Abstract
One quarter of the global population is estimated to have nonalcoholic fatty liver disease (NAFLD). The incidence of nonalcoholic steatohepatitis (NASH) is projected to increase by up to 56% in the next 10 years. NAFLD is already the fastest growing cause of hepatocellular carcinoma (HCC) in the USA, France and the UK. Globally, the prevalence of NAFLD-related HCC is likely to increase concomitantly with the growing obesity epidemic. The estimated annual incidence of HCC ranges from 0.5% to 2.6% among patients with NASH cirrhosis. The incidence of HCC among patients with non-cirrhotic NAFLD is lower, approximately 0.1 to 1.3 per 1,000 patient-years. Although the incidence of NAFLD-related HCC is lower than that of HCC of other aetiologies such as hepatitis C, more people have NAFLD than other liver diseases. Urgent measures that increase global awareness and tackle the metabolic risk factors are necessary to reduce the impending burden of NAFLD-related HCC. Emerging evidence indicates that reduced immune surveillance, increased gut inflammation and gut dysbiosis are potential key steps in tumorigenesis. In this Review, we discuss the global epidemiology, projections and risk factors for NAFLD-related HCC, and propose preventive strategies to tackle this growing problem.
Key points
-
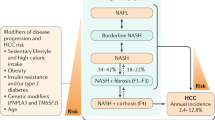
Nonalcoholic fatty liver disease (NAFLD) includes simple steatosis and nonalcoholic steatohepatitis (NASH); NASH can be progressive and predisposes individuals to the development of fibrosis and cancer.
-
NAFLD-related hepatocellular carcinoma (HCC) can develop in the absence of cirrhosis.
-
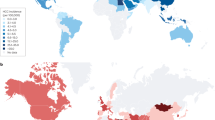
NAFLD is the fastest growing cause of HCC in many parts of the world, including the USA and parts of Europe.
-
The incidence of NAFLD-related HCC is projected to increase dramatically by 2030, with increases of 82%, 117% and 122% from 2016 in China, France and the USA, respectively.
-
Diabetes is the most important risk factor for HCC development in patients with NAFLD; thus, screening and early treatment are essential.
-
Dysregulation of the gut microbiota and reduced immune surveillance are two new mechanisms that have been implicated in NAFLD hepatocarcinogenesis, and further research is warranted.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease–meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016). This meta-analysis of studies from 1989 to 2015 reported that the global prevalence of NAFLD is 25%.
Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 686–690 (2013).
Zhou, F. et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology 70, 1119–1133 (2019).
Li, J. et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 4, 389–398 (2019).
Adams, L. A. et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129, 113–121 (2005).
White, D. L., Kanwal, F. & El-Serag, H. B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 10, 1342–1359.e2 (2012).
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018). This modelling study projected a rapid increase in incidence and prevalence of NAFLD-related HCC in the USA, Europe and China by 2030.
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Baffy, G., Brunt, E. M. & Caldwell, S. H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 56, 1384–1391 (2012).
Eslam, M., Sanyal, A. J. & George, J., & International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158, 1999–2014.e1 (2020).
Younossi, Z. M. et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology https://doi.org/10.1002/hep.31420 (2020).
Global Burden of Disease Liver Cancer Collaboration et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 3, 1683–1691 (2017).
Yang, J. D. et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16, 589–604 (2019).
Global Burden of Disease Cancer Collaboration et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 5, 1749–1768 (2019).
Henley, S. J. et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 126, 2225–2249 (2020).
Younossi, Z. M. et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62, 1723–1730 (2015). This article reported a 9% yearly increase in NAFLD-related HCC prevalence in the USA from 2004 to 2009.
Stine, J. G. et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment. Pharmacol. Ther. 48, 696–703 (2018). This meta-analysis of 19 studies and 168,571 individuals with NASH reported that the prevalence of NAFLD-related HCC in patients with NASH but without cirrhosis is approximately 38% compared with 14% for other liver diseases.
