Abstract
Chronic liver injury leads to liver inflammation and fibrosis, through which activated myofibroblasts in the liver secrete extracellular matrix proteins that generate the fibrous scar. The primary source of these myofibroblasts are the resident hepatic stellate cells. Clinical and experimental liver fibrosis regresses when the causative agent is removed, which is associated with the elimination of these activated myofibroblasts and resorption of the fibrous scar. Understanding the mechanisms of liver fibrosis regression could identify new therapeutic targets to treat liver fibrosis. This Review summarizes studies of the molecular mechanisms underlying the reversibility of liver fibrosis, including apoptosis and the inactivation of hepatic stellate cells, the crosstalk between the liver and the systems that orchestrate the recruitment of bone marrow-derived macrophages (and other inflammatory cells) driving fibrosis resolution, and the interactions between various cell types that lead to the intracellular signalling that induces fibrosis or its regression. We also discuss strategies to target hepatic myofibroblasts (for example, via apoptosis or inactivation) and the myeloid cells that degrade the matrix (for example, via their recruitment to fibrotic liver) to facilitate fibrosis resolution and liver regeneration.
Key points
-
Most chronic liver diseases, such as hepatitis C virus infection or non-alcoholic hepatic steatohepatitis, can progress to liver fibrosis with the formation of a fibrous scar.
-
Experimental and clinical liver fibrosis regresses with the removal of the aetiological agent or with new therapeutic interventions.
-
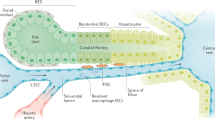
Chronic liver injury leads to activation of hepatic stellate cells, the major source of the fibrous scar in liver fibrosis.
-
Hepatic stellate cells have four known phenotypes — quiescent, activated, inactivated and senescent — each of which has a critical role in liver fibrosis and its regression.
-
During regression of liver fibrosis, activated hepatic stellate cells can undergo apoptosis or revert to an inactivated phenotype; the inactivated cells have a phenotype that is similar to but distinct from quiescent hepatic stellate cells.
-
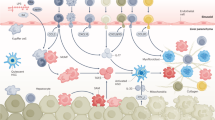
Macrophages can promote fibrogenesis by the secretion of TGFβ and other agonists, but they also support the regression of fibrosis through the secretion of collagenases that resorb the fibrous scar.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Friedman, S. L. Liver fibrosis – from bench to bedside. J. Hepatol. 38 (Suppl. 1), S38–S53 (2003).
Bataller, R. & Brenner, D. A. Liver fibrosis. J. Clin. Invest. 115, 209–218 (2005).
Lo, R. C. & Kim, H. Histopathological evaluation of liver fibrosis and cirrhosis regression. Clin. Mol. Hepatol. 23, 302–307 (2017).
Troeger, J. S. et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143, 1073–1083.e22 (2012).
Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl Acad. Sci. USA 109, 9448–9453 (2012).
Iredale, J. P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 (1998).
Lu, M. et al. Serum biomarkers indicate long-term reduction in liver fibrosis in patients with sustained virological response to treatment for HCV infection. Clin. Gastroenterol. Hepatol. 14, 1044–1055.e3 (2016).
Kisseleva, T. & Brenner, D. A. Mechanisms of fibrogenesis. Exp. Biol. Med. 233, 109–122 (2008).
Schmitt-Graff, A., Kruger, S., Bochard, F., Gabbiani, G. & Denk, H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am. J. Pathol. 138, 1233–1242 (1991).
Kisseleva, T. & Brenner, D. A. Hepatic stellate cells and the reversal of fibrosis. J. Gastroenterol. Hepatol. 21 (Suppl. 3), S84–S87 (2006).
Kalluri, R. & Neilson, E. G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112, 1776–1784 (2003).
Gomperts, B. N. & Strieter, R. M. Fibrocytes in lung disease. J. Leukoc. Biol. 82, 449–456 (2007).
Fallowfield, J. A. et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 178, 5288–5295 (2007).
Taura, K. et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology 51, 1027–1036 (2010).
Scholten, D. et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 139, 987–998 (2010).
Ueno, T. et al. Hepatic stellate cells and intralobular innervation in human liver cirrhosis. Hum. Pathol. 28, 953–959 (1997).
Goddard, C. J. et al. Localisation and semiquantitative assessment of hepatic procollagen mRNA in primary biliary cirrhosis. Gut 43, 433–440 (1998).
Iwaisako, K. et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl Acad. Sci. USA 111, E3297–E3305 (2014).
