Abstract
Probiotics and prebiotics are microbiota-management tools for improving host health. They target gastrointestinal effects via the gut, although direct application to other sites such as the oral cavity, vaginal tract and skin is being explored. Here, we describe gut-derived effects in humans. In the past decade, research on the gut microbiome has rapidly accumulated and has been accompanied by increased interest in probiotics and prebiotics as a means to modulate the gut microbiota. Given the importance of these approaches for public health, it is timely to reiterate factual and supporting information on their clinical application and use. In this Review, we discuss scientific evidence on probiotics and prebiotics, including mechanistic insights into health effects. Strains of Lactobacillus, Bifidobacterium and Saccharomyces have a long history of safe and effective use as probiotics, but Roseburia spp., Akkermansia spp., Propionibacterium spp. and Faecalibacterium spp. show promise for the future. For prebiotics, glucans and fructans are well proven, and evidence is building on the prebiotic effects of other substances (for example, oligomers of mannose, glucose, xylose, pectin, starches, human milk and polyphenols).
Key points
-
The human gut microbiota is integral to health and is associated with a variety of diseases.
-
Therapeutic and prophylactic effects of some probiotics and prebiotics for a variety of gut-related disorders might be, at least in part, mediated through modification of the microbiota and/or its function.
-
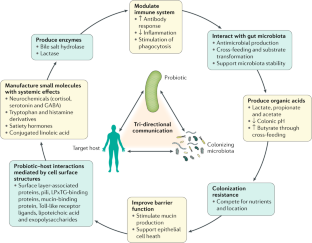
Probiotic microorganisms act via a variety of means, including modulation of immune function, production of organic acids and antimicrobial compounds, interaction with resident microbiota, interfacing with the host, improving gut barrier integrity and enzyme formation.
-
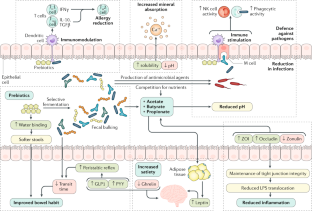
Prebiotics are substrates that are selectively utilized by host microorganisms conferring a health benefit; prebiotic effects include defence against pathogens, immune modulation, mineral absorption, bowel function, metabolic effects and satiety.
-
Use of some probiotics and prebiotics is justified by robust assessments of efficacy, but not all products have been validated; the goal is evidence-based use by healthcare professionals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
09 August 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Food and Agriculture Organization of the United Nations & World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. FAO http://www.fao.org/3/a-a0512e.pdf (2001).
Hill, C. et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014). This Consensus Statement examines the definition, evolution, uses, types and health attributes of probiotics.
Rook, G., Backhed, F., Levin, B. R., McFall-Ngai, M. J. & McLean, A. R. Evolution, human-microbe interactions, and life history plasticity. Lancet 390, 521–530 (2017). This article discusses how some microorganisms have co-evolved with humans and have crucial roles in host physiology and metabolism, whereas others are intrusive.
Reid, G. et al. Expanding the reach of probiotics through social enterprises. Benef. Microbes 9, 707–715 (2018).
Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412 (1995).
Gibson, G. R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017). This Consensus Statement examines the definition, evolution, uses, types and health attributes of prebiotics.
Collins, S. L. et al. Promising prebiotic candidate established by evaluation of lactitol, lactulose, raffinose, and oligofructose for maintenance of a Lactobacillus-dominated vaginal microbiota. Appl. Environ. Microbiol. 84, e02200-17 (2018).
Rodriguez, J. M. et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050 (2015).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 (2016).
Rowland, I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24 (2018).
Thursby, E. & Juge, N. Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836 (2017). This article provides current understanding of the development and composition of the human gut microbiota, and its effects on gut integrity and host health.
Fava, F. et al. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. (Lond.) 37, 216–223 (2013).
Dicks, L. M. T., Geldenhuys, J., Mikkelsen, L. S., Brandsborg, E. & Marcotte, H. Our gut microbiota: a long walk to homeostasis. Benef. Microbes 9, 3–20 (2018).
Dethlefsen, L. & Relman, D. A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. U. S. A. 108 (Suppl. 1), 4554–4561 (2011).
Gagliardi, A. et al. Rebuilding the gut microbiota ecosystem. Int. J. Environ. Res. Public Health 15, E1679 (2018).