Desai, A., Sandhu, S., Lai, J.-P. & Sandhu, D. S. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J. Hepatol. 11, 1–18 (2019).
Ward, Z. J. et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381, 2440–2450 (2019).
Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 131, 1–8 (2013).
Younossi, Z. et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 17, 748–755.e3 (2019).
Cho, E. J. et al. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion 84, 17–22 (2011).
Liew, Z.-H., Goh, G. B.-B., Hao, Y., Chang, P.-E. & Tan, C.-K. Comparison of hepatocellular carcinoma in patients with cryptogenic versus hepatitis B etiology: a study of 1079 cases over 3 decades. Dig. Dis. Sci. 64, 585–590 (2019).
Dyson, J. et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 60, 110–117 (2014). This study from the UK showed a substantial increase in the proportion of NAFLD-related HCC from <10% in 2000 to 34.8% in 2010.
Pais, R. et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment. Pharmacol. Ther. 46, 856–863 (2017).
Ascha, M. S. et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 51, 1972–1978 (2010).
Sanyal, A. J. et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 43, 682–689 (2006).
Bhala, N. et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 54, 1208–1216 (2011).
Yang, J. D. et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology 71, 907–916 (2020).
Kanwal, F. et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155, 1828–1837.e2 (2018).
Yatsuji, S. et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 24, 248–254 (2009).
Thrift, A. P., El-Serag, H. B. & Kanwal, F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat. Rev. Gastroenterol. Hepatol. 14, 122–132 (2017).
Amarapurkar, D. N., Dharod, M., Gautam, S. & Patel, N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop. Gastroenterol. 34, 159–163 (2013).
Mittal, S. et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 13, 594–601.e1 (2015).
Sanyal, A., Poklepovic, A., Moyneur, E. & Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 26, 2183–2191 (2010).
Mittal, S. et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 14, 124–131.e1 (2016).
Tateishi, R. et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J. Gastroenterol. 50, 350–360 (2015).
Piscaglia, F. et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 63, 827–838 (2016).
Yasui, K. et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 9, 428–433 (2011).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370 (2017).
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Alexander, M. et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 17, 95 (2019).
Kawamura, Y. et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 107, 253–261 (2012).
Ito, T. et al. Utility and limitations of noninvasive fibrosis markers for predicting prognosis in biopsy-proven Japanese non-alcoholic fatty liver disease patients. J. Gastroenterol. Hepatol. 34, 207–214 (2019).
Seko, Y. et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol. Res. 47, 1083–1092 (2017).
Kim, G.-A. et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 68, 140–146 (2018).
Lee, T.-Y. et al. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int. J. Cancer 141, 1307–1314 (2017).
Alexander, M. et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 16, 130 (2018).
Mercado-Irizarry, A. & Torres, E. A. Cryptogenic cirrhosis: current knowledge and future directions. Clin. Liver Dis. 7, 69–72 (2016).
Caldwell, S. & Marchesini, G. Cryptogenic vs. NASH-cirrhosis: the rose exists well before its name... J. Hepatol. 68, 391–392 (2018).
Thuluvath, P. J., Kantsevoy, S., Thuluvath, A. J. & Savva, Y. Is cryptogenic cirrhosis different from NASH cirrhosis? J. Hepatol. 68, 519–525 (2018).
Blüher, M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298 (2019).
Ganslmayer, M. et al. A large cohort of patients with hepatocellular carcinoma in a single European centre: aetiology and prognosis now and in a historical cohort. Swiss Med. Wkly. 144, w13900 (2014).
Aljumah, A. A. et al. Clinical presentation, risk factors, and treatment modalities of hepatocellular carcinoma: a single tertiary care center experience. Gastroenterol. Res. Pract. 2016, 1989045 (2016).
Yapali, S. & Tozun, N. Epidemiology and viral risk factors for hepatocellular carcinoma in the Eastern Mediterranean countries. Hepatoma Res. 4, 24 (2018).
Yang, J. D. et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol. Hepatol. 2, 103–111 (2017).
Paul, S. B. et al. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology 77, 162–171 (2009).