Nishio, T. et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J. Hepatol. 71, 573–585 (2019).
Dranoff, J. A. & Wells, R. G. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 51, 1438–1444 (2010).
Zavadil, J. et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc. Natl Acad. Sci. USA 98, 6686–6691 (2001).
Chu, A. S. et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53, 1685–1695 (2011).
Russo, F. P. et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 130, 1807–1821 (2006).
Kallis, Y. N. & Forbes, S. J. The bone marrow and liver fibrosis: friend or foe? Gastroenterology 137, 1218–1221 (2009).
Short, B. J., Brouard, N. & Simmons, P. J. Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol. Biol. 482, 259–268 (2009).
Simmons, P. J., Przepiorka, D., Thomas, E. D. & Torok-Storb, B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature 328, 429–432 (1987).
Song, L. & Tuan, R. S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 18, 980–982 (2004).
Hashimoto, N., Jin, H., Liu, T., Chensue, S. W. & Phan, S. H. Bone marrow-derived progenitor cells in pulmonary fibrosis. J. Clin. Invest. 113, 243–252 (2004).
Iredale, J. P. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 117, 539–548 (2007).
Thorgeirsson, S. S. & Grisham, J. W. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology 43, 2–8 (2006).
Alison, M. R., Islam, S. & Lim, S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J. Pathol. 217, 282–298 (2009).
Kisseleva, T. et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 45, 429–438 (2006).
Kharaziha, P. et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur. J. Gastroenterol. Hepatol. 21, 1199–1205 (2009).
Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 21, 311–335 (2001).
Senoo, H., Kojima, N. & Sato, M. Vitamin A-storing cells (stellate cells). Vitam. Horm. 75, 131–159 (2007).
Hazra, S. et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J. Biol. Chem. 279, 11392–11401 (2004).
She, H., Xiong, S., Hazra, S. & Tsukamoto, H. Adipogenic transcriptional regulation of hepatic stellate cells. J. Biol. Chem. 280, 4959–4967 (2005).
Koyama, Y. et al. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J. Clin. Invest. 127, 1254–1270 (2017).
Xu, F., Liu, C., Zhou, D. & Zhang, L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 64, 157–167 (2016).
Dooley, S. et al. Transforming growth factor beta signal transduction in hepatic stellate cells via Smad2/3 phosphorylation, a pathway that is abrogated during in vitro progression to myofibroblasts. TGFβ signal transduction during transdifferentiation of hepatic stellate cells. FEBS Lett. 502, 4–10 (2001).
Meng, F. et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776.e3 (2012).
Liu, Y. et al. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-beta-independent Smad signaling. J. Immunol. 187, 2814–2823 (2011).
Kordes, C., Sawitza, I., Gotze, S., Herebian, D. & Haussinger, D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J. Clin. Invest. 124, 5503–5515 (2014).
Lua, I., James, D., Wang, J., Wang, K. S. & Asahina, K. Mesodermal mesenchymal cells give rise to myofibroblasts, but not epithelial cells, in mouse liver injury. Hepatology 60, 311–322 (2014).
Zhu, C. et al. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci. Transl Med. 10, eaat0344 (2018).
Wang, X. et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 24, 848–862 (2016).
Lan, T., Kisseleva, T. & Brenner, D. A. Deficiency of NOX1 or NOX4 prevents liver inflammation and fibrosis in mice through inhibition of hepatic stellate cell activation. PLoS ONE 10, e0129743 (2015).
Xie, G. et al. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology 58, 1801–1813 (2013).
Lee, Y. S. et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci. Rep. 7, 3710 (2017).
Mridha, A. R. et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 66, 1037–1046 (2017).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Meier, A. et al. Inhibition of human neutrophil extracellular trap (NET) production by propofol and lipid emulsion. Front. Pharmacol. 10, 323 (2019).
Saijou, E. et al. Neutrophils alleviate fibrosis in the CCl4-induced mouse chronic liver injury model. Hepatol. Commun. 2, 703–717 (2018).
Moles, A. et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J. Hepatol. 60, 782–791 (2014).
Gehrke, N. et al. Loss of cellular FLICE-inhibitory protein promotes acute cholestatic liver injury and inflammation from bile duct ligation. Am. J. Physiol. Gastrointest. Liver Physiol. 314, G319–G333 (2018).
Gao, B. & Bataller, R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585 (2011).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Krenkel, O. & Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 17, 306–321 (2017).
