Abstract
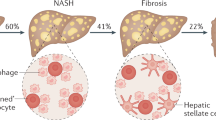
Nonalcoholic fatty liver disease (NAFLD) has reached epidemic proportions worldwide. NAFLD and type 2 diabetes mellitus (T2DM) are known to frequently coexist and act synergistically to increase the risk of adverse (hepatic and extra-hepatic) clinical outcomes. T2DM is also one of the strongest risk factors for the faster progression of NAFLD to nonalcoholic steatohepatitis, advanced fibrosis or cirrhosis. However, the link between NAFLD and T2DM is more complex than previously believed. Strong evidence indicates that NAFLD is associated with an approximate twofold higher risk of developing T2DM, irrespective of obesity and other common metabolic risk factors. This risk parallels the severity of NAFLD, such that patients with more advanced stages of liver fibrosis are at increased risk of incident T2DM. In addition, the improvement or resolution of NAFLD (on ultrasonography) is associated with a reduction of T2DM risk, adding weight to causality and suggesting that liver-focused treatments might reduce the risk of developing T2DM. This Review describes the evidence of an association and causal link between NAFLD and T2DM, discusses the putative pathophysiological mechanisms linking NAFLD to T2DM and summarizes the current pharmacological treatments for NAFLD or T2DM that might benefit or adversely affect the risk of T2DM or NAFLD progression.
Key points
-
An updated meta-analysis of 33 observational studies showed that nonalcoholic fatty liver disease (NAFLD) is associated with an approximate doubled risk of type 2 diabetes mellitus (T2DM), irrespective of obesity and other metabolic risk factors.
-
Patients with more advanced stages of liver fibrosis are at increased risk of T2DM; some observational studies have shown that improvement or resolution of NAFLD on ultrasonography is closely associated with a reduction of diabetes risk.
-
NAFLD exacerbates hepatic and peripheral insulin resistance, predisposes to atherogenic dyslipidaemia and causes the systemic release of pro-inflammatory cytokines and hepatokines that can promote the development of T2DM.
-
Treatment of NAFLD and T2DM is based on lifestyle modifications aiming at substantial weight loss in individuals with overweight or obesity.
-
Although no pharmacotherapy is currently approved for NAFLD, some antihyperglycaemic drugs, such as pioglitazone, glucagon-like peptide 1 analogues and sodium–glucose cotransporter 2 inhibitors, have some efficacy.
-
Multiple investigational compounds for NAFLD treatment, including modulators of bile acid and lipid metabolism, are in phase II and phase III randomized controlled trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Younossi, Z. M. Non-alcoholic fatty liver disease — a global public health perspective. J. Hepatol. 70, 531–544 (2019).
Byrne, C. D., Patel, J., Scorletti, E. & Targher, G. Tests for diagnosing and monitoring non-alcoholic fatty liver disease in adults. BMJ 362, k2734 (2018).
Targher, G., Lonardo, A. & Byrne, C. D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat. Rev. Endocrinol. 14, 99–114 (2018).
Mantovani, A. et al. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 111S, 154170 (2020).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71, 793–801 (2019).
Lonardo, A., Nascimbeni, F., Mantovani, A. & Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 68, 335–352 (2018).
Lonardo, A. et al. A round trip from nonalcoholic fatty liver disease to diabetes: molecular targets to the rescue? Acta Diabetol. 56, 385–396 (2019).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Tilg, H., Moschen, A. R. & Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 14, 32–42 (2017).
Valenti, L., Bugianesi, E., Pajvani, U. & Targher, G. Nonalcoholic fatty liver disease: cause or consequence of type 2 diabetes? Liver Int. 36, 1563–1579 (2016).
Hallsworth, K. & Adams, L. A. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 1, 468–479 (2019).
Knowler, W. C. et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 374, 1677–1686 (2009).
Lindström, J. et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368, 1673–1679 (2006).
DeFronzo, R. A. et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364, 1104–1115 (2011).