Patkar, S., Parray, A., Mahendra, B., Kurunkar, S. & Goel, M. Performance of Hong Kong liver cancer staging system in patients of hepatocellular carcinoma treated with surgical resection: an Indian validation study. J. Surg. Oncol. 120, 1119–1125 (2019).
Yuen, M.-F., Hou, J.-L. & Chutaputti, A., Asia Pacific Working Party on Prevention of Hepatocellular Carcinoma. Hepatocellular carcinoma in the Asia Pacific region. J. Gastroenterol. Hepatol. 24, 346–353 (2009).
Goh, K.-L. et al. Liver cancer in Malaysia: epidemiology and clinical presentation in a multiracial Asian population. J. Dig. Dis. 16, 152–158 (2015).
Jasirwan, C. O. M. et al. Risk factors of mortality in the patients with hepatocellular carcinoma: a multicenter study in Indonesia. Curr. Probl. Cancer 44, 100480 (2019).
Somboon, K., Siramolpiwat, S. & Vilaichone, R.-K. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac. J. Cancer Prev. 15, 3567–3570 (2014).
Wong, R. J., Cheung, R. & Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the US. Hepatology 59, 2188–2195 (2014).
Heffernan, A., Cooke, G. S., Nayagam, S., Thursz, M. & Hallett, T. B. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet 393, 1319–1329 (2019).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Hanumanthappa, N. et al. Epidemiology, clinical treatment patterns, and survival of hepatocellular carcinoma in Manitoba. Can. Liv. J. 3, 194–202 (2020).
Swain, M. G. et al. Burden of nonalcoholic fatty liver disease in Canada, 2019-2030: a modelling study. CMAJ Open 8, E429–E436 (2020).
Debes, J. D. et al. Hepatocellular carcinoma in South America: evaluation of risk factors, demographics and therapy. Liver Int. 38, 136–143 (2018).
Fassio, E. et al. Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann. Hepatol. 9, 63–69 (2010).
Park, J.-W. et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 35, 2155–2166 (2015).
van der Poorten, D. et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology 57, 2180–2188 (2013).
Tokushige, K., Hashimoto, E., Horie, Y., Taniai, M. & Higuchi, S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J. Gastroenterol. 46, 1230–1237 (2011).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71, 793–801 (2019).
Ma, C. et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257 (2016).
Shalapour, S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017). This study demonstrated in a mouse model that IgA cells accumulated in patients with NASH and suppressed CD8+ T cells, which reduced immune surveillance and promoted hepatocarcinogenesis.
Davila, J. A., Morgan, R. O., Shaib, Y., McGlynn, K. A. & El-Serag, H. B. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 54, 533–539 (2005).
El-Serag, H. B., Hampel, H. & Javadi, F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 4, 369–380 (2006).
Kanwal, F. et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 71, 808–819 (2020). In this study of 271,906 patients with NAFLD diagnosed between 2004 and 2008, diabetes had the strongest association with HCC development (adjusted HR 2.77, 95% CI 2.03–3.77) among the metabolic risk factors.
Naugler, W. E. et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 (2007).
Chen, Y., Wang, X., Wang, J., Yan, Z. & Luo, J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur. J. Cancer 48, 2137–2145 (2012).
Saunders, D., Seidel, D., Allison, M. & Lyratzopoulos, G. Systematic review: the association between obesity and hepatocellular carcinoma – epidemiological evidence. Aliment. Pharmacol. Ther. 31, 1051–1063 (2010).
Hassan, M. M. et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology 149, 119–129 (2015).
Nair, S., Mason, A., Eason, J., Loss, G. & Perrillo, R. P. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology 36, 150–155 (2002).
Petrick, J. L. et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br. J. Cancer 118, 1005–1012 (2018).
Abdel-Rahman, O. et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J. Evid. Based Med. 10, 245–254 (2017).
Miele, L. et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49, 1877–1887 (2009).
Zhang, H.-L. et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J. Hepatol. 57, 803–812 (2012).