Hellerbrand, C., Stefanovic, B., Giordano, F., Burchardt, E. R. & Brenner, D. A. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J. Hepatol. 30, 77–87 (1999).
de Gouville, A. C. et al. Inhibition of TGF-β signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br. J. Pharmacol. 145, 166–177 (2005).
Bonniaud, P. et al. TGF-β and Smad3 signaling link inflammation to chronic fibrogenesis. J. Immunol. 175, 5390–5395 (2005).
Kulkarni, A. B. & Karlsson, S. Inflammation and TGF beta 1: lessons from the TGF beta 1 null mouse. Res. Immunol. 148, 453–456 (1997).
Miura, K. et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139, 323–334.e7 (2010).
Sudo, K., Yamada, Y., Moriwaki, H., Saito, K. & Seishima, M. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 29, 236–244 (2005).
Liu, C. et al. Transcriptional repression of the transforming growth factor beta (TGF-beta) Pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by Nuclear Factor kappaB (NF-kappaB) p50 enhances TGF-beta signaling in hepatic stellate cells. J. Biol. Chem. 289, 7082–7091 (2014).
Kolls, J. K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Zenewicz, L. A. et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659 (2007).
Radaeva, S., Sun, R., Pan, H. N., Hong, F. & Gao, B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39, 1332–1342 (2004).
Baeck, C. et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61, 416–426 (2012).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012).
Iredale, J. P. Hepatic stellate cell behavior during resolution of liver injury. Semin. Liver Dis. 21, 427–436 (2001).
Shetty, S., Lalor, P. F. & Adams, D. H. Liver sinusoidal endothelial cells - gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 15, 555–567 (2018).
Xie, G. et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 142, 918–927.e6 (2012).
Deleve, L. D., Wang, X. & Guo, Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48, 920–930 (2008).
DeLeve, L. D., Wang, X., Hu, L., McCuskey, M. K. & McCuskey, R. S. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G757–G763 (2004).
Maretti-Mira, A. C., Wang, X., Wang, L. & DeLeve, L. D. Incomplete differentiation of engrafted bone marrow endothelial progenitor cells initiates hepatic fibrosis in the rat. Hepatology 69, 1259–1272 (2019).
Ding, B. S. et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505, 97–102 (2014).
Desmouliere, A. et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab. Invest. 76, 765–778 (1997).
Bucala, R., Spiegel, L. A., Chesney, J., Hogan, M. & Cerami, A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1, 71–81 (1994).
Abe, R., Donnelly, S. C., Peng, T., Bucala, R. & Metz, C. N. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 166, 7556–7562 (2001).
Kisseleva, T. & Brenner, D. A. Fibrogenesis of parenchymal organs. Proc. Am. Thorac. Soc. 5, 338–342 (2008).
Strieter, R. M., Gomperts, B. N. & Keane, M. P. The role of CXC chemokines in pulmonary fibrosis. J. Clin. Invest. 117, 549–556 (2007).
Karhadkar, T. R., Pilling, D., Cox, N. & Gomer, R. H. Sialidase inhibitors attenuate pulmonary fibrosis in a mouse model. Sci. Rep. 7, 15069 (2017).
Quan, T. E., Cowper, S., Wu, S. P., Bockenstedt, L. K. & Bucala, R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int. J. Biochem. Cell Biol. 36, 598–606 (2004).
Phillips, R. J. et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 114, 438–446 (2004).
Scholten, D. et al. Migration of fibrocytes in fibrogenic liver injury. Am. J. Pathol. 179, 189–198 (2011).
Xu, J. et al. Contribution of bone marrow-derived fibrocytes to liver fibrosis. Hepatobiliary Surg. Nutr. 4, 34–47 (2015).
Kisseleva, T. et al. Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection. J. Mol. Med. 89, 997–1013 (2011).
El-Serag, H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273.e1 (2012).
Tornesello, M. L., Buonaguro, L., Izzo, F. & Buonaguro, F. M. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget 7, 25087–25102 (2016).
Liu, W., Baker, R. D., Bhatia, T., Zhu, L. & Baker, S. S. Pathogenesis of nonalcoholic steatohepatitis. Cell. Mol. Life Sci. 73, 1969–1987 (2016).
Mazzanti, R., Arena, U. & Tassi, R. Hepatocellular carcinoma: Where are we? World J. Exp. Med. 6, 21–36 (2016).
Byass, P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 12, 159 (2014).