Sung, K. C., Jeong, W. S., Wild, S. H. & Byrne, C. D. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 35, 717–722 (2012).
Mantovani, A. P. G., Beatrice, G., Tilg, H., Byrne, C. D. & Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut 70, 962–969 (2021).
Morrison, A. E., Zaccardi, F., Khunti, K. & Davies, M. J. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: a meta-analysis with bias analysis. Liver Int. 39, 557–567 (2019).
Bjorkstrom, K., Stal, P., Hultcrantz, R. & Hagstrom, H. Histologic scores for fat and fibrosis associate with development of type 2 diabetes in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 15, 1461–1468 (2017).
Nasr, P., Ignatova, S., Kechagias, S. & Ekstedt, M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol. Commun. 2, 199–210 (2018).
Nasr, P., Fredrikson, M., Ekstedt, M. & Kechagias, S. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int. 40, 1069–1078 (2020).
Ampuero, J. et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J. Hepatol. 73, 17–25 (2020).
Bae, J. C. et al. The persistence of fatty liver has a differential impact on the development of diabetes: The Kangbuk Samsung Health Study. Diabetes Res. Clin. Pract. 135, 1–6 (2018).
Lee, J. et al. The impact of NAFLD and waist circumference changes on diabetes development in prediabetes subjects. Sci. Rep. 9, 17258 (2019).
Cho, H. J. et al. Improvement of nonalcoholic fatty liver disease reduces the risk of type 2 diabetes mellitus. Gut liver 13, 440–449 (2019).
Brunner, K. T. et al. Increasing liver fat is associated with progression of cardiovascular risk factors. Liver Int. 40, 1339–1343 (2020).
Fukuda, T. et al. Transient remission of nonalcoholic fatty liver disease decreases the risk of incident type 2 diabetes mellitus in Japanese men. Eur. J. Gastroenterol. Hepatol. 28, 1443–1449 (2016).
Yamazaki, H., Tsuboya, T., Tsuji, K., Dohke, M. & Maguchi, H. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care 38, 1673–1679 (2015).
Sung, K. C., Wild, S. H. & Byrne, C. D. Resolution of fatty liver and risk of incident diabetes. J. Clin. Endocrinol. Metab. 98, 3637–3643 (2013).
Zaharia, O. P. et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 7, 684–694 (2019).
Zaharia, O. P. et al. Role of patatin-like phospholipase domain-containing 3 gene for hepatocellular lipid content and insulin resistance in diabetes. Diabetes Care 43, 2161–2168 (2020).
Liu, Z. et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J. Hepatol. 73, 263–276 (2020).
Dongiovanni, P. et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J. Intern. Med. 283, 356–370 (2018).
Eslam, M. & George, J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat. Rev. Gastroenterol. Hepatol. 17, 40–52 (2020).
Liu, D. J. et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 49, 1758–1766 (2017).
Katsiki, N., Mikhailidis, D. P. & Mantzoros, C. S. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism 65, 1109–1123 (2016).
Al-Mrabeh, A. et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab. 31, 233–249.e4 (2020).
Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol. 62 (Suppl. 1), S47–S64 (2015).
Yki-Jarvinen, H. Ceramides: a cause of insulin resistance in nonalcoholic fatty liver disease in both murine models and humans. Hepatology 71, 1499–1501 (2020).
Liao, W., Hui, T. Y., Young, S. G. & Davis, R. A. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. J. Lipid Res. 44, 978–985 (2003).
Yamaguchi, K. et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45, 1366–1374 (2007).
Bechmann, L. P. et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology 57, 1394–1406 (2013).
Kalhan, S. C. et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 60, 404–413 (2011).
Legry, V. et al. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J. Clin. Endocrinol. Metab. 102, 3783–3794 (2017).
Caussy, C. et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment. Pharmacol. Ther. 49, 183–193 (2019).
de Aguiar Vallim, T. Q., Tarling, E. J. & Edwards, P. A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 17, 657–669 (2013).
