Abstract
Normal eating behaviour is coordinated by the tightly regulated balance between intestinal and extra-intestinal homeostatic and hedonic mechanisms. By contrast, food addiction is a complex, maladaptive eating behaviour that reflects alterations in brain–gut–microbiome (BGM) interactions and a shift of this balance towards hedonic mechanisms. Each component of the BGM axis has been implicated in the development of food addiction, with both brain to gut and gut to brain signalling playing a role. Early-life influences can prime the infant gut microbiome and brain for food addiction, which might be further reinforced by increased antibiotic usage and dietary patterns throughout adulthood. The ubiquitous availability and marketing of inexpensive, highly palatable and calorie-dense food can further shift this balance towards hedonic eating through both central (disruptions in dopaminergic signalling) and intestinal (vagal afferent function, metabolic endotoxaemia, systemic immune activation, changes to gut microbiome and metabolome) mechanisms. In this Review, we propose a systems biology model of BGM interactions, which incorporates published reports on food addiction, and provides novel insights into treatment targets aimed at each level of the BGM axis.
Key points
-
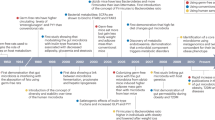
Food addiction refers to maladaptive ingestive behaviours resulting from a shift from primarily homeostatic to hedonic regulatory mechanisms of food intake; this shift reflects alterations at all levels of the brain–gut–microbiome (BGM) axis.
-
Normal ingestive behaviour is the result of the tightly regulated interplay between orexigenic and anorexigenic gut hormones, leptin signalling from adipose tissue, hypothalamic nuclei, the dopaminergic reward system and prefrontal inhibitory influences.
-
In food addiction, a disinhibition of reward and anorexigenic mechanisms at all levels of the BGM axis results in unrestrained craving for food.
-
Several adverse early-life events, including nutrition, stress and antibiotic intake, can influence the development of BGM interactions and of ingestive behaviour.
-
Lifelong dietary choices can modulate BGM interactions and eating behaviours; for example, chronic ingestion of a Western diet can result in systemic low-grade immune system activation, reducing feedback inhibitory mechanisms restraining food intake.
-
Pharmacological treatment options for food addition are limited and bariatric surgery is the only therapy providing long-term benefits; however, novel treatment approaches, including time-restricted eating and cognitive behavioural interventions, are being evaluated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Centers for Disease Control and Prevention Overweight & Obesity http://www.cdc.gov/obesity/data/adult.html (2014).
World Health Organization Obesity and Overweight http://www.who.int/mediacentre/factsheets/fs311/en/ (2016).
State of Childhood Obesity Obesity Rates & Trend Data http://stateofobesity.org/rates/ (2016).
Biener, A., Cawley, J. & Meyerhoefer, C. The high and rising costs of obesity to the US health care system. J. Gen. Intern. Med. 32, S6–S8 (2017).
Mancini, M. C. & de Melo, M. E. The burden of obesity in the current world and the new treatments available: focus on liraglutide 3.0 mg. Diabetol. Metab. Syndr. 9, 44 (2017).
Zhang, Y. et al. Obesity: pathophysiology and intervention. Nutrients 6, 5153–5183 (2014).
Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376, 254–266 (2017).
Osadchiy, V., Martin, C. R. & Mayer, E. A. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 17, 322–332 (2019).
Mayer, E. A. et al. Functional GI disorders: from animal models to drug development. Gut 57, 384–404 (2008).
Keita, A. V. & Soderholm, J. D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 22, 718–733 (2010).
Yu, M. et al. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 138, 231–239 (2017).
Moreira, C. G. et al. Bacterial adrenergic sensors regulate virulence of enteric pathogens in the gut. mBio 7, e00826–16 (2016).
Houlden, A. et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 57, 10–20 (2016).
Sovran, B. et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 9, 1437 (2019).
Barrett, E., Ross, R. P., O’Toole, P. W., Fitzgerald, G. F. & Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417 (2012).
Shishov, V. A., Kirovskaia, T. A., Kudrin, V. S. & Oleskin, A. V. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikl. Biokhim Mikrobiol. 45, 550–554 (2009).
Asano, Y. et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol 303, G1288–G1295 (2012).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
McLoughlin, R. F., Berthon, B. S., Jensen, M. E., Baines, K. J. & Wood, L. G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am. J. Clin. Nutr. 106, 930–945 (2017).
Byrne, C. S. et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 104, 5–14 (2016).
Lal, S., Kirkup, A. J., Brunsden, A. M., Thompson, D. G. & Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol 281, G907–G915 (2001).
Diaz Heijtz, R. Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal Med. 21, 410–417 (2016).
Bliss, E. S. & Whiteside, E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front. Physiol. 9, 900 (2018).
Torres-Fuentes, C., Schellekens, H., Dinan, T. G. & Cryan, J. F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2, 747–756 (2017).
Ochoa-Reparaz, J. & Kasper, L. H. The second brain: is the gut microbiota a link between obesity and central nervous system disorders? Curr. Obes. Rep. 5, 51–64 (2016).
Buhmann, H., le Roux, C. W. & Bueter, M. The gut-brain axis in obesity. Best. Pract. Res. Clin. Gastroenterol. 28, 559–571 (2014).
Myers, M. G. Jr., Leibel, R. L., Seeley, R. J. & Schwartz, M. W. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol. Metab. 21, 643–651 (2010).
Guyenet, S. J. & Schwartz, M. W. Clinical review: regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J. Clin. Endocrinol. Metab. 97, 745–755 (2012).
Rossi, M. A. & Stuber, G. D. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 27, 42–56 (2018).
Volkow, N. D., Wang, G. J., Fowler, J. S., Tomasi, D. & Baler, R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 11, 1–24 (2012).
Volkow, N. D., Wang, G. J., Tomasi, D. & Baler, R. D. Obesity and addiction: neurobiological overlaps. Obes. Rev. 14, 2–18 (2013).
Lindgren, E. et al. Food addiction: a common neurobiological mechanism with drug abuse. Front. Biosci. 23, 811–836 (2018).