Paquissi, F. C. Immunity and fibrogenesis: the role of Th17/IL-17 axis in HBV and HCV-induced chronic hepatitis and progression to cirrhosis. Front. Immunol. 8, 1195 (2017).
Kanda, T., Goto, T., Hirotsu, Y., Moriyama, M. & Omata, M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int. J. Mol. Sci. 20, 1358 (2019).
Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 686–690 (2013).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Di Rosa, M. & Malaguarnera, L. Genetic variants in candidate genes influencing NAFLD progression. J. Mol. Med. 90, 105–118 (2012).
Kim, J. Y. et al. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell 175, 133–145.e15 (2018).
Musso, G. et al. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology 42, 1175–1183 (2005).
Malaguarnera, M., Di Rosa, M., Nicoletti, F. & Malaguarnera, L. Molecular mechanisms involved in NAFLD progression. J. Mol. Med. 87, 679–695 (2009).
Maricic, I. et al. Differential activation of hepatic invariant NKT cell subsets plays a key role in progression of nonalcoholic steatohepatitis. J. Immunol. 201, 3017–3035 (2018).
Jang, C. et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 27, 351–361.e3 (2018).
Stanhope, K. L. et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 119, 1322–1334 (2009).
Seki, E. & Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J. Physiol. 590, 447–458 (2012).
Moschen, A. R., Kaser, S. & Tilg, H. Non-alcoholic steatohepatitis: a microbiota-driven disease. Trends Endocrinol. Metab. 24, 537–545 (2013).
Llorente, C. et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat. Commun. 8, 837 (2017).
Zhou, D. et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 7, 1529 (2017).
Shalapour, S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017).
Teschke, R. Alcoholic liver disease: alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 6, 106 (2018).
O’Shea, R. S., Dasarathy, S., McCullough, A. J., Practice Guideline Committee of the American Association for the Study of Liver Diseases & Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 51, 307–328 (2010).
Lucey, M. R., Mathurin, P. & Morgan, T. R. Alcoholic hepatitis. N. Engl. J. Med. 360, 2758–2769 (2009).
Maltby, J., Wright, S., Bird, G. & Sheron, N. Chemokine levels in human liver homogenates: associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology 24, 1156–1160 (1996).
Dominguez, M. et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 136, 1639–1650 (2009).
Lemmers, A. et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology 49, 646–657 (2009).
Friedman, S. L. Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669 (2008).
Ikenaga, N. et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut 66, 1697–1708 (2017).
Chen, P. et al. Microbiota and alcoholic liver disease. Alcohol. Clin. Exp. Res. 40, 1791–1792 (2016).
Hirschfield, G. M. & Heathcote, E. J. Cholestasis and cholestatic syndromes. Curr. Opin. Gastroenterol. 25, 175–179 (2009).
Wagner, M., Zollner, G. & Trauner, M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin. Liver Dis. 30, 160–177 (2010).
Vavassori, P., Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 183, 6251–6261 (2009).
Fickert, P. et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am. J. Pathol. 175, 2392–2405 (2009).
Gulamhusein, A. F. & Hirschfield, G. M. Primary biliary cholangitis: pathogenesis and therapeutic opportunities. Nat. Rev. Gastroenterol. Hepatol. 17, 93–110 (2020).
Eaton, J. E., Talwalkar, J. A., Lazaridis, K. N., Gores, G. J. & Lindor, K. D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 145, 521–536 (2013).
Feldman, A. G. & Sokol, R. J. Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics. Nat. Rev. Gastroenterol. Hepatol. 16, 346–360 (2019).
Shneider, B. L. et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J. Pediatr. 148, 467–474 (2006).
Ramachandran, P. & Iredale, J. P. Reversibility of liver fibrosis. Ann. Hepatol. 8, 283–291 (2009).
Hammel, P. et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N. Engl. J. Med. 344, 418–423 (2001).
Arthur, M. J. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology 122, 1525–1528 (2002).
Kweon, Y. O. et al. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J. Hepatol. 35, 749–755 (2001).
Dixon, J. B., Bhathal, P. S., Hughes, N. R. & O’Brien, P. E. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology 39, 1647–1654 (2004).
Czaja, A. J. & Carpenter, H. A. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J. Hepatol. 40, 646–652 (2004).
Pares, A., Caballeria, J., Bruguera, M., Torres, M. & Rodes, J. Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. J. Hepatol. 2, 33–42 (1986).
Hafeez, S. & Ahmed, M. H. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J. Obes. 2013, 839275 (2013).