Grabherr, F., Grander, C., Effenberger, M., Adolph, T. E. & Tilg, H. Gut dysfunction and non-alcoholic fatty liver disease. Front. Endocrinol. 10, 611 (2019).
Chavez-Talavera, O., Tailleux, A., Lefebvre, P. & Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–1694.e3 (2017).
Kuipers, F., Bloks, V. W. & Groen, A. K. Beyond intestinal soap–bile acids in metabolic control. Nat. Rev. Endocrinol. 10, 488–498 (2014).
Han, C. Y. Update on FXR biology: promising therapeutic target? Int. J. Mol. Sci. 19, 2069 (2018).
Utzschneider, K. M., Kahn, S. E. & Polidori, D. C. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J. Clin. Endocrinol. Metab. 104, 1855–1865 (2019).
Wahlstrom, A., Sayin, S. I., Marschall, H. U. & Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Jiao, Y., Lu, Y. & Li, X. Y. Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 36, 44–50 (2015).
Kim, H. & Fang, S. Crosstalk between FXR and TGR5 controls glucagon-like peptide 1 secretion to maintain glycemic homeostasis. Lab. Anim. Res. 34, 140–146 (2018).
Pathak, P. et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 292, 11055–11069 (2017).
Roden, M. & Shulman, G. I. The integrative biology of type 2 diabetes. Nature 576, 51–60 (2019).
Koliaki, C. et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 21, 739–746 (2015).
Ter Horst, K. W. et al. Hepatic diacylglycerol-associated protein kinase Cε translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Rep. 19, 1997–2004 (2017).
Magkos, F. et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 142, 1444–1446.e2 (2012).
Kumashiro, N. et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA 108, 16381–16385 (2011).
Luukkonen, P. K. et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 64, 1167–1175 (2016).
Cantley, J. L. et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl Acad. Sci. USA 110, 1869–1874 (2013).
Jelenik, T. et al. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes 66, 2241–2253 (2017).
Nardo, A. D. et al. Impact of osteopontin on the development of non-alcoholic liver disease and related hepatocellular carcinoma. Liver Int. 40, 1620–1633 (2020).
Bikman, B. T. & Summers, S. A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Invest. 121, 4222–4230 (2011).
Sharma, A. X. & Holland, W. L. Adiponectin and its hydrolase-activated receptors. J. Nat. Sci. 3, e396 (2017).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Raichur, S. et al. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Chaurasia, B. et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–392 (2019).
Summers, S. A. Could ceramides become the new cholesterol? Cell Metab. 27, 276–280 (2018).
Apostolopoulou, M. et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care 41, 1235–1243 (2018).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e1 (2013).
Scorletti, E. et al. Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: Results from the WELCOME trial. J. Hepatol. 63, 1476–1483 (2015).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Ghosh-Swaby, O. R. et al. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: an updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol. 8, 418–435 (2020).
Mantovani, A., Byrne, C. D., Scorletti, E., Mantzoros, C. S. & Targher, G. Efficacy and safety of anti-hyperglycaemic drugs in patients with non-alcoholic fatty liver disease with or without diabetes: an updated systematic review of randomized controlled trials. Diabetes Metab. 46, 427–441 (2020).
Samuel, V. T. & Shulman, G. I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 27, 22–41 (2018).
Loomba, R. et al. Multicenter validation of association between decline in MRI-PDFF and histologic response in nonalcoholic steatohepatitis. Hepatology 72, 1219–1229 (2020).
Bugianesi, E. et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 100, 1082–1090 (2005).
Haukeland, J. W. et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand. J. Gastroenterol. 44, 853–860 (2009).
Lavine, J. E. et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 305, 1659–1668 (2011).
Said, A. & Akhter, A. Meta-analysis of randomized controlled trials of pharmacologic agents in non-alcoholic steatohepatitis. Ann. Hepatol. 16, 538–547 (2017).