Sharpton, S. R., Ajmera, V. & Loomba, R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin. Gastroenterol. Hepatol. 17, 296–306 (2019).
Luther, J. et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell. Mol. Gastroenterol. Hepatol. 1, 222–232 (2015).
Gäbele, E. et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 55, 1391–1399 (2011).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Meng, Z. et al. FXR regulates liver repair after CCl4-induced toxic injury. Mol. Endocrinol. 24, 886–897 (2010).
Yang, F. et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 67, 863–867 (2007).
Fickert, P. et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am. J. Pathol. 175, 2392–2405 (2009).
Ponziani, F. R. et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 69, 107–120 (2019). This study from Italy demonstrated that Akkermansia and Bifidobacterium species are decreased in patients with NAFLD-related HCC compared with patients with NASH cirrhosis, highlighting that dysregulation of the gut microbiome might influence NAFLD-related hepatocarcinogenesis.
Wu, W. et al. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front. Microbiol. 8, 1804 (2017).
Fang, D. et al. Bifidobacterium pseudocatenulatum LI09 and Bifidobacterium catenulatum LI10 attenuate D-galactosamine-induced liver injury by modifying the gut microbiota. Sci. Rep. 7, 8770 (2017).
Stender, S. & Loomba, R. PNPLA3 genotype and risk of liver and all-cause mortality. Hepatology 71, 777–779 (2020).
Hassan, M. M. et al. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: risk and prognosis prediction. Mol. Carcinog. 52, E139–E147 (2013).
Singal, A. G. et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am. J. Gastroenterol. 109, 325–334 (2014).
Liu, Y.-L. et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 61, 75–81 (2014).
Liu, Y.-L. et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 5, 4309 (2014).
Donati, B. et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 7, 4492 (2017).
Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68, 723–750 (2018).
Loomba, R., Lim, J. K., Patton, H. & El-Serag, H. B. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 158, 1822–1830 (2020).
Eslam, M. et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. https://doi.org/10.1007/s12072-020-10094-2 (2020).
Simmons, O. et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 45, 169–177 (2017).
Morgan, T. A. et al. US LI-RADS: ultrasound liver imaging reporting and data system for screening and surveillance of hepatocellular carcinoma. Abdom. Radiol. 43, 41–55 (2018).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Chitturi, S. et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017–Part 2: management and special groups. J. Gastroenterol. Hepatol. 33, 86–98 (2018).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378.e5 (2015).
Promrat, K. et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51, 121–129 (2010).
Koutoukidis, D. A. et al. Association of weight loss interventions with changes in biomarkers of nonalcoholic fatty liver disease: a systematic review and meta-analysis. JAMA Intern. Med. 179, 1262–1271 (2019).
Lee, Y. et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 17, 1040–1060.e11 (2019).
Demierre, M.-F., Higgins, P. D. R., Gruber, S. B., Hawk, E. & Lippman, S. M. Statins and cancer prevention. Nat. Rev. Cancer 5, 930–942 (2005).
Singh, S., Singh, P. P., Singh, A. G., Murad, M. H. & Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144, 323–332 (2013).
Thrift, A. P., Natarajan, Y., Liu, Y. & El-Serag, H. B. Statin use after diagnosis of hepatocellular carcinoma is associated with decreased mortality. Clin. Gastroenterol. Hepatol. 17, 2117–2125.e3 (2019).
Cholesterol Treatment Trialists’ (CTT) Collaboration et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 7, e29849 (2012).
Blandino, G. et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 3, 865 (2012).
Singh, S., Singh, P. P., Singh, A. G., Murad, M. H. & Sanchez, W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am. J. Gastroenterol. 108, 881–891 (2013).
Ma, S., Zheng, Y., Xiao, Y., Zhou, P. & Tan, H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine 96, e6888 (2017).
Zhou, Y.-Y. et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci. Rep. 6, 33743 (2016).
Suissa, S. & Azoulay, L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35, 2665–2673 (2012).
Tseng, C.-H. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 38, 2018–2027 (2018).