Issa, R. et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126, 1795–1808 (2004).
Issa, R. et al. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 48, 548–557 (2001).
Schnabl, B. et al. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology 34, 89–100 (2001).
Kendall, T. J. et al. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology 49, 901–910 (2009).
Patel, R. et al. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation 104, 317–324 (2001).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Lee, C. G. et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J. Exp. Med. 200, 377–389 (2004).
Huby, A. C. et al. Restoration of podocyte structure and improvement of chronic renal disease in transgenic mice overexpressing renin. PLoS ONE 4, e6721 (2009).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Schnabl, B., Purbeck, C. A., Choi, Y. H., Hagedorn, C. H. & Brenner, D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology 37, 653–664 (2003).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Collado, M. & Serrano, M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 10, 51–57 (2010).
Takahashi, A. et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun. 9, 1249 (2018).
Kong, X., Feng, D., Mathews, S. & Gao, B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J. Gastroenterol. Hepatol. 28, 56–60 (2013).
Novo, E. et al. Overexpression of Bcl-2 by activated human hepatic stellate cells: resistance to apoptosis as a mechanism of progressive hepatic fibrogenesis in humans. Gut 55, 1174–1182 (2006).
Gao, B., Radaeva, S. & Park, O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J. Leukoc. Biol. 86, 513–528 (2009).
Radaeva, S. et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 130, 435–452 (2006).
Glassner, A. et al. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab. Invest. 92, 967–977 (2012).
Puche, J. E. et al. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology 57, 339–350 (2013).
Parsons, C. J. et al. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology 40, 1106–1115 (2004).
Oakley, F. et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 128, 108–120 (2005).
Jeong, W. I., Park, O., Radaeva, S. & Gao, B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 44, 1441–1451 (2006).
Mohar, I., Brempelis, K. J., Murray, S. A., Ebrahimkhani, M. R. & Crispe, I. N. Isolation of non-parenchymal cells from the mouse liver. Methods Mol. Biol. 1325, 3–17 (2015).
Geissmann, F. et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3, e113 (2005).
Wehr, A. et al. Chemokine receptor CXCR6-dependent hepatic NK T cell accumulation promotes inflammation and liver fibrosis. J. Immunol. 190, 5226–5236 (2013).
Ishikawa, S. et al. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J. Hepatol. 54, 1195–1204 (2011).
Popov, Y. et al. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G323–G334 (2010).
Uchinami, H., Seki, E., Brenner, D. A. & D’Armiento, J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology 44, 420–429 (2006).
Wei, J. et al. IkappaB kinase-beta inhibitor attenuates hepatic fibrosis in mice. World J. Gastroenterol. 17, 5203–5213 (2011).
Kisseleva, T. & Brenner, D. A. Inactivation of myofibroblasts during regression of liver fibrosis. Cell Cycle 12, 381–382 (2013).
Hernandez-Gea, V. et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142, 938–946 (2012).
Kluwe, J. et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut 60, 1260–1268 (2011).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Wells, R. G. The role of matrix stiffness in regulating cell behavior. Hepatology 47, 1394–1400 (2008).
Gaca, M. D. et al. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 22, 229–239 (2003).
Mann, J. et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 138, 705–714 (2010).
Perugorria, M. J. et al. Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast transdifferentiation. Hepatology 56, 1129–1139 (2012).
Liu, X. et al. Identification of lineage-specific transcription factors that prevent activation of hepatic stellate cells and promote fibrosis resolution. Gastroenterology 158, 1728–1744.e14 (2020).
Schuppan, D., Ashfaq-Khan, M., Yang, A. T. & Kim, Y. O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 68–69, 435–451 (2018).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018).
Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14, 397–411 (2017).
Feld, J. J. et al. Sofosbuvir and Velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N. Engl. J. Med. 373, 2599–2607 (2015).
Lynch, S. M. & Wu, G. Y. Hepatitis C virus: a review of treatment guidelines, cost-effectiveness, and access to therapy. J. Clin. Transl Hepatol. 4, 310–319 (2016).
Chen Yi Mei, S. L. G. et al. Sustained virological response halts fibrosis progression: A long-term follow-up study of people with chronic hepatitis C infection. PLoS ONE 12, e0185609 (2017).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378.e5 (2015).
Lassailly, G. et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 149, 379–388 (2015).
Kim, C. W. et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 26, 576 (2017).