Macauley, M. et al. Effect of vildagliptin on hepatic steatosis. J. Clin. Endocrinol. Metab. 100, 1578–1585 (2015).
Cui, J. et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J. Hepatol. 65, 369–376 (2016).
Fei, Y., Tsoi, M. F. & Cheung, B. M. Y. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc. Diabetol. 18, 112 (2019).
Brunton, S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int. J. Clin. Pract. 68, 557–567 (2014).
Petit, J. M. et al. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: The Lira-NAFLD study. J. Clin. Endocrinol. Metab. 102, 407–415 (2017).
Yan, J. et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology 69, 2414–2426 (2019).
Bizino, M. B. et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia 63, 65–74 (2020).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03648554 (2019).
Frias, J. P. et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392, 2180–2193 (2018).
Hartman, M. L. et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 43, 1352–1355 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03900429 (2021).
Cusi, K. et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes. Metab. 21, 812–821 (2019).
Eriksson, J. W. et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 61, 1923–1934 (2018).
Latva-Rasku, A. et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care 42, 931–937 (2019).
Kuchay, M. S. et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 41, 1801–1808 (2018).
Kahl, S. et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care 43, 298–305 (2020).
Lai, L. L., Vethakkan, S. R., Nik Mustapha, N. R., Mahadeva, S. & Chan, W. K. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig. Dis. Sci. 65, 623–631 (2020).
de Boer, R. A. et al. Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozin vs placebo or empagliflozin in patients with type 2 diabetes and heart failure. Br. J. Clin. Pharmacol. 86, 1346–1356 (2020).
Harrison S. A., et al. LIK066 (licogliflozin), an SGLT1/2 inhibitor, robustly decreases ALT and improves markers of hepatic and metabolic health in patients with nonalcoholic fatty liver disease: interim analysis of a 12-week, randomized, placebo-controlled, phase 2a study. Liver Meet. https://www.natap.org/2019/AASLD/AASLD_75.htm (2019).
Zhou, Y. et al. Pioglitazone for the primary and secondary prevention of cardiovascular and renal outcomes in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis. J. Clin. Endocrinol. Metab. 105, dgz252 (2020).
Musso, G., Cassader, M., Paschetta, E. & Gambino, R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern. Med. 177, 633–640 (2017).
Belfort, R. et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 355, 2297–2307 (2006).
Aithal, G. P. et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135, 1176–1184 (2008).
Cusi, K. et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann. Intern. Med. 165, 305–315 (2016).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Ratziu, V. et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51, 445–453 (2010).
Ratziu, V. et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150, 1147–1159.e5 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02704403 (2020).
Kaul, U. et al. New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence. Cardiovasc. Diabetol. 18, 80 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03008070 (2021).
Joy, T. R. et al. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J. Gastroenterol. 23, 141–150 (2017).
Bolinder, J. et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J. Clin. Endocrinol. Metab. 97, 1020–1031 (2012).
Ratziu, V. et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135, 100–110 (2008).
Trauner, M. et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol. Hepatol. 4, 445–453 (2019).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Siddiqui, M. S. et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J. Hepatol. 72, 25–33 (2020).
Pockros, P. J. et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 39, 2082–2093 (2019).
Kim, C. W. et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 26, 394–406.e6 (2017).
Kim, W. et al. Randomised clinical trial: the efficacy and safety of oltipraz, a liver X receptor alpha-inhibitory dithiolethione in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 45, 1073–1083 (2017).
Struik, D., Dommerholt, M. B. & Jonker, J. W. Fibroblast growth factors in control of lipid metabolism: from biological function to clinical application. Curr. Opin. Lipidol. 30, 235–243 (2019).
Harrison, S. A. et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 391, 1174–1185 (2018).
Harrison, S. A. et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 71, 1198–1212 (2020).
Rinella, M. E. et al. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. J. Hepatol. 70, 735–744 (2019).
Sanyal, A. et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 392, 2705–2717 (2019).