Hatton, G. B., Madla, C. M., Rabbie, S. C. & Basit, A. W. All disease begins in the gut: influence of gastrointestinal disorders and surgery on oral drug performance. Int. J. Pharm. 548, 408–422 (2018).
John, G. K. et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel) 9, (E167 (2018).
Yoshida, N., Yamashita, T. & Hirata, K. I. Gut microbiome and cardiovascular diseases. Diseases 6, E56 (2018).
Hansen, L. B. S. et al. A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat. Commun. 9, 4630 (2018).
Parker, R. B. Probiotics, the other half of the antibiotic story. Anim. Nutr. Health 29, 4–8 (1974).
Havenaar, R. & Huis In’t Veld, J. M. J. in Lactic Acid Bacteria in Health and Disease Vol. 1 (ed. Wood, B. J. B.) 151–170 (Elsevier Applied Science Publishers, 1992).
Ng, S. C. et al. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis. Inflamm. Bowel Dis. 16, 1286–1298 (2010).
Mujagic, Z. et al. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci. Rep. 7, 40128 (2017).
Del Piano, M. et al. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: a double-blind, randomized, placebo-controlled study. J. Clin. Gastroenterol. 44 (Suppl. 1), S30–S34 (2010).
Reid, G., Gadir, A. A. & Dhir, R. Probiotics: reiterating what they are and what they are not. Front. Microbiol. 10, 424 (2019).
Cabre, E. & Gassull, M. A. Probiotics for preventing relapse or recurrence in Crohn’s disease involving the ileum: are there reasons for failure? J. Crohns Colitis 1, 47–52 (2007).
Kelly, J. R. et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 61, 50–59 (2017).
Panigrahi, P. et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548, 407–412 (2017).
Costeloe, K. et al. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 387, 649–660 (2016).
Sorbara, M. T. & Pamer, E. G. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 34, 1608 (2018).
Chiu, L. et al. Protective microbiota: from localized to long-reaching co-immunity. Front. Immunol. 8, 1678 (2017).
Maldonado-Gomez, M. X. et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 20, 515–526 (2016).
Murphy, R. et al. Eczema-protective probiotic alters infant gut microbiome functional capacity but not composition: sub-sample analysis from a RCT. Benef. Microbes 10, 5–17 (2019).
Korpela, K. et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 6, 182 (2018).
Clarke, G. et al. Gut reactions: breaking down xenobiotic-microbiome interactions. Pharmacol. Rev. 71, 198–224 (2019).
Klaenhammer, T. R., Kleerebezem, M., Kopp, M. V. & Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 12, 728–734 (2012). Here, four experts discuss probiotics, prebiotics and immunity, then provide their thoughts on the future application as a disease therapy.
Przemska-Kosicka, A. et al. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun. Ageing 13, 6 (2016).
Vitetta, L., Saltzman, E. T., Thomsen, M., Nikov, T. & Hall, S. Adjuvant probiotics and the intestinal microbiome: enhancing vaccines and immunotherapy outcomes. Vaccines (Basel) 5, (E50 (2017).
Childs, C. E. et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br. J. Nutr. 111, 1945–1956 (2014).
Flint, H. J., Duncan, S. H., Scott, K. P. & Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 74, 13–22 (2015).
Aoudia, N. et al. Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol. 53, 51–59 (2016).
Rios-Covian, D. et al. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 7, 185 (2016).
Canfora, E. E., Jocken, J. W. & Blaak, E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591 (2015).
Sanna, S. et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605 (2019).
Stefan, N., Fritsche, A., Schick, F. & Haring, H. U. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol. 4, 789–798 (2016).
van Baarlen, P., Wells, J. M. & Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 34, 208–215 (2013).
Hegarty, J. W., Guinane, C. M., Ross, R. P., Hill, C. & Cotter, P. D. Bacteriocin production: a relatively unharnessed probiotic trait? F1000Res. 5, 2587 (2016).
Mokoena, M. P. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 22, E1255 (2017).
Bali, V., Panesar, P. S., Bera, M. B. & Kennedy, J. F. Bacteriocins: recent trends and potential applications. Crit. Rev. Food Sci. Nutr. 56, 817–834 (2016).
Riviere, A., Selak, M., Lantin, D., Leroy, F. & De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7, 979 (2016).
Abdulkadir, B. et al. Routine use of probiotics in preterm infants: longitudinal impact on the microbiome and metabolome. Neonatology 109, (239–247 (2016).
