Abstract
Alcoholic liver disease, which ranges from mild disease to alcoholic hepatitis and cirrhosis, is a leading cause of