Gearhardt, A. N., Corbin, W. R. & Brownell, K. D. Food addiction: an examination of the diagnostic criteria for dependence. J. Addict. Med. 3, 1–7 (2009).
Gearhardt, A. N., Grilo, C. M., DiLeone, R. J., Brownell, K. D. & Potenza, M. N. Can food be addictive? Public health and policy implications. Addiction 106, 1208–1212 (2011).
Schulte, E. M. & Gearhardt, A. N. Associations of food addiction in a sample recruited to be nationally representative of the United States. Eur. Eat. Disord. Rev. 26, 112–119 (2018).
Schulte, E. M., Potenza, M. N. & Gearhardt, A. N. A commentary on the “eating addiction” versus “food addiction” perspectives on addictive-like food consumption. Appetite 115, 9–15 (2017).
Gearhardt, A. N., Davis, C., Kuschner, R. & Brownell, K. D. The addiction potential of hyperpalatable foods. Curr. Drug Abuse Rev. 4, 140–145 (2011).
Randolph, T. G. The descriptive features of food addiction; addictive eating and drinking. Q. J. Stud. Alcohol. 17, 198–224 (1956).
Meule, A. & Gearhardt, A. N. Food addiction in the light of DSM-5. Nutrients 6, 3653–3671 (2014).
Corsica, J. A. & Pelchat, M. L. Food addiction: true or false? Curr. Opin. Gastroenterol. 26, 165–169 (2010).
Hone-Blanchet, A. & Fecteau, S. Overlap of food addiction and substance use disorders definitions: analysis of animal and human studies. Neuropharmacology 85, 81–90 (2014).
Gearhardt, A. N., Corbin, W. R. & Brownell, K. D. Development of the Yale Food Addiction Scale version 2.0. Psychol. Addict. Behav. 30, 113–121 (2016).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 5th edn (American Psychiatric Association, 2013).
Sengor, G. & Gezer, C. Food addiction and its relationship with disordered eating behaviours and obesity. Eat. Weight Disord. 24, 1031–1039 (2019).
Penzenstadler, L., Soares, C., Karila, L. & Khazaal, Y. Systematic review of food addiction as measured with the Yale Food Addiction Scale: implications for the food addiction construct. Curr. Neuropharmacol. 17, 526–538 (2019).
Burrows, T., Kay-Lambkin, F., Pursey, K., Skinner, J. & Dayas, C. Food addiction and associations with mental health symptoms: a systematic review with meta-analysis. J. Hum. Nutr. Diet. 31, 544–572 (2018).
Volkow, N. D., Wang, G. J., Tomasi, D. & Baler, R. D. The addictive dimensionality of obesity. Biol. Psychiatry 73, 811–818 (2013).
Chen, M., Sun, Y., Lu, L. & Shi, J. Similarities and differences in neurobiology. Adv. Exp. Med. Biol. 1010, 45–58 (2017).
Kalon, E., Hong, J. Y., Tobin, C. & Schulte, T. Psychological and neurobiological correlates of food addiction. Int. Rev. Neurobiol. 129, 85–110 (2016).
DiLeone, R. J., Taylor, J. R. & Picciotto, M. R. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat. Neurosci. 15, 1330–1335 (2012).
Rogers, P. J. Food and drug addictions: similarities and differences. Pharmacol. Biochem. Behav. 153, 182–190 (2017).
Ouellette, A. S. et al. Establishing a food addiction diagnosis using the Yale Food Addiction Scale: a closer look at the clinically significant distress/functional impairment criterion. Appetite 129, 55–61 (2018).
Davis, C. et al. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 57, 711–717 (2011).
Meule, A. How prevalent is “food addiction”? Front. Psychiatry 2, 61 (2011).
Avena, N. M., Gearhardt, A. N., Gold, M. S., Wang, G. J. & Potenza, M. N. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat. Rev. Neurosci. 13, 514 (2012).
Ziauddeen, H. & Fletcher, P. C. Is food addiction a valid and useful concept? Obes. Rev 14, 19–28 (2013).
Ziauddeen, H., Farooqi, I. S. & Fletcher, P. C. Obesity and the brain: how convincing is the addiction model? Nat. Rev. Neurosci. 13, 279–286 (2012).
Muller, A. et al. Food addiction and other addictive behaviours in bariatric surgery candidates. Eur. Eat. Disord. Rev. 26, 585–596 (2018).
Sevincer, G. M., Konuk, N., Bozkurt, S. & Coskun, H. Food addiction and the outcome of bariatric surgery at 1-year: prospective observational study. Psychiatry Res. 244, 159–164 (2016).
Gearhardt, A. N., Boswell, R. G. & White, M. A. The association of “food addiction” with disordered eating and body mass index. Eat. Behav. 15, 427–433 (2014).
Gearhardt, A. N., White, M. A. & Potenza, M. N. Binge eating disorder and food addiction. Curr. Drug Abuse Rev. 4, 201–207 (2011).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Wren, A. M. et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50, 2540–2547 (2001).
Jiang, H., Betancourt, L. & Smith, R. G. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol. Endocrinol. 20, 1772–1785 (2006).
Shah, M. & Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 15, 181–187 (2014).
Karra, E., Chandarana, K. & Batterham, R. L. The role of peptide YY in appetite regulation and obesity. J. Physiol. 587, 19–25 (2009).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Cani, P. D. et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 90, 1236–1243 (2009).
Parnell, J. A. & Reimer, R. A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 89, 1751–1759 (2009).
Rodin, J., Wack, J., Ferrannini, E. & DeFronzo, R. A. Effect of insulin and glucose on feeding behavior. Metabolism 34, 826–831 (1985).
Jiao, N. et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol. Genomics 50, 244–254 (2018).
Perry, R. J. et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Fang, S. et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 21, 159–165 (2015).
Pathak, P. et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588 (2018).
Vrieze, A. et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 60, 824–831 (2014).
Leitao-Goncalves, R. et al. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 15, e2000862 (2017).