Fang, H. R., Zhang, G. Q., Cheng, J. Y. & Li, Z. Y. Efficacy of Lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: a meta-analysis of randomized controlled trials. Eur. J. Pediatr. 178, 7–16 (2019).
Sanders, M. E., Benson, A., Lebeer, S., Merenstein, D. J. & Klaenhammer, T. R. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr. Opin. Biotechnol. 49, 207–216 (2018).
Petrova, M. I. et al. Comparative genomic and phenotypic analysis of the vaginal probiotic Lactobacillus rhamnosus GR-1. Front. Microbiol. 9, 1278 (2018).
La Fata, G., Weber, P. & Mohajeri, M. H. Probiotics and the gut immune system: indirect regulation. Probiot. Antimicrob. Proteins 10, 11–21 (2018).
Han, X. et al. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes 10, 59–76 (2019).
Mack, D. R., Michail, S., Wei, S., McDougall, L. & Hollingsworth, M. A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276, G941–G950 (1999).
Yan, F. et al. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J. Biol. Chem. 288, 30742–30751 (2013).
Stadlbauer, V. et al. Lactobacillus casei Shirota supplementation does not restore gut microbiota composition and gut barrier in metabolic syndrome: a randomized pilot study. PLOS ONE 10, e0141399 (2015).
Kim, N., Yun, M., Oh, Y. J. & Choi, H. J. Mind-altering with the gut: modulation of the gut-brain axis with probiotics. J. Microbiol. 56, 172–182 (2018).
Janik, R. et al. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 125, 988–995 (2016).
Reid, G. Disentangling what we know about microbes and mental health. Front. Endocrinol. 10, 81 (2019).
Liang, S. et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577 (2015).
Kotz, C. M., Furne, J. K., Savaiano, D. A. & Levitt, M. D. Factors affecting the ability of a high beta-galactosidase yogurt to enhance lactose absorption. J. Dairy Sci. 77, 3538–3544 (1994).
Costabile, A. et al. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLOS ONE 12, e0187964 (2017).
European Food Safety Authority Panel on Dietetic Products. Scientific opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to article 13(1) of regulation (EC) No 1924/2006. EFSA J. 8, 1763 (2010).
Li, D., Wang, P., Wang, P., Hu, X. & Chen, F. The gut microbiota: a treasure for human health. Biotechnol. Adv. 34, 1210–1224 (2016).
Kasubuchi, M., Hasegawa, S., Hiramatsu, T., Ichimura, A. & Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7, 2839–2849 (2015).
Verbeke, K. A. et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 28, 42–66 (2015).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Fogliano, V. et al. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 55 (Suppl. 1), S44–S55 (2011).
Falony, G. et al. In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by Bifidobacterium species reveals four different phenotypes. Appl. Environ. Microbiol. 75, 454–461 (2009).
Riviere, A., Selak, M., Geirnaert, A., Van den Abbeele, P. & De Vuyst, L. Complementary mechanisms for degradation of inulin-type fructans and arabinoxylan oligosaccharides among bifidobacterial strains suggest bacterial cooperation. Appl. Environ. Microbiol. 84, e02893-17 (2018).
Flint, H. J., Scott, K. P., Duncan, S. H., Louis, P. & Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3, 289–306 (2012).
Hamaker, B. R. & Tuncil, Y. E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 426, 3838–3850 (2014).
Ze, X., Le Mougen, F., Duncan, S. H., Louis, P. & Flint, H. J. Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 4, 236–240 (2013).
Ze, X., Duncan, S. H., Louis, P. & Flint, H. J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 6, 1535–1543 (2012).
Hosseini, E., Grootaert, C., Verstraete, W. & Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 69, 245–258 (2011).
Louis, P. & Flint, H. J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294, 1–8 (2009).
Falony, G., Calmeyn, T., Leroy, F. & De Vuyst, L. Coculture fermentations of Bifidobacterium species and Bacteroides thetaiotaomicron reveal a mechanistic insight into the prebiotic effect of inulin-type fructans. Appl. Environ. Microbiol. 75, 2312–2319 (2009).
Scott, K. P., Martin, J. C., Duncan, S. H. & Flint, H. J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 87, 30–40 (2014).
Flint, H. J., Duncan, S. H. & Louis, P. The impact of nutrition on intestinal bacterial communities. Curr. Opin. Microbiol. 38, 59–65 (2017).
