. S., McAlindon, M. E., Sanders, D. S. & Sidhu, R. Assessment of disease severity on capsule endoscopy in patients with small bowel villous atrophy. J. Gastroenterol. Hepatol. 36, 1015–1021 (2021).
Perez-Cuadrado-Robles, E. et al. Role of capsule endoscopy in alarm features and non-responsive celiac disease: a European multicenter study. Dig. Endosc. 30, 461–466 (2018).
Shah, S. et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am. J. Gastroenterol. 109, 1304–1311 (2014).
Mearns, E. S. et al. Systematic literature review of the economic burden of celiac disease. Pharmacoeconomics 37, 45–61 (2019).
White, L. E., Bannerman, E. & Gillett, P. M. Coeliac disease and the gluten-free diet: a review of the burdens; factors associated with adherence and impact on health-related quality of life, with specific focus on adolescence. J. Hum. Nutr. Diet. 29, 593–606 (2016).
Collin, P., Thorell, L., Kaukinen, K. & Mäki, M. The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease? Aliment. Pharmacol. Ther. 19, 1277–1283 (2004).
Catassi, C. et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 85, 160–166 (2007).
Akobeng, A. K. & Thomas, A. G. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 27, 1044–1052 (2008).
Costa, A. F. et al. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J. Gastroenterol. 25, 1409–1420 (2019).
FAO. Food and Agriculture Organization Standard for foods for special dietary use for persons intolerant to gluten CXS 118-1979. FAO https://www.fao.org/fao-who-codexalimentarius (1979).
European Union. Commission Regulation (EC) No 41/2009 of 20 January 2009 concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten (Text with EEA relevance). EU http://data.europa.eu/eli/reg/2009/41/oj (2009).
FDA. Food Labeling; Gluten-Free Labeling of Foods. FDA https://www.federalregister.gov/documents/2013/08/05/2013-18813/food-labeling-gluten-free-labeling-of-foods (2013).
García-Molina, M. D., Giménez, M. J., Sánchez-León, S. & Barro, F. Gluten free wheat: are we there? Nutrients 11, 487 (2019).
Scherf, K. A. et al. Recent progress and recommendations on celiac disease from the working group on prolamin analysis and toxicity. Front. Nutr. 7, 29 (2020).
Scherf, K. A. et al. Statement of the prolamin working group on the determination of gluten in fermented foods containing partially hydrolyzed gluten. Front. Nutr. 7, 626712 (2020).
Lee, A. R., Wolf, R. L., Lebwohl, B., Ciaccio, E. J. & Green, P. H. R. Persistent economic burden of the gluten free diet. Nutrients 11, 399 (2019).
Falcomer, A. L. et al. Worldwide public policies for celiac disease: are patients well assisted? Int. J. Public Health 65, 937–945 (2020).
Rostami, K., Bold, J., Parr, A. & Johnson, M. W. Gluten-free diet indications, safety, quality, labels, and challenges. Nutrients 9, 846 (2017).
Rodrigues, M., Yonamine, G. H. & Fernandes Satiro, C. A. Rate and determinants of non-adherence to a gluten-free diet and nutritional status assessment in children and adolescents with celiac disease in a tertiary Brazilian referral center: a cross-sectional and retrospective study. BMC Gastroenterol. 18, 15 (2018).
Lerner, B. A. et al. Detection of gluten in gluten-free labeled restaurant food: analysis of crowd-sourced data. Am. J. Gastroenterol. 114, 792–797 (2019).
Bustamante, M. Á. et al. Evolution of gluten content in cereal-based gluten-free products: an overview from 1998 to 2016. Nutrients 9, 21 (2017).
Lee, H. J., Anderson, Z. & Ryu, D. Gluten contamination in foods labeled as ‘gluten free’ in the United States. J. Food Prot. 77, 1830–1833 (2014).
Farage, P. et al. Gluten contamination in gluten-free bakery products: a risk for coeliac disease patients. Public Health Nutr. 20, 413–416 (2017).
Thompson, T., Lee, A. R. & Grace, T. Gluten contamination of grains, seeds, and flours in the United States: a pilot study. J. Am. Diet. Assoc. 110, 937–940 (2010).
Halmos, E. P., Clarke, D., Pizzey, C. & Tye-Din, J. A. Gluten in ‘gluten-free’ manufactured foods in Australia: a cross-sectional study. Med. J. Aust. 209, 448–449 (2018).
Verma, A. K. et al. Gluten contamination in naturally or labeled gluten-free products marketed in Italy. Nutrients 9, 115 (2017).
Pinto-Sanchez, M. I. et al. Tax-deductible provisions for gluten-free diet in Canada compared with systems for gluten-free diet coverage available in various countries. Can. J. Gastroenterol. Hepatol. 29, 104–110 (2015).
Mårild, K. et al. Costs and use of health care in patients with celiac disease: a population-based longitudinal study. Am. J. Gastroenterol. 115, 1253–1263 (2020).
Cappell, K. et al. Healthcare resource utilization and costs in celiac disease: a US claims analysis. Am. J. Gastroenterol. 115, 1821–1829 (2020).
Guandalini, S. et al. Direct costs in patients with celiac disease in the USA: a retrospective claims analysis. Dig. Dis. Sci. 61, 2823–2830 (2016).
van Wanrooij, R. L. J. et al. Outcome of referrals for non-responsive celiac disease in a tertiary center: low incidence of refractory celiac disease in the Netherlands. Clin. Transl. Gastroenterol. 8, e218 (2017).