Ribeiro, C. & Dickson, B. J. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 20, 1000–1005 (2010).
Storelli, G. et al. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 14, 403–414 (2011).
Kahathuduwa, C. N., Boyd, L. A., Davis, T., O’Boyle, M. & Binks, M. Brain regions involved in ingestive behavior and related psychological constructs in people undergoing calorie restriction. Appetite 107, 348–361 (2016).
Berthoud, H. R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr. Opin. Neurobiol. 21, 888–896 (2011).
Simon, J. J. et al. Integration of homeostatic signaling and food reward processing in the human brain. JCI Insight 2, e92970 (2017).
Abizaid, A., Gao, Q. & Horvath, T. L. Thoughts for food: brain mechanisms and peripheral energy balance. Neuron 51, 691–702 (2006).
Gao, Q. & Horvath, T. L. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 30, 367–398 (2007).
Berthoud, H. R., Munzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152, 1728–1738 (2017).
Berthoud, H. R. & Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 59, 55–92 (2008).
Harding, I. H. et al. Brain substrates of unhealthy versus healthy food choices: influence of homeostatic status and body mass index. Int. J. Obes. 42, 448–454 (2018).
Gupta, A. et al. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. NeuroImage. Clin. 7, 506–517 (2015).
Gupta, A. et al. Sex differences in the influence of body mass index on anatomical architecture of brain networks. Int. J. Obes. 41, 1185–1195 (2017).
Kenny, P. J. Reward mechanisms in obesity: new insights and future directions. Neuron 69, 664–679 (2011).
Volkow, N. D., Wang, G. J. & Baler, R. D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 15, 37–46 (2011).
Bartholdy, S., Dalton, B., O’Daly, O. G., Campbell, I. C. & Schmidt, U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci. Biobehav. Rev. 64, 35–62 (2016).
Gearhardt, A. N., Yokum, S., Stice, E., Harris, J. L. & Brownell, K. D. Relation of obesity to neural activation in response to food commercials. Soc. Cogn. Affect. Neurosci. 9, 932–938 (2014).
Steward, T. et al. Food addiction and impaired executive functions in women with obesity. Eur. Eat. Disord. Rev. 26, 574–584 (2018).
Stice, E., Yokum, S., Burger, K. S., Epstein, L. H. & Small, D. M. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci. 31, 4360–4366 (2011).
Olivo, G. et al. Resting-state brain and the FTO obesity risk allele: default mode, sensorimotor, and salience network connectivity underlying different somatosensory integration and reward processing between genotypes. Front. Hum. Neurosci. 10, 52 (2016).
Morrow, J. D., Maren, S. & Robinson, T. E. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav. Brain Res. 220, 238–243 (2011).
Garcia-Garcia, I. et al. Alterations of the salience network in obesity: a resting-state fMRI study. Hum. Brain Mapp. 34, 2786–2797 (2013).
Volkow, N. D. & Baler, R. D. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 38, 345–352 (2015).
Wijngaarden, M. A. et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav. Brain Res. 287, 127–134 (2015).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667 (2010).
Zald, D. H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Brain Res. Rev. 41, 88–123 (2003).
Kilpatrick, L. A. et al. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology 146, 1212–1221 (2014).
Park, B. Y., Chung, C. S., Lee, M. J. & Park, H. Accurate neuroimaging biomarkers to predict body mass index in adolescents: a longitudinal study. Brain Imaging Behav. https://doi.org/10.1007/s11682-019-00101-y (2019).
Meng, Q. et al. Disrupted topological organization of the frontal-mesolimbic network in obese patients. Brain Imaging Behav. 12, 1544–1555 (2018).
Baek, K., Morris, L. S., Kundu, P. & Voon, V. Disrupted resting-state brain network properties in obesity: decreased global and putaminal cortico-striatal network efficiency. Psychol. Med. 47, 585–596 (2017).
Harrold, J. A. & Halford, J. C. The hypothalamus and obesity. Recent. Pat. CNS Drug Discov. 1, 305–314 (2006).
Munzberg, H., Qualls-Creekmore, E., Berthoud, H. R., Morrison, C. D. & Yu, S. Neural control of energy expenditure. Handb. Exp. Pharmacol. 233, 173–194 (2016).
King, B. M. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 87, 221–244 (2006).
Purnell, J. Q., Lahna, D. L., Samuels, M. H., Rooney, W. D. & Hoffman, W. F. Loss of pons-to-hypothalamic white matter tracks in brainstem obesity. Int. J. Obes. 38, 1573–1577 (2014).
Carmo-Silva, S. & Cavadas, C. Hypothalamic dysfunction in obesity and metabolic disorders. Adv. Neurobiol. 19, 73–116 (2017).
Fu, O. et al. Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat. Commun. 10, 4560 (2019).
Zagmutt, S., Mera, P., Soler-Vazquez, M. C., Herrero, L. & Serra, D. Targeting AgRP neurons to maintain energy balance: lessons from animal models. Biochem. Pharmacol. 155, 224–232 (2018).
Morrison, C. D. & Berthoud, H. R. Neurobiology of nutrition and obesity. Nutr. Rev. 65, 517–534 (2007).
Pedram, P. et al. Food addiction: its prevalence and significant association with obesity in the general population. PLoS ONE 8, e74832 (2013).
Chao, A. M. et al. Prevalence and psychosocial correlates of food addiction in persons with obesity seeking weight reduction. Compr. Psychiatry 73, 97–104 (2017).
Pursey, K. M., Stanwell, P., Gearhardt, A. N., Collins, C. E. & Burrows, T. L. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients 6, 4552–4590 (2014).
Eichen, D. M., Lent, M. R., Goldbacher, E. & Foster, G. D. Exploration of “food addiction” in overweight and obese treatment-seeking adults. Appetite 67, 22–24 (2013).
Michaelides, M., Thanos, P. K., Volkow, N. D. & Wang, G. J. Translational neuroimaging in drug addiction and obesity. ILAR J. 53, 59–68 (2012).
Hommel, J. D. et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810 (2006).