Chen, T. et al. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 7, 2594 (2017).
Wu, Q. et al. Fermentation properties of isomaltooligosaccharides are affected by human fecal enterotypes. Anaerobe 48, 206–214 (2017).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. U. S. A. 104, 13780–13785 (2007).
Larsen, N. et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLOS ONE 5, e9085 (2010).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012). This study provides a definition and description of the minimal gut metagenome and bacterial genome in terms of functions.
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Carroll, I. M., Chang, Y. H., Park, J., Sartor, R. B. & Ringel, Y. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. 2, 19 (2010).
Krogius-Kurikka, L. et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 9, 95 (2009).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Zhang, H. et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl Acad. Sci. U. S. A. 106, 2365–2370 (2009).
Ha, C. W., Lam, Y. Y. & Holmes, A. J. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Gastroenterol. 20, 16498–16517 (2014).
Moya, A. & Ferrer, M. Functional redundancy-induced stability of gut microbiota subjected to disturbance. Trends Microbiol. 24, 402–413 (2016).
Fooks, L. J. & Gibson, G. R. In vitro investigations of the effect of probiotics and prebiotics on selected human intestinal pathogens. FEMS Microbiol. Ecol. 39, 67–75 (2002).
Tzortzis, G., Baillon, M. L., Gibson, G. R. & Rastall, R. A. Modulation of anti-pathogenic activity in canine-derived Lactobacillus species by carbohydrate growth substrate. J. Appl. Microbiol. 96, 552–559 (2004).
Vulevic, J., Drakoularakou, A., Yaqoob, P., Tzortzis, G. & Gibson, G. R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 88, 1438–1446 (2008).
Vulevic, J. et al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 114, 586–595 (2015).
Moro, G. et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 91, 814–819 (2006).
Ivakhnenko, O. S. & Nyankovskyy, S. L. Effect of the specific infant formula mixture of oligosaccharides on local immunity and development of allergic and infectious disease in young children: randomized study. Pediatr. Pol. 88, 398–404 (2013).
Arslanoglu, S. et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J. Biol. Regul. Homeost. Agents 26, 49–59 (2012).
Diaz de Barboza, G. & Guizzardi, S. & Tolosa de Talamoni, N. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 21, 7142–7154 (2015).
Goss, S. L., Lemons, K. A., Kerstetter, J. E. & Bogner, R. H. Determination of calcium salt solubility with changes in pH and P(CO(2)), simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 59, 1485–1492 (2007).
Abrams, S. A., Griffin, I. J., Hawthorne, K. M. & Ellis, K. J. Effect of prebiotic supplementation and calcium intake on body mass index. J. Pediatr. 151, 293–298 (2007).
Abrams, S. A., Griffin, I. J. & Hawthorne, K. M. Young adolescents who respond to an inulin-type fructan substantially increase total absorbed calcium and daily calcium accretion to the skeleton. J. Nutr. 137, 2524S–2526S (2007).
Whisner, C. M. et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br. J. Nutr. 110, 1292–1303 (2013).
Chonan, O., Matsumoto, K. & Watanuki, M. Effect of galactooligosaccharides on calcium absorption and preventing bone loss in ovariectomized rats. Biosci. Biotechnol. Biochem. 59, 236–239 (1995).
Kanauchi, O., Andoh, A. & Mitsuyama, K. Effects of the modulation of microbiota on the gastrointestinal immune system and bowel function. J. Agric. Food Chem. 61, 9977–9983 (2013).
Hurst, N. R., Kendig, D. M., Murthy, K. S. & Grider, J. R. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol. Motil. 26, 1586–1596 (2014).
Lamsal, B. P. Production, health aspects and potential food uses of dairy prebiotic galactooligosaccharides. J. Sci. Food Agric. 92, 2020–2028 (2012).
Hager, A.-S. et al. Influence of the soluble fibres inulin and oat β-glucan on quality of dough and bread. Eur. Food Res. Technol. 232, 405–413 (2011).
Collado Yurrita, L., San Mauro Martin, I., Ciudad-Cabanas, M. J., Calle-Puron, M. E. & Hernandez Cabria, M. Effectiveness of inulin intake on indicators of chronic constipation; a meta-analysis of controlled randomized clinical trials. Nutr. Hosp. 30, 244–252 (2014).