Leffler, D. A. et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin. Gastroenterol. Hepatol. 5, 445–450 (2007).
Kelly, C. P. et al. TAK-101 nanoparticles induce gluten-specific tolerance in celiac disease: a randomized, double-blind, placebo-controlled study. Gastroenterology 161, 66–80 (2021).
Syage, J. A. et al. Latiglutenase treatment for celiac disease: symptom and quality of life improvement for seropositive patients on a gluten-free diet. GastroHep 1, 293–301 (2019).
Pultz, I. S. et al. Gluten degradation, pharmacokinetics, safety, and tolerability of TAK-062, an engineered enzyme to treat celiac disease. Gastroenterology 161, 81–93 (2021).
Schuppan, D. et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N. Engl. J. Med. 385, 35–45 (2021).
Seiler, C. L. et al. Probiotics for celiac disease: a systematic review and meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 115, 1584–1595 (2020).
Kivelä, L. et al. Current and emerging therapies for coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 18, 181–195 (2021).
Ludvigsson, J. F. et al. The Oslo definitions for coeliac disease and related terms. Gut 62, 43–52 (2013).
Nachman, F. et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur. J. Gastroenterol. Hepatol. 23, 473–480 (2011).
Leffler, D. A. & Schuppan, D. Update on serologic testing in celiac disease. Am. J. Gastroenterol. 105, 2520–2524 (2010).
Nachman, F. et al. Quality of life in celiac disease patients: prospective analysis on the importance of clinical severity at diagnosis and the impact of treatment. Dig. Liver Dis. 41, 15–25 (2009).
Nachman, F. et al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig. Liver Dis. 42, 685–691 (2010).
Corazza, G. R., Stefano, M. D., Mauriño, E. & Bai, J. C. Bones in coeliac disease: diagnosis and treatment. Best Pract. Res. Clin. Gastroenterol. 19, 453–465 (2005).
Zanchetta, M. B. et al. Improved bone microarchitecture in patients with celiac disease after 3 years on a gluten-free diet. Clin. Gastroenterol. Hepatol. 16, 774–775 (2018).
Zanchetta, M. B., Longobardi, V. & Bai, J. C. Bone and celiac disease. Curr. Osteoporos. Rep. 14, 43–48 (2016).
Sánchez, M. I. P. et al. Risk of fracture in celiac disease: gender, dietary compliance, or both? World J. Gastroenterol. 17, 3035–3042 (2011).
Hadjivassiliou, M. et al. Neurological dysfunction in coeliac disease and non-coeliac gluten sensitivity. Am. J. Gastroenterol. 111, 561–567 (2016).
Croall, I. D., Sanders, D. S., Hadjivassiliou, M. & Hoggard, N. Cognitive deficit and white matter changes in persons with celiac disease: a population-based study. Gastroenterology 158, 2112–2122 (2020).
Rubio-Tapia, A. et al. Creation of a model to predict survival in patients with refractory coeliac disease using a multinational registry. Aliment. Pharmacol. Ther. 44, 704–714 (2016).
Ludvigsson, J. F. Mortality and malignancy in celiac disease. Gastrointest. Endosc. Clin. N. Am. 22, 705–722 (2012).
Cellier, C. et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 356, 203–208 (2000).
Holmes, G. K. T. & Muirhead, A. Mortality in coeliac disease: a population-based cohort study from a single centre in Southern Derbyshire, UK. BMJ Open Gastroenterol. 5, e000201 (2018).
Rubio-Tapia, A. & Murray, J. A. The liver and celiac disease. Clin. Liver Dis. 23, 167–176 (2019).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article and to writing, reviewing and/or editing the manuscript before submission. In addition, G.K.M., P.S., C.C., D.S.S., D.L. and J.C.B. contributed substantially to discussions of its content.
Corresponding author
Ethics declarations
Competing interests
C.C. declares that he has acted as a scientific consultant for Dr Schär Food (a gluten-free diet manufacturer), Nóos and Takeda. D.S.S. declares that he has received consultancy fees and an educational grant from Dr Schär Food. D.L. declares that he has received salary support from Takeda. J.C.B. declares that he has acted as a scientific consultant for Innovate, Proventionbio and Takeda. G.K.M., P.S. and R.A.R.A. declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks J. Ludviasson, P. Green and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
United Nations Food and Agriculture Organization: http://faostat.fao.org
Glossary
- Gluten
-
The storage proteins of wheat (gliadins and glutenins), rye (secalins), barley (hordeins) and oats (avenins), which are composed of prolamin and glutelin moieties.
Rights and permissions
About this article
Cite this article
Makharia, G.K., Singh, P., Catassi, C. et al. The global burden of coeliac disease: opportunities and challenges. Nat Rev Gastroenterol Hepatol 19, 313–327 (2022). https://doi.org/10.1038/s41575-021-00552-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-021-00552-z
This article is cited by
-
The financial impact on people with coeliac disease of withdrawing gluten-free food from prescriptions in England: findings from a cross-sectional survey
BMC Health Services Research (2024)
-
Fermented foods and gastrointestinal health: underlying mechanisms
Nature Reviews Gastroenterology & Hepatology (2024)
-
Guidelines for best practices in monitoring established coeliac disease in adult patients
Nature Reviews Gastroenterology & Hepatology (2024)