Sonoda, J., Chen, M. Z. & Baruch, A. FGF21-receptor agonists: an emerging therapeutic class for obesity-related diseases. Horm. Mol. Biol. Clin. Investig. https://doi.org/10.1515/hmbci-2017-0002 (2017).
Gao, B., Ahmad, M. F., Nagy, L. E. & Tsukamoto, H. Inflammatory pathways in alcoholic steatohepatitis. J. Hepatol. 70, 249–259 (2019).
Canbay, A., Feldstein, A., Baskin-Bey, E., Bronk, S. F. & Gores, G. J. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J. Pharmacol. Exp. Ther. 308, 1191–1196 (2004).
Iwaisako, K. et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proc. Natl Acad. Sci. USA 109, E1369–E1376 (2012).
Zhang, S., Wang, J., Liu, Q. & Harnish, D. C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 51, 380–388 (2009).
Xiang, M. et al. Targeting hepatic TRAF1-ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J. Hepatol. 64, 1365–1377 (2016).
Ratziu, V. et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150, 1147–1159 (2016).
Ratziu, V. et al. REGENERATE: design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 84, 105803 (2019).
Brandl, K. et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J. Hepatol. 69, 396–405 (2018).
Gross, O., Thomas, C. J., Guarda, G. & Tschopp, J. The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 (2011).
Schroder, K., Zhou, R. & Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010).
Ahn, H. et al. Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine 71, 223–231 (2015).
Lamkanfi, M. et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Watanabe, A. et al. Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol 296, G1248–G1257 (2009).
Linton, S. D. Caspase inhibitors: a pharmaceutical industry perspective. Curr. Top. Med. Chem. 5, 1697–1717 (2005).
MacKenzie, S. H., Schipper, J. L. & Clark, A. C. The potential for caspases in drug discovery. Curr. Opin. Drug Discov. Devel. 13, 568–576 (2010).
Shiffman, M. et al. Randomised clinical trial: emricasan versus placebo significantly decreases ALT and caspase 3/7 activation in subjects with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 49, 64–73 (2019).
Mandrekar, P., Ambade, A., Lim, A., Szabo, G. & Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54, 2185–2197 (2011).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489 (2012).
Seki, E. et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 50, 185–197 (2009).
Seki, E. et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 119, 1858–1870 (2009).
Pilling, D. et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J. Immunol. 179, 4035–4044 (2007).
Verna, E. C. et al. Novel association between serum pentraxin-2 levels and advanced fibrosis in well-characterised patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 42, 582–590 (2015).
Gomes, A. L. et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 30, 161–175 (2016).
Hammerich, L., Heymann, F. & Tacke, F. Role of IL-17 and Th17 cells in liver diseases. Clin. Dev. Immunol. 2011, 345803 (2011).
Ivanov, I. I. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Zhang, X., Jin, J., Peng, X., Ramgolam, V. S. & Markovic-Plese, S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J. Immunol. 180, 6988–6996 (2008).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Ma, H. Y. et al. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease. J. Hepatol. 72, 946–959 (2020).
Ki, S. H. et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52, 1291–1300 (2010).
Weston, C. J. et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J. Clin. Invest. 125, 501–520 (2015).
Fan, X. et al. Attenuation of CCl4-induced hepatic fibrosis in mice by vaccinating against TGF-beta1. PLoS ONE 8, e82190 (2013).
Ling, H. et al. Transforming growth factor beta neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS ONE 8, e54499 (2013).
Akhurst, R. J. & Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 11, 790–811 (2012).
Munger, J. S. et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Henderson, N. C. et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Peng, Z. W. et al. Integrin alphavbeta6 critically regulates hepatic progenitor cell function and promotes ductular reaction, fibrosis, and tumorigenesis. Hepatology 63, 217–232 (2016).
Meurer, S. K. et al. Overexpression of endoglin modulates TGF-beta1-signalling pathways in a novel immortalized mouse hepatic stellate cell line. PLoS ONE 8, e56116 (2013).
Blanco, F. J. et al. Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J. Cell Physiol. 204, 574–584 (2005).
Duffy, A. G. et al. Phase I and preliminary phase II study of TRC105 in combination with sorafenib in hepatocellular carcinoma. Clin. Cancer Res. 23, 4633–4641 (2017).
Rancoule, C. et al. Lysophosphatidic acid-1-receptor targeting agents for fibrosis. Expert Opin. Investig. Drugs 20, 657–667 (2011).
Mazzocca, A. et al. Tumor-secreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology 54, 920–930 (2011).