Buddington, R. K., Kapadia, C., Neumer, F. & Theis, S. Oligofructose provides laxation for irregularity associated with low fiber intake. Nutrients 9, E1372 (2017).
Krumbeck, J. A. et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6, 121 (2018).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Kellow, N. J., Coughlan, M. T. & Reid, C. M. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br. J. Nutr. 111, 1147–1161 (2014).
Beserra, B. T. et al. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 34, 845–858 (2015).
Liu, F., Prabhakar, M., Ju, J., Long, H. & Zhou, H. W. Effect of inulin-type fructans on blood lipid profile and glucose level: a systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 71, 9–20 (2017).
Guo, Z. et al. Effects of inulin on the plasma lipid profile of normolipidemic and hyperlipidemic subjects: a meta-analysis of randomized controlled trials. Clin. Lipidol 7, 215–222 (2012).
Vulevic, J., Juric, A., Tzortzis, G. & Gibson, G. R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 143, 324–331 (2013).
Dewulf, E. M. et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62, 1112–1121 (2013).
Bhatia, S. et al. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol. Nutr. Food Res. 59, 566–573 (2015).
Akbari, P. et al. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: insight into the role of structure and size: structure-activity relationships of non-digestible oligosaccharides. Eur. J. Nutr. 56, 1919–1930 (2017).
Neyrinck, A. M. et al. Intestinal sucrase as a novel target contributing to the regulation of glycemia by prebiotics. PLOS ONE 11, e0160488 (2016).
Stoddart, L. A., Smith, N. J. & Milligan, G. International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol. Rev. 60, 405–417 (2008).
Bolognini, D. et al. Chemogenetics defines receptor-mediated functions of short chain free fatty acids. Nat. Chem. Biol. 15, 489–498 (2019).
Chambers, E. S., Morrison, D. J. & Frost, G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc. Nutr. Soc. 74, 328–336 (2015).
Mithieux, G. Metabolic effects of portal vein sensing. Diabetes Obes. Metab. 16 (Suppl. 1), 56–60 (2014).
Frost, G. et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5, 3611 (2014).
Jackson, S. A. et al. Improving end-user trust in the quality of commercial probiotic products. Front. Microbiol. 10, 739 (2019).
AlFaleh, K. & Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 4, CD005496 (2014).
Vanderhoof, J. A. et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J. Pediatr. 135, 564–568 (1999).
Szajewska, H., Albrecht, P. & Topczewska-Cabanek, A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J. Pediatr. Gastroenterol. Nutr. 48, 431–436 (2009).
Goldenberg, J. Z. et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 4, CD004827 (2015).
Eskesen, D. et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12®, on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br. J. Nutr. 114, 1638–1646 (2015).
Yang, Y. X. et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J. Gastroenterol. 14, 6237–6243 (2008).
Sung, V. et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics 141, e20171811 (2018).
Mardini, H. E. & Grigorian, A. Y. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm. Bowel Dis. 20, 1562–1567 (2014).
Whorwell, P. J. et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 101, 1581–1590 (2006).
Szajewska, H. et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children - a 2019 update. Aliment. Pharmacol. Ther. 49, 1376–1384 (2019).
Goldenberg, J. Z. et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 12, CD006095 (2017).
King, S. et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur. J. Public Health 29, 494–499 (2019).
Cruchet, S. et al. The use of probiotics in pediatric gastroenterology: a review of the literature and recommendations by Latin-American experts. Paediatr. Drugs 17, 199–216 (2015).
Cameron, D. et al. Probiotics for gastrointestinal disorders: proposed recommendations for children of the Asia-Pacific region. World J. Gastroenterol. 23, 7952–7964 (2017).
Hao, Q., Dong, B. R. & Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 9, CD006895 (2015).
King, S., Glanville, J., Sanders, M. E., Fitzgerald, A. & Varley, D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br. J. Nutr. 112, 41–54 (2014).
Yang, L. et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137, 588–597 (2009).
Szymanski, H. & Szajewska, H. Lack of efficacy of Lactobacillus reuteri DSM 17938 for the treatment of acute gastroenteritis: a randomized controlled trial. Pediatr. Infect. Dis. J. https://doi.org/10.1097/INF.0000000000002355 (2019).
van den Akker, C. H. P. et al. Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. J. Pediatr. Gastroenterol. Nutr. 67, 103–122 (2018).