Lawitz, E. J. et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 16, 1983–1991.e3 (2018).
Loomba, R. et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology 155, 1463–1473.e6 (2018).
Safadi, R. et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 12, 2085–2091.e1 (2014).
Ratziu, V. et al. On behalf of the ARREST investigator study group. One-year results of the Global Phase 2b randomized placebo-controlled ARREST trial of aramchol, a stearoyl CoA desaturase modulator in NASH patients. Liver Meet. https://www.natap.org/2018/AASLD/AASLD_222.htm (2018).
Amin, N. B. et al. Targeting diacylglycerol acyltransferase 2 for the treatment of nonalcoholic steatohepatitis. Sci. Transl Med. 11, eaav9701 (2019).
Harrison, S. A. et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 394, 2012–2024 (2019).
Ratziu, V. et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR Study. Hepatology 72, 892–905 (2020).
Anstee, Q. M. et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp. Clin. Trials 89, 105922 (2020).
Harrison, S. A. et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J. Hepatol. 73, 26–39 (2020).
Harrison, S. A. et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology 155, 1140–1153 (2018).
Johnston, M. P., Patel, J. & Byrne, C. D. Multi-drug approaches to NASH: what’s in the development pipeline? Expert Opin. Investig. Drugs 29, 143–150 (2020).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Byrne, C. D. Dorothy Hodgkin Lecture 2012: non-alcoholic fatty liver disease, insulin resistance and ectopic fat: a new problem in diabetes management. Diabet. Med. 29, 1098–1107 (2012).
Scorletti, E. & Byrne, C. D. Extrahepatic Diseases and NAFLD: the triangular relationship between NAFLD, type 2-diabetes and dysbiosis. Dig. Dis. 34 (Suppl. 1), 11–18 (2016).
Acknowledgements
The work of G.T. is supported in part by grants from the University School of Medicine of Verona, Verona, Italy. The work of C.D.B. is supported in part by grants from the Southampton National Institute for Health Research Biomedical Research Centre. The work of M.R. is supported by grants from the German Federal Ministry of Health (BMG) and the Ministry of Culture and Science of the state North Rhine-Westphalia (MKW NRW) to the German Diabetes Center (DDZ), the German Federal Ministry of Education and Research (BMBF) to DZD e. V., the European Funds for Regional Development (EFRE-0400191), EUREKA Eurostars-2 (E. 113230 DIA-PEP), the German Science Foundation (DFG; CRC/SFB 1116/2 B12) and the Schmutzler Stiftung.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, made a substantial contribution to discussion of content, and wrote and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.R. is on the scientific advisory boards of Allergan, Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly, Gilead Sciences, Inventiva, Intercept Pharma, Novartis, NovoNordisk, Servier Laboratories, Target Pharmasolutions, and Terra Firma and receives investigator-initiated support from Boehringer Ingelheim, Nutricia/Danone and Sanofi–Aventis. K.C. is on the scientific advisory boards for Bristol-Myers Squibb and NovoNordisk and has received grant funding from Boehringer-Ingelheim, Bristol-Myers Squibb and Novartis. G.T. and C.D.B. declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Geoff Farrell, Vincent Wong and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Targher, G., Corey, K.E., Byrne, C.D. et al. The complex link between NAFLD and type 2 diabetes mellitus — mechanisms and treatments. Nat Rev Gastroenterol Hepatol 18, 599–612 (2021). https://doi.org/10.1038/s41575-021-00448-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-021-00448-y
This article is cited by
-
Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: a systematic review and meta-analysis
BMC Medicine (2024)
-
Advances in management of metabolic dysfunction-associated steatotic liver disease: from mechanisms to therapeutics
Lipids in Health and Disease (2024)
-
Evaluating the coding accuracy of type 2 diabetes mellitus among patients with non-alcoholic fatty liver disease
BMC Health Services Research (2024)
-
Inter-organ crosstalk during development and progression of type 2 diabetes mellitus
Nature Reviews Endocrinology (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)