Giannone, F. A. et al. Reversal of liver fibrosis by the antagonism of endocannabinoid CB1 receptor in a rat model of CCl4-induced advanced cirrhosis. Lab. Invest. 92, 384–395 (2012).
Mejias, M. et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 49, 1245–1256 (2009).
Qu, K. et al. New insight into the anti-liver fibrosis effect of multitargeted tyrosine kinase inhibitors: from molecular target to clinical trials. Front. Pharmacol. 6, 300 (2015).
Aoyama, T. et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56, 2316–2327 (2012).
Moreno, M. et al. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology 51, 942–952 (2012).
Yang, L. et al. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J. Hepatol. 43, 317–323 (2005).
Yokohama, S. et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 40, 1222–1225 (2004).
Yoshiji, H. et al. Renin-angiotensin system inhibitors as therapeutic alternatives in the treatment of chronic liver diseases. Curr. Med. Chem. 14, 2749–2754 (2007).
Yoshiji, H. et al. Angiotensin-II induces the tissue inhibitor of metalloproteinases-1 through the protein kinase-C signaling pathway in rat liver fibrosis development. Hepatol. Res. 27, 51–56 (2003).
Abu Dayyeh, B. K., Yang, M., Dienstag, J. L. & Chung, R. T. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT-C trial cohort. Dig. Dis. Sci. 56, 564–568 (2011).
Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 (2010).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Massey, V. L. et al. The hepatic “matrisome” responds dynamically to injury: characterization of transitional changes to the extracellular matrix in mice. Hepatology 65, 969–982 (2017).
Mohammadi, M., Olsen, S. K. & Goetz, R. A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr. Opin. Struct. Biol. 15, 506–516 (2005).
Shi, Y. & Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Rahman, S. et al. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 6, 8 (2005).
Wijelath, E. S. et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ. Res. 99, 853–860 (2006).
Wells, R. G. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J. Clin. Gastroenterol. 39, S158–S161 (2005).
Bollyky, P. L. et al. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J. Leukoc. Biol. 86, 567–572 (2009).
Bollyky, P. L. et al. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J. Immunol. 179, 744–747 (2007).
Meran, S. et al. Involvement of hyaluronan in regulation of fibroblast phenotype. J. Biol. Chem. 282, 25687–25697 (2007).
Webber, J., Meran, S., Steadman, R. & Phillips, A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J. Biol. Chem. 284, 9083–9092 (2009).
Iredale, J. P. & Pellicoro, A. “It is a good morning exercise for a research scientist to discard a pet hypothesis every day before breakfast: it keeps him young” (Konrad Lorenz, 1903–1989). Gastroenterology 140, 1395–1398 (2011).
Hynes, R. O. & Naba, A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 4, a004903 (2012).
Brenner, D. A. et al. New aspects of hepatic fibrosis. J. Hepatol. 32, 32–38 (2000).
Popov, Y. et al. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology 140, 1642–1652 (2011).
Liu, S. B. et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 30, 1599–1609 (2016).
Barry-Hamilton, V. et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 (2010).
Loomba, R. et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 67, 549–559 (2018).
Rowbottom, M. W. et al. Identification of 4-(aminomethyl)-6-(trifluoromethyl)-2-(phenoxy)pyridine derivatives as potent, selective, and orally efficacious inhibitors of the copper-dependent amine oxidase, lysyl oxidase-like 2 (LOXL2). J. Med. Chem. 60, 4403–4423 (2017).
Lu, P., Takai, K., Weaver, V. M. & Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3, a005058 (2011).
Brew, K. & Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta 1803, 55–71 (2010).
Murphy, F. R. et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J. Biol. Chem. 277, 11069–11076 (2002).
Zhang, L. P. et al. Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells. J. Hepatol. 31, 703–711 (1999).
Flevaris, P. & Vaughan, D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin. Thromb. Hemost. 43, 169–177 (2017).
Hu, P. F. et al. Inhibition of plasminogen activator inhibitor-1 expression by siRNA in rat hepatic stellate cells. J. Gastroenterol. Hepatol. 23, 1917–1925 (2008).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Mahady, S. E., Webster, A. C., Walker, S., Sanyal, A. & George, J. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review and meta analysis. J. Hepatol. 55, 1383–1390 (2011).
Duran, A. et al. p62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Cancer Cell 30, 595–609 (2016).
Wahsh, E., Abu-Elsaad, N., El-Karef, A. & Ibrahim, T. The vitamin D receptor agonist, calcipotriol, modulates fibrogenic pathways mitigating liver fibrosis in-vivo: an experimental study. Eur. J. Pharmacol. 789, 362–369 (2016).