Arslanoglu, S., Moro, G. E. & Boehm, G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 137, 2420–2424 (2007).
Arslanoglu, S. et al. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 138, 1091–1095 (2008).
Boehm, G. et al. Prebiotics in infant formulas. J. Clin. Gastroenterol. 38, S76–S79 (2004).
Shahramian, I. et al. The effects of prebiotic supplementation on weight gain, diarrhoea, constipation, fever and respiratory tract infections in the first year of life. J. Paediatr. Child Health 54, 875–880 (2018).
Drakoularakou, A., Tzortzis, G., Rastall, R. A. & Gibson, G. R. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. Eur. J. Clin. Nutr. 64, 146–152 (2010).
Micka, A., Siepelmeyer, A., Holz, A., Theis, S. & Schon, C. Effect of consumption of chicory inulin on bowel function in healthy subjects with constipation: a randomized, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 68, 82–89 (2017).
European Food Safety Authority Panel on Dietetic Products. Scientific opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to article 13.5 of regulation (EC) No 1924/2006. EFSA J. 13, 3951 (2015).
Hume, M. P., Nicolucci, A. C. & Reimer, R. A. Prebiotic supplementation improves appetite control in children with overweight and obesity: a randomized controlled trial. Am. J. Clin. Nutr. 105, 790–799 (2017).
Nicolucci, A. C. et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 153, 711–722 (2017).
Pol, K., de Graaf, C., Meyer, D. & Mars, M. The efficacy of daily snack replacement with oligofructose-enriched granola bars in overweight and obese adults: a 12-week randomised controlled trial. Br. J. Nutr. 119, 1076–1086 (2018).
Liber, A. & Szajewska, H. Effect of oligofructose supplementation on body weight in overweight and obese children: a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 112, 2068–2074 (2014).
European Food Safety Authority Panel on Dietetic Products. Scientific opinion on the substantiation of a health claim related to non-digestible carbohydrates and a reduction of post-prandial glycaemic responses pursuant to article 13(5) of regulation (EC) No 1924/2006. EFSA J. 12, 3513 (2015).
Lightowler, H., Thondre, S., Holz, A. & Theis, S. Replacement of glycaemic carbohydrates by inulin-type fructans from chicory (oligofructose, inulin) reduces the postprandial blood glucose and insulin response to foods: report of two double-blind, randomized, controlled trials. Eur. J. Nutr. 57, 1259–1268 (2018).
Olbjorn, C. et al. Fecal microbiota profiles in treatment-naive pediatric inflammatory bowel disease - associations with disease phenotype, treatment, and outcome. Clin. Exp. Gastroenterol. 12, 37–49 (2019).
Backhed, F. et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe 12, 611–622 (2012).
Dey, M. Toward a personalized approach in prebiotics research. Nutrients 9, 92 (2017).
Healey, G. et al. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 119, 176–189 (2018).
Tandon, D. et al. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci. Rep. 9, 5473 (2019).
Bian, G. et al. The gut microbiota of healthy aged Chinese is similar to that of the healthy young. mSphere 2, e00327–17 (2017).
Gloor, G. B., Macklaim, J. M., Pawlowsky-Glahn, V. & Egozcue, J. J. Microbiome datasets are compositional: and this is not optional. Front. Microbiol. 8, 2224 (2017).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012). This study considers the gut microbiome in evaluating human development, nutritional needs, physiological variations and the effects of westernization.
Marques, T. M. et al. Programming infant gut microbiota: influence of dietary and environmental factors. Curr. Opin. Biotechnol. 21, 149–156 (2010).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Clarke, S. F. et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63, 1913–1920 (2014).
Engen, P. A., Green, S. J., Voigt, R. M., Forsyth, C. B. & Keshavarzian, A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. 37, 223–236 (2015).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Picoraro, J. A. & LeLeiko, N. S. Omes of inflammatory bowel disease: a primer for clinicians. J. Pediatr. Gastroenterol. Nutr. 66, 374–377 (2018).
Weiser, M. et al. Molecular classification of Crohn’s disease reveals two clinically relevant subtypes. Gut 67, 36–42 (2018).
Bourreille, A. et al. Saccharomyces boulardii does not prevent relapse of Crohn’s disease. Clin. Gastroenterol. Hepatol. 11, 982–987 (2013).
Van Gossum, A. et al. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn’s disease after ileo-caecal resection. Inflamm. Bowel Dis. 13, 135–142 (2007).