Lutter, M. & Nestler, E. J. Homeostatic and hedonic signals interact in the regulation of food intake. J. Nutr. 139, 629–632 (2009).
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S. & Schwartz, M. W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Sinha, R. & Jastreboff, A. M. Stress as a common risk factor for obesity and addiction. Biol. Psychiatry 73, 827–835 (2013).
Sinha, R. Role of addiction and stress neurobiology on food intake and obesity. Biol. Psychol. 131, 5–13 (2018).
Onaolapo, A. Y. & Onaolapo, O. J. Food additives, food and the concept of ‘food addiction’: is stimulation of the brain reward circuit by food sufficient to trigger addiction? Pathophysiology 25, 263–276 (2018).
Stice, E., Spoor, S., Bohon, C., Veldhuizen, M. G. & Small, D. M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol. 117, 924–935 (2008).
Stoeckel, L. E. et al. Effective connectivity of a reward network in obese women. Brain Res. Bull. 79, 388–395 (2009).
Stoeckel, L. E. et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage 41, 636–647 (2008).
Volkow, N. D. et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 26, 6583–6588 (2006).
Blum, K., Thanos, P. K. & Gold, M. S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 5, 919 (2014).
Jastreboff, A. M. et al. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care 36, 394–402 (2013).
Loeber, S. et al. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int. J. Obes. 36, 1334–1339 (2012).
Martin, L. E. et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 18, 254–260 (2010).
Blum, K., Oscar-Berman, M., Barh, D., Giordano, J. & Gold, M. Dopamine genetics and function in food and substance abuse. J. Genet. Syndr. Gene. Ther. 4, 1000121 (2013).
Hardman, C. A., Herbert, V. M., Brunstrom, J. M., Munafo, M. R. & Rogers, P. J. Dopamine and food reward: effects of acute tyrosine/phenylalanine depletion on appetite. Physiol. Behav. 105, 1202–1207 (2012).
Volkow, N. D. et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage 42, 1537–1543 (2008).
Gaiser, E. C. et al. Elevated dopamine D2/3 receptor availability in obese individuals: a PET imaging study with [(11)C](+)PHNO. Neuropsychopharmacology 41, 3042–3050 (2016).
Volkow, N. D., Wang, G. J., Fowler, J. S. & Telang, F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos. Trans. R. Soc. B Biol. Sci. 363, 3191–3200 (2008).
Sinha, R., Gu, P., Hart, R. & Guarnaccia, J. B. Food craving, cortisol and ghrelin responses in modeling highly palatable snack intake in the laboratory. Physiol. Behav. 208, 112563 (2019).
Johnson, P. M. & Kenny, P. J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 13, 635–641 (2010).
Pepino, M. Y. et al. Sweet dopamine: sucrose preferences relate differentially to striatal D2 receptor binding and age in obesity. Diabetes 65, 2618–2623 (2016).
Molteni, R., Barnard, R. J., Ying, Z., Roberts, C. K. & Gomez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 112, 803–814 (2002).
Roessmann, U. & Gambetti, P. Astrocytes in the developing human brain. An immunohistochemical study. Acta Neuropathol. 70, 308–313 (1986).
Zhang, S. C. Defining glial cells during CNS development. Nat. Rev. Neurosci. 2, 840–843 (2001).
Hoban, A. E. et al. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 6, e774 (2016).
Diaz Heijtz, R. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011).
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004).
Zellner, D. A. et al. Food selection changes under stress. Physiol. Behav. 87, 789–793 (2006).
Oliver, G., Wardle, J. & Gibson, E. L. Stress and food choice: a laboratory study. Psychosom. Med. 62, 853–865 (2000).
Epel, E., Lapidus, R., McEwen, B. & Brownell, K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26, 37–49 (2001).
Bose, M., Olivan, B. & Laferrere, B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr. Opin. Endocrinol. Diabetes Obes. 16, 340–346 (2009).
Lee, M. J., Pramyothin, P., Karastergiou, K. & Fried, S. K. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta 1842, 473–481 (2014).
Cong, X., Henderson, W. A., Graf, J. & McGrath, J. M. Early life experience and gut microbiome: the brain-gut-microbiota signaling system. Adv. Neonatal Care 15, 314–323 (2015).
Neuman, H., Forsythe, P., Uzan, A., Avni, O. & Koren, O. Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol. Rev. 42, 489–499 (2018).
Lundgren, S. N. et al. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 6, 109 (2018).
Chu, D. M. et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 8, 77 (2016).
Bhagavata Srinivasan, S. P., Raipuria, M., Bahari, H., Kaakoush, N. O. & Morris, M. J. Impacts of diet and exercise on maternal gut microbiota are transferred to offspring. Front. Endocrinol. 9, 716 (2018).
Hohwu, L., Li, J., Olsen, J., Sorensen, T. I. & Obel, C. Severe maternal stress exposure due to bereavement before, during and after pregnancy and risk of overweight and obesity in young adult men: a Danish National Cohort Study. PLoS ONE 9, e97490 (2014).
Jasarevic, E. et al. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 21, 1061–1071 (2018).
Mueller, N. T. et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 39, 665–670 (2015).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Marcobal, A. & Sonnenburg, J. L. Human milk oligosaccharide consumption by intestinal microbiota. Clin. Microbiol. Infect. 18, 12–15 (2012).
Roger, L. C., Costabile, A., Holland, D. T., Hoyles, L. & McCartney, A. L. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 156, 3329–3341 (2010).
Tamburini, S., Shen, N., Wu, H. C. & Clemente, J. C. The microbiome in early life: implications for health outcomes. Nat. Med. 22, 713–722 (2016).
Hart, A. L. et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 53, 1602–1609 (2004).
O’Sullivan, A., Farver, M. & Smilowitz, J. T. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr. Metab. Insights 8, 1–9 (2015).
Bogen, D. L., Hanusa, B. H. & Whitaker, R. C. The effect of breast-feeding with and without formula use on the risk of obesity at 4 years of age. Obes. Res. 12, 1527–1535 (2004).
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108, 4578–4585 (2011).
Forbes, J. D. et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 172, e181161 (2018).
Yan, J., Liu, L., Zhu, Y., Huang, G. & Wang, P. P. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public. Health 14, 1267 (2014).
Monteiro, C. A. et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public. Health Nutr. 21, 18–26 (2018).
Martinez Steele, E. et al. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 6, e009892 (2016).
Hall, K. D. Did the food environment cause the obesity epidemic? Obesity 26, 11–13 (2018).
Sadeghirad, B., Duhaney, T., Motaghipisheh, S., Campbell, N. R. & Johnston, B. C. Influence of unhealthy food and beverage marketing on children’s dietary intake and preference: a systematic review and meta-analysis of randomized trials. Obes. Rev. 17, 945–959 (2016).
Uribe, R. & Fuentes-Garcia, A. The effects of TV unhealthy food brand placement on children. Its separate and joint effect with advertising. Appetite 91, 165–172 (2015).
Hicks, L. A., Taylor, T. H. Jr. & Hunkler, R. J. U.S. outpatient antibiotic prescribing, 2010. N. Engl. J. Med. 368, 1461–1462 (2013).
Stark, C. M., Susi, A., Emerick, J. & Nylund, C. M. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut 68, 62–69 (2019).
Yassour, M. et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl Med. 8, 343ra381 (2016).
Ajslev, T. A., Andersen, C. S., Gamborg, M., Sorensen, T. I. & Jess, T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int. J. Obes. 35, 522–529 (2011).
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Candon, S. et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 10, e0125448 (2015).
Gaskins, H. R., Collier, C. T. & Anderson, D. B. Antibiotics as growth promotants: mode of action. Anim. Biotechnol. 13, 29–42 (2002).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Ruiz, V. E. et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat. Commun. 8, 518 (2017).
Hemmingsson, E. Early childhood obesity risk factors: socioeconomic adversity, family dysfunction, offspring distress, and junk food self-medication. Curr. Obes. Rep. 7, 204–209 (2018).
Farr, O. M. et al. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr. Metab. Cardiovasc. Dis. 25, 479–488 (2015).
Hemmingsson, E., Johansson, K. & Reynisdottir, S. Effects of childhood abuse on adult obesity: a systematic review and meta-analysis. Obes. Rev. 15, 882–893 (2014).
Forster, G. L., Anderson, E. M., Scholl, J. L., Lukkes, J. L. & Watt, M. J. Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity. Neurobiol. Stress. 9, 29–39 (2018).
Whitesell, N. R. et al. Childhood exposure to adversity and risk of substance-use disorder in two American Indian populations: the meditational role of early substance-use initiation. J. Stud. Alcohol. Drugs 70, 971–981 (2009).
Moffett, M. C. et al. Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol. 73, 321–330 (2007).
Moussaoui, N. et al. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. J. Neurogastroenterol. Motil. 23, 135–143 (2017).
Isohookana, R., Marttunen, M., Hakko, H., Riipinen, P. & Riala, K. The impact of adverse childhood experiences on obesity and unhealthy weight control behaviors among adolescents. Compr. Psychiatry 71, 17–24 (2016).
Windle, M. et al. A multivariate analysis of adverse childhood experiences and health behaviors and outcomes among college students. J. Am. Coll. Health 66, 246–251 (2018).
Campbell, J. A., Farmer, G. C., Nguyen-Rodriguez, S., Walker, R. J. & Egede, L. E. Using path analysis to examine the relationship between sexual abuse in childhood and diabetes in adulthood in a sample of US adults. Prev. Med. 108, 1–7 (2018).
Van Niel, C., Pachter, L. M., Wade, R. Jr., Felitti, V. J. & Stein, M. T. Adverse events in children: predictors of adult physical and mental conditions. J. Dev. Behav. Pediatr. 35, 549–551 (2014).
Osadchiy, V. et al. History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obes. Sci. Pract. 5, 416–436 (2019).
Martin, B. et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology 148, 4318–4333 (2007).
Inam, Q. U., Ikram, H., Shireen, E. & Haleem, D. J. Effects of sugar rich diet on brain serotonin, hyperphagia and anxiety in animal model of both genders. Pak. J. Pharm. Sci. 29, 757–763 (2016).
Osadchiy, V. et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE 13, e0201772 (2018).
Leigh, S. J., Lee, F. & Morris, M. J. Hyperpalatability and the generation of obesity: roles of environment, stress exposure and individual difference. Curr. Obes. Rep. 7, 6–18 (2018).
Chao, A., Grilo, C. M., White, M. A. & Sinha, R. Food cravings, food intake, and weight status in a community-based sample. Eat. Behav. 15, 478–482 (2014).
Mozaffarian, D., Hao, T., Rimm, E. B., Willett, W. C. & Hu, F. B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 364, 2392–2404 (2011).
Schulte, E. M., Joyner, M. A., Potenza, M. N., Grilo, C. M. & Gearhardt, A. N. Current considerations regarding food addiction. Curr. Psychiatry Rep. 17, 563 (2015).
Nunes-Neto, P. R. et al. Food addiction: prevalence, psychopathological correlates and associations with quality of life in a large sample. J. Psychiatr. Res. 96, 145–152 (2018).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Xu, Z. & Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 113, S1–S5 (2015).
English, L., Lasschuijt, M. & Keller, K. L. Mechanisms of the portion size effect. What is known where do we go here? Appetite 88, 39–49 (2015).
Kerr, M. A., McCann, M. T. & Livingstone, M. B. Food and the consumer: could labelling be the answer? Proc. Nutr. Soc. 74, 158–163 (2015).
Lucan, S. C., Maroko, A. R., Sanon, O. C. & Schechter, C. B. Unhealthful food-and-beverage advertising in subway stations: targeted marketing, vulnerable groups, dietary intake, and poor health. J. Urban. Health 94, 220–232 (2017).
Coates, A. E., Hardman, C. A., Halford, J. C. G., Christiansen, P. & Boyland, E. J. Social media influencer marketing and children’s food intake: a randomized trial. Pediatrics 143, e20182554 (2019).
Norman, J. et al. Sustained impact of energy-dense TV and online food advertising on children’s dietary intake: a within-subject, randomised, crossover, counter-balanced trial. Int. J. Behav. Nutr. Phys. Act. 15, 37 (2018).
Folkvord, F., Anschutz, D. J., Wiers, R. W. & Buijzen, M. The role of attentional bias in the effect of food advertising on actual food intake among children. Appetite 84, 251–258 (2015).
Deglaire, A. et al. Associations between weight status and liking scores for sweet, salt and fat according to the gender in adults (the Nutrinet-Sante study). Eur. J. Clin. Nutr. 69, 40–46 (2015).
Geiker, N. R. W. et al. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes. Rev. 19, 81–97 (2018).
Chao, A., Grilo, C. M., White, M. A. & Sinha, R. Food cravings mediate the relationship between chronic stress and body mass index. J. Health Psychol. 20, 721–729 (2015).
Sinha, R. Chronic stress, drug use, and vulnerability to addiction. Ann. NY Acad. Sci. 1141, 105–130 (2008).
Chao, A. M., Jastreboff, A. M., White, M. A., Grilo, C. M. & Sinha, R. Stress, cortisol, and other appetite-related hormones: prospective prediction of 6-month changes in food cravings and weight. Obesity 25, 713–720 (2017).
Dallman, M. F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 21, 159–165 (2010).
Christiansen, A. M., Dekloet, A. D., Ulrich-Lai, Y. M. & Herman, J. P. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol. Behav. 103, 111–116 (2011).
Ulrich-Lai, Y. M. et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc. Natl Acad. Sci. USA 107, 20529–20534 (2010).
Bharwani, A. et al. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 63, 217–227 (2016).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
Gheorghe, C. E. et al. Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 48, 137–145 (2019).
Osadchiy, V., Martin, C. R. & Mayer, E. A. Gut microbiome and modulation of CNS function. Compr. Physiol. 10, 57–72 (2019).
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G. & Cryan, J. F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48 (2015).
Wikoff, W. R. et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl Acad. Sci. USA 106, 3698–3703 (2009).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Kim, D. Y. & Camilleri, M. Serotonin: a mediator of the brain-gut connection. Am. J. Gastroenterol. 95, 2698–2709 (2000).
Hood, S. D., Bell, C. J. & Nutt, D. J. Acute tryptophan depletion. Part I: rationale and methodology. Aust. N. Z. J. Psychiatry 39, 558–564 (2005).
Pagoto, S. L. et al. Acute tryptophan depletion and sweet food consumption by overweight adults. Eat. Behav. 10, 36–41 (2009).
Roager, H. M. & Licht, T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9, 3294 (2018).
Bender, D. A. Biochemistry of tryptophan in health and disease. Mol. Asp. Med. 6, 101–197 (1983).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H. Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012).
Marin, I. A. et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 7, 43859 (2017).
Stavrum, A. K., Heiland, I., Schuster, S., Puntervoll, P. & Ziegler, M. Model of tryptophan metabolism, readily scalable using tissue-specific gene expression data. J. Biol. Chem. 288, 34555–34566 (2013).
Favennec, M. et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 23, 2066–2074 (2015).
Kennedy, P. J., Cryan, J. F., Dinan, T. G. & Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112, 399–412 (2017).
Chimerel, C. et al. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 9, 1202–1208 (2014).
Mangge, H. et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity 22, 195–201 (2014).
Buckman, L. B. et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav. Immun. 35, 33–42 (2014).
Teixeira, T. F., Collado, M. C., Ferreira, C. L., Bressan, J. & Peluzio, M. do C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 32, 637–647 (2012).
Sonnenburg, E. D. et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016).
Rasoamanana, R., Even, P. C., Darcel, N., Tome, D. & Fromentin, G. Dietary fibers reduce food intake by satiation without conditioned taste aversion in mice. Physiol. Behav. 110-111, 13–19 (2013).
Tailford, L. E., Crost, E. H., Kavanaugh, D. & Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 6, 81 (2015).
Shen, W. et al. Intestinal and systemic inflammatory responses are positively associated with sulfidogenic bacteria abundance in high-fat-fed male C57BL/6J mice. J. Nutr. 144, 1181–1187 (2014).
Ding, S. et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 5, e12191 (2010).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Chang, M., Alsaigh, T., Kistler, E. B. & Schmid-Schonbein, G. W. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS ONE 7, e40087 (2012).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Wang, Q., Liu, D., Song, P. & Zou, M. H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. 20, 1116–1143 (2015).
Larraufie, P., Dore, J., Lapaque, N. & Blottiere, H. M. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol. 19, e12648 (2017).
Palazzo, M. et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J. Immunol. 178, 4296–4303 (2007).
Kidd, M., Gustafsson, B. I., Drozdov, I. & Modlin, I. M. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol. Motil. 21, 439–450 (2009).
de Lartigue, G., Ronveaux, C. C. & Raybould, H. E. Vagal plasticity the key to obesity. Mol. Metab. 3, 855–856 (2014).
de Lartigue, G., Barbier de la Serre, C., Espero, E., Lee, J. & Raybould, H. E. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am. J. Physiol. Endocrinol. Metab. 301, E187–E195 (2011).
de Lartigue, G., de La Serre, C. B. & Raybould, H. E. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 105, 100–105 (2011).
Qin, Y. et al. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 19, 7 (2018).
Cani, P. D. et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50, 2374–2383 (2007).
Peterli, R. et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA 319, 255–265 (2018).
Chang, S. H. et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 149, 275–287 (2014).
Scholtz, S. et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 63, 891–902 (2014).
Pepino, M. Y. et al. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 22, E13–E20 (2014).
Sanmiguel, C. et al. Bariatric surgery is associated with changes in the brain’s reward system architecture and eating behaviors. Gastroenterology 150, S824 (2016).
Kanerva, N., Larsson, I., Peltonen, M., Lindroos, A. K. & Carlsson, L. M. Changes in total energy intake and macronutrient composition after bariatric surgery predict long-term weight outcome: findings from the Swedish Obese Subjects (SOS) study. Am. J. Clin. Nutr. 106, 136–145 (2017).
Konttinen, H., Peltonen, M., Sjostrom, L., Carlsson, L. & Karlsson, J. Psychological aspects of eating behavior as predictors of 10-y weight changes after surgical and conventional treatment of severe obesity: results from the Swedish Obese Subjects intervention study. Am. J. Clin. Nutr. 101, 16–24 (2015).
Makaronidis, J. M. et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 107, 93–105 (2016).
Pepino, M. Y., Stein, R. I., Eagon, J. C. & Klein, S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity 22, 1792–1798 (2014).
Basso, N. et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg. Endosc. 25, 3540–3550 (2011).
le Roux, C. W. et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 246, 780–785 (2007).
Faulconbridge, L. F. et al. Changes in neural responsivity to highly palatable foods following Roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: an fMRI study. Obesity 24, 1054–1060 (2016).
Li, J. V. et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 60, 1214–1223 (2011).
Li, M. et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl Acad. Sci. USA 105, 2117–2122 (2008).
Arora, T. et al. Roux-en-Y gastric bypass surgery induces early plasma metabolomic and lipidomic alterations in humans associated with diabetes remission. PLoS ONE 10, e0126401 (2015).
Gralka, E. et al. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 102, 1313–1322 (2015).
Ryan, K. K. et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188 (2014).
Coveleskie, K. et al. The effect of the GLP-1 analogue exenatide on functional connectivity within an NTS-based network in women with and without obesity. Obes. Sci. Pract. 3, 434–445 (2017).
Braas, D. et al. Dynamic changes in gut microbial derived indole and phenol products after bariatric surgery and its relationship to weight loss. Gastroenterology 154, S158 (2018).
Jacobs, J. et al. Glutamate and hedonic eating: role of the brain-gut-microbiome axis on changes on hedonic eating after bariatric surgery. Gastroenterology 154, s201 (2018).
Lee, C. J. et al. Changes in gut microbiome after bariatric surgery versus medical weight loss in a pilot randomized trial. Obes. Surg. 29, 3239–3245 (2019).
Guo, Y. et al. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur. J. Endocrinol. 178, 43–56 (2018).
Luijten, J., Vugts, G., Nieuwenhuijzen, G. A. P. & Luyer, M. D. P. The importance of the microbiome in bariatric surgery: a systematic review. Obes. Surg. 29, 2338–2349 (2019).
Monte, S. V. et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 151, 587–593 (2012).
Iannelli, A., Anty, R., Schneck, A. S., Tran, A. & Gugenheim, J. Inflammation, insulin resistance, lipid disturbances, anthropometrics, and metabolic syndrome in morbidly obese patients: a case control study comparing laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy. Surgery 149, 364–370 (2011).
Iannelli, A. et al. Body composition, anthropometrics, energy expenditure, systemic inflammation, in premenopausal women 1 year after laparoscopic Roux-en-Y gastric bypass. Surg. Endosc. 28, 500–507 (2014).
Yadav, R. et al. Effect of Roux-en-Y bariatric surgery on lipoproteins, insulin resistance, and systemic and vascular inflammation in obesity and diabetes. Front. Immunol. 8, 1512 (2017).
Peng, Y., Li, J. Z., You, M. & Murr, M. M. Roux-en-Y gastric bypass improves glucose homeostasis, reduces oxidative stress and inflammation in livers of obese rats and in Kupffer cells via an AMPK-dependent pathway. Surgery 162, 59–67 (2017).
Lindegaard, K. K., Jorgensen, N. B., Just, R., Heegaard, P. M. & Madsbad, S. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol. Metab. Syndr. 7, 12 (2015).
van de Sande-Lee, S. et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 60, 1699–1704 (2011).
Blackburn, A. N., Hajnal, A. & Leggio, L. The gut in the brain: the effects of bariatric surgery on alcohol consumption. Addict. Biol. 22, 1540–1553 (2017).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012).
Kootte, R. S. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619 (2017).
Dailey, F. E., Turse, E. P., Daglilar, E. & Tahan, V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr. Opin. Pharmacol. 49, 29–33 (2019).
Longo, V. D. & Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 (2016).
Melkani, G. C. & Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 595, 3691–3700 (2017).
Di Francesco, A., Di Germanio, C., Bernier, M. & de Cabo, R. A time to fast. Science 362, 770–775 (2018).
Kohsaka, A. et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 6, 414–421 (2007).
Hatori, M. et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 (2012).
Gill, S. & Panda, S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 22, 789–798 (2015).
Thaiss, C. A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014).
Racz, B., Duskova, M., Starka, L., Hainer, V. & Kunesova, M. Links between the circadian rhythm, obesity and the microbiome. Physiol. Res. 67, S409–S420 (2018).
Ara, R. et al. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technol. Assess. 16, 1–195 (2012).
Shin, J. H. & Gadde, K. M. Clinical utility of phentermine/topiramate (Qsymia) combination for the treatment of obesity. Diabetes Metab. Syndr. Obes. 6, 131–139 (2013).
Hainer, V. & Aldhoon-Hainerova, I. Tolerability and safety of the new anti-obesity medications. Drug. Saf. 37, 693–702 (2014).
Billes, S. K., Sinnayah, P. & Cowley, M. A. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol. Res. 84, 1–11 (2014).
Wellman, P. J. & Maher, T. J. Synergistic interactions between fenfluramine and phentermine. Int. J. Obes. Relat. Metab. Disord. 23, 723–732 (1999).
Lam, D. D. et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149, 1323–1328 (2008).
McElroy, S. L. et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol. Psychiatry 61, 1039–1048 (2007).
Anderberg, R. H. et al. Glucagon-like peptide 1 and its analogs act in the dorsal raphe and modulate central serotonin to reduce appetite and body weight. Diabetes 66, 1062–1073 (2017).
Wang, Z. et al. Gut microbiome differences between metformin- and liraglutide-treated T2DM subjects. Endocrinol. Diabetes Metab. 1, e00009 (2018).
Foster, D., Sanchez-Collins, S. & Cheskin, L. J. Multidisciplinary team-based obesity treatment in patients with diabetes: current practices and the state of the science. Diabetes Spectr. 30, 244–249 (2017).
Cooper, Z. & Fairburn, C. G. A new cognitive behavioural approach to the treatment of obesity. Behav. Res. Ther. 39, 499–511 (2001).
Klumpp, H., Fitzgerald, D. A., Angstadt, M., Post, D. & Phan, K. L. Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychol. Med. 44, 3109–3121 (2014).
Jensen, K. B. et al. Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153, 1495–1503 (2012).
Seminowicz, D. A. et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain. 14, 1573–1584 (2013).
Brunoni, A. R. et al. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J. Affect. Disord. 162, 43–49 (2014).
Clay, S. W., Allen, J. & Parran, T. A review of addiction. Postgrad. Med. 120, E01–E07 (2008).
Labus, J. et al. Randomised clinical trial: symptoms of the irritable bowel syndrome are improved by a psycho-education group intervention. Aliment. Pharmacol. Ther. 37, 304–315 (2013).
An, H., He, R. H., Zheng, Y. R. & Tao, R. Cognitive-behavioral therapy. Adv. Exp. Med. Biol. 1010, 321–329 (2017).
Sawamoto, R. et al. Predictors of successful long-term weight loss maintenance: a two-year follow-up. Biopsychosoc. Med. 11, 14 (2017).
O’reilly, G. A., Cook, L., Spruijt-Metz, D. & Black, D. S. Mindfulness-based interventions for obesity-related eating behaviours: a literature review. Obes. Rev. 15, 453–461 (2014).
Lappalainen, R. et al. The effectiveness and applicability of different lifestyle interventions for enhancing wellbeing: the study design for a randomized controlled trial for persons with metabolic syndrome risk factors and psychological distress. BMC Public Health 14, 310 (2014).
Cani, P. D. & Everard, A. Talking microbes: when gut bacteria interact with diet and host organs. Mol. Nutr. Food Res. 60, 58–66 (2016).
Acknowledgements
We acknowledge C. P. Sanmiguel for her contributions in making editorial suggestions to the gut-directed therapies section of this review and C. Liu for invaluable editorial services. E.A.M. has been supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK048351, DK064539 and DK096606). A.G. has been supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK106528) and CURE at the University of California, Los Angeles/Clinical and Translational Science Institute (ULTR001881/DK041301).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.A.M. serves on the scientific advisory boards of Amare, APC Microbiome Ireland, Axial Biotherapeutics, Bloom Science, Danone, Mahana Therapeutics, Pendulum and Viome. A.G. and V.O. declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks R. Brown, E. Jerlhag, P. Kenny and L. Leggio for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Systems biology
-
An interdisciplinary field of study that focuses on complex interactions within multiple biological systems, rather than focusing on individual mechanisms.
- Hedonic-driven eating behaviour
-
The continued consumption of highly palatable foods even after energy requirements have been met (also known as ‘food addiction’).
- Dopaminergic reward system
-
The extensive network of neurons in the extended reward network that depend on dopamine as the primary neurotransmitter for reward-related processing.
- Extended reward network
-
A network comprising interconnecting brain networks such as reward and salience networks, associated with processing of reward stimuli and modulation of food-seeking behaviours (used interchangeably with ‘greater reward system’).
- Neural substrates
-
A brain region or network associated with a specific behaviour.
- Cortical performance monitoring
-
Processes associated with reward sensitivity, motivation, interoceptive awareness, stress reactivity and self-control.
- Nucleus accumbens
-
Region of the basal ganglia and a key hub for the core reward system, responsible for many dopaminergic processes, especially those related to pleasure, motivation and aversion.
- Ventral tegmental area
-
Key region of the midbrain that houses the dopaminergic cell bodies that project to all regions of the core and extended reward network.
- Salience network
-
The brain network responsible for monitoring the homeostatic state of the body to make adaptive adjustments to real or expected disturbances in homeostasis through the autonomic nervous system and behavioural responses.
- Corticostriatal communication
-
The extensive communication network between the cortex, which houses the extended reward network (including the frontal cortex and insula) and the striatum, which houses the core reward network (nucleus accumbens, basal ganglia).
- Prebiotic
-
Dietary fibre or other substrates that can only be digested by commensal gut microorganisms, thereby promoting gut microbiota diversity and health.
- Maladaptive coping
-
Behaviours used to cope with stressful situations to alleviate the stress or symptoms, but are not necessarily healthy and do not address the core cause of the stress.
- Psychosocial stress
-
Stress originating from the environment that is sufficient to cause dysregulation of homeostatic responses and physical or psychological symptoms.
- Perceived stress
-
Stress from events in an individual’s life perceived as stressful. The most widely used scale for perceived stress is the Perceived Stress Scale.
Rights and permissions
About this article
Cite this article
Gupta, A., Osadchiy, V. & Mayer, E.A. Brain–gut–microbiome interactions in obesity and food addiction. Nat Rev Gastroenterol Hepatol 17, 655–672 (2020). https://doi.org/10.1038/s41575-020-0341-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-0341-5
This article is cited by
-
The relationship between psychological distress and weight maintenance in weight cycling: mediating role of eating behavior
BMC Public Health (2024)
-
Impact of Bacillus licheniformis from yaks following antibiotic therapy in mouse model
Applied Microbiology and Biotechnology (2024)
-
Effects of probiotic supplementation with weight reducing intervention on anthropometric measures, body composition, eating behavior, and related hormone levels in patients with food addiction and weight regain after bariatric surgery: a study protocol for a randomized clinical trial
BMC Nutrition (2023)
-
Identification of adipose tissue transcriptomic memory of anorexia nervosa
Molecular Medicine (2023)
-
Metagenomic analysis reveals distinct changes in the gut microbiome of obese Chinese children
BMC Genomics (2023)