Hah, N., Sherman, M. H., Yu, R. T., Downes, M. & Evans, R. M. Targeting transcriptional and epigenetic reprogramming in stromal cells in fibrosis and cancer. Cold Spring Harb. Symp. Quant. Biol. 80, 249–255 (2015).
Ding, N., Liddle, C., Evans, R. M. & Downes, M. Hepatic actions of vitamin D receptor ligands: a sunshine option for chronic liver disease? Expert Rev. Clin. Pharmacol. 6, 597–599 (2013).
Varga, J., Brenner D. & Phan S. E. Fibrosis Research. Methods and Protocols (Humana Press, 2005).
Pellicoro, A. et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology 55, 1965–1975 (2012).
Lee, Y. A., Wallace, M. C. & Friedman, S. L. Pathobiology of liver fibrosis: a translational success story. Gut 64, 830–841 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT00013598.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01672866.
Du, K. et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology 154, 1465–1479.e13 (2018).
Lalor, P. F., Shields, P., Grant, A. & Adams, D. H. Recruitment of lymphocytes to the human liver. Immunol. Cell Biol. 80, 52–64 (2002).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01051219.
Yoshiji, H., Kuriyama, S. & Fukui, H. Blockade of renin-angiotensin system in antifibrotic therapy. J. Gastroenterol. Hepatol. 22 (Suppl. 1), S93–S95 (2007).
Kalluri, R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 119, 1417–1419 (2009).
Okada, H., Danoff, T. M., Kalluri, R. & Neilson, E. G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. 273, F563–F574 (1997).
Zavadil, J., Haley, J., Kalluri, R., Muthuswamy, S. K. & Thompson, E. Epithelial-mesenchymal transition. Cancer Res. 68, 9574–9577 (2008).
Friedman, S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008).
Tuchweber, B., Desmouliere, A., Bochaton-Piallat, M. L., Rubbia-Brandt, L. & Gabbiani, G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab. Invest. 74, 265–278 (1996).
Bosselut, N. et al. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics 10, 1017–1028 (2010).
Dudas, J., Mansuroglu, T., Batusic, D. & Ramadori, G. Thy-1 is expressed in myofibroblasts but not found in hepatic stellate cells following liver injury. Histochem. Cell Biol. 131, 115–127 (2009).
Strieter, R. M., Keeley, E. C., Hughes, M. A., Burdick, M. D. & Mehrad, B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J. Leukoc. Biol. 86, 1111–1118 (2009).
Pilling, D., Fan, T., Huang, D., Kaul, B. & Gomer, R. H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 4, e7475 (2009).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Forbes, S. J. et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology 126, 955–963 (2004).
Harting, M. T., Jimenez, F. & Cox, C. S. Jr. Isolation of mesenchymal stem cells (MSCs) from green fluorescent protein positive (GFP+) transgenic rodents: The grass is not always green(er). Stem Cells Dev. 18, 127–135 (2008).
Wang, Y. et al. TLR4 inhibits mesenchymal stem cell (MSC) STAT3 activation and thereby exerts deleterious effects on MSC-mediated cardioprotection. PLoS ONE 5, e14206 (2010).
Acknowledgements
Supported by the NIH (AA011999, DK101737, AA022614, DK099205, DK111866, AA018663). The authors thank Christopher Glass, Xiao Liu, Karin Diggle and Kevin Lam for help with the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks S. Dooley, N. Henderson and Y. Popov for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kisseleva, T., Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol 18, 151–166 (2021). https://doi.org/10.1038/s41575-020-00372-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-00372-7
This article is cited by
-
miR-541 is associated with the prognosis of liver cirrhosis and directly targets JAG2 to inhibit the activation of hepatic stellate cells
BMC Gastroenterology (2024)
-
Fibroblasts inhibit osteogenesis by regulating nuclear-cytoplasmic shuttling of YAP in mesenchymal stem cells and secreting DKK1
Biological Research (2024)
-
MyD88 in myofibroblasts enhances nonalcoholic fatty liver disease-related hepatocarcinogenesis via promoting macrophage M2 polarization
Cell Communication and Signaling (2024)
-
Clinical applications of stem cell-derived exosomes
Signal Transduction and Targeted Therapy (2024)
-
The crosstalk between exosomes and ferroptosis: a review
Cell Death Discovery (2024)