Tursi, A. et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 105, 2218–2227 (2010).
O’Toole, P. W., Marchesi, J. R. & Hill, C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2, 17057 (2017).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Lee, C. H. et al. Frozen versus fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 315, 142–149 (2016).
Kelly, C. R. et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann. Intern. Med. 165, 609–616 (2016).
Halkjaer, S. I. et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut 67, 2107–2115 (2018).
Delaune, V. et al. Fecal microbiota transplantation: a promising strategy in preventing the progression of non-alcoholic steatohepatitis and improving the anti-cancer immune response. Expert Opin. Biol. Ther. 18, 1061–1071 (2018).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109 (2015).
Gupta, S., Allen-Vercoe, E. & Petrof, E. O. Fecal microbiota transplantation: in perspective. Therap. Adv. Gastroenterol. 9, 229–239 (2016).
Reid, G. et al. How do probiotics and prebiotics function at distant sites? Benef. Microbes 8, 521–533 (2017). An ISAPP working group article that looks at how microbiome programming in early life influences the gut microbiota communication with distant sites, such as airways, heart and brain, and influences metabolism.
Hiippala, K. et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 10, E988 (2018).
Crusell, M. K. W. et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6, 89 (2018).
Cousin, F. J. et al. The probiotic Propionibacterium freudenreichii as a new adjuvant for TRAIL-based therapy in colorectal cancer. Oncotarget 7, 7161–7178 (2016).
Zullo, B. A. & Ciafardini, G. Evaluation of physiological properties of yeast strains isolated from olive oil and their in vitro probiotic trait. Food Microbiol. 78, 179–187 (2019).
Gonzalez-Rodriguez, I. et al. Catabolism of glucose and lactose in Bifidobacterium animalis subsp. lactis, studied by 13C nuclear magnetic resonance. Appl. Environ. Microbiol. 79, 7628–7638 (2013).
Kostinek, M. et al. Characterisation and biochemical properties of predominant lactic acid bacteria from fermenting cassava for selection as starter cultures. Int. J. Food Microbiol. 114, 342–351 (2007).
Hancock, T., Capon, A., Dooris, M. & Patrick, R. One planet regions: planetary health at the local level. Lancet Planet. Health 1, e92–e93 (2017).
Garchitorena, A. et al. Disease ecology, health and the environment: a framework to account for ecological and socio-economic drivers in the control of neglected tropical diseases. Philos. Trans. R. Soc. B. 372, 20160128 (2017).
Acknowledgements
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks H. Szajewska, and the other, anonymous, reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.E.S. declares personal fees related to probiotics from the following entities: California Dairy Research Foundation, Clorox, Danone, Danone USA, Dutch Mill, General Mills, JHeimbach, Kelley Drye & Warren, Kellogg, Kerry, Medscape, Nestle, New Chapter, Pepsico, Pfizer, Pharmavite, Probi, Procter & Gamble, Trouw Nutrition, Visalia Dairy Company, Williams Mullen, Winclove Probiotics and Yakult. D.J.M. declares personal fees for consulting for Bayer and Pharmavite. G.R. declares that he helped develop and commercialize probiotic strains GR-1 and RC-14, but has had no financial interest in them for over 10 years. He is Chief Scientific Officer for Seed, a company producing probiotic products. Over the past 3 years, he has consulted on probiotics with Acerus Pharmaceuticals, Altmann, Chr. Hansen, Danone, KGK Science, Kimberly-Clark, Metagenics and Seed. G.R.G. and R.A.R. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanders, M.E., Merenstein, D.J., Reid, G. et al. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 16, 605–616 (2019). https://doi.org/10.1038/s41575-019-0173-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-019-0173-3
This article is cited by
-
The effect of in vitro simulated colonic pH gradients on microbial activity and metabolite production using common prebiotics as substrates
BMC Microbiology (2024)
-
Metabolic network of the gut microbiota in inflammatory bowel disease
Inflammation and Regeneration (2024)
-
The role of gut microbiota in human metabolism and inflammatory diseases: a focus on elderly individuals
Annals of Microbiology (2024)
-
Bridging the gap: associations between gut microbiota and psychiatric disorders
Middle East Current Psychiatry (2024)
-
Prebiotic inulin ameliorates SARS-CoV-2 infection in hamsters by modulating the gut microbiome
npj Science of Food (2024)