Sitia, G. et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc. Natl Acad. Sci. USA 109, E2165–E2172 (2012).
Sahasrabuddhe, V. V. et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J. Natl Cancer Inst. 104, 1808–1814 (2012).
Singh, P. & Singh, S. Re: nonsteroidal antiinflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J. Natl Cancer Inst. 105, 666–667 (2013).
Simon, T. G. et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 4, 1683–1690 (2018).
Ioannou, G. N., Green, P., Lowy, E., Mun, E. J. & Berry, K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS ONE 13, e0204412 (2018).
Paranaguá-Vezozzo, D. C. et al. Epidemiology of HCC in Brazil: incidence and risk factors in a ten-year cohort. Ann. Hepatol. 13, 386–393 (2014).
Hsiang, J. C. et al. Epidemiology, disease burden and outcomes of cirrhosis in a large secondary care hospital in South Auckland, New Zealand. Intern. Med. J. 45, 160–169 (2015).
Kimura, T. et al. Mild drinking habit is a risk factor for hepatocarcinogenesis in non-alcoholic fatty liver disease with advanced fibrosis. World J. Gastroenterol. 24, 1440–1450 (2018).
Wong, V. W.-S. et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: a prospective cohort study. Hepatology 63, 754–763 (2016).
Lopes, F. et al. Influence of hepatocellular carcinoma etiology in the survival after resection. Arq. Bras. Cir. Dig. 29, 105–108 (2016).
Raptis, I., Koskinas, J., Emmanouil, T. & Hadziyannis, S. Changing relative roles of hepatitis B and C viruses in the aetiology of hepatocellular carcinoma in Greece. Epidemiological and clinical observations. J. Viral Hepat. 10, 450–454 (2003).
Liu, P.-H. et al. Hong Kong liver cancer staging system is associated with better performance for hepatocellular carcinoma: special emphasis on viral etiology. Medicine 94, e1772 (2015).
Hong, T. P. et al. Novel population-based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology 63, 1205–1212 (2016).
Acknowledgements
The authors acknowledge K.H. Sippel and S.M. Kim for reading the manuscript and providing comments. R.L. receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835), and DOD PRCRP (W81XWH-18-2-0026). D.Q.H. receives funding support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship and Exxon Mobil-NUS Research Fellowship for Clinicians. H.B.E.-S. receives funding support from the Department of Veterans Affairs (5I01CX001616-04), the Cancer Prevention and Research Institute of Texas (grant RP150587) and the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK 56338).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made a substantial contribution to discussion of content, wrote the article, and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.L. serves as a consultant or advisory board member for Anylam/Regeneron, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Inipharm, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Promethera, Sagimet, 89 bio, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer Ingelheim, Bristol Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer, pH Pharma, and Siemens. He is also co-founder of Liponexus, Inc.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks K. Tokushige and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, D.Q., El-Serag, H.B. & Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 18, 223–238 (2021). https://doi.org/10.1038/s41575-020-00381-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-00381-6
This article is cited by
-
Discovery of dual rho-associated protein kinase 1 (ROCK1)/apoptosis signal–regulating kinase 1 (ASK1) inhibitors as a novel approach for non-alcoholic steatohepatitis (NASH) treatment
BMC Chemistry (2024)
-
PARD3 drives tumorigenesis through activating Sonic Hedgehog signalling in tumour-initiating cells in liver cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
Topical rhubarb charcoal-crosslinked chitosan/silk fibroin sponge scaffold for the repair of diabetic ulcers improves hepatic lipid deposition in db/db mice via the AMPK signalling pathway
Lipids in Health and Disease (2024)
-
Liver fat volume fraction measurements based on multi-material decomposition algorithm in patients with nonalcoholic fatty liver disease: the influences of blood vessel, location, and iodine contrast
BMC Medical Imaging (2024)
-
Integration of transcriptomic analysis and multiple machine learning approaches identifies NAFLD progression-specific hub genes to reveal distinct genomic patterns and actionable targets
Journal of Big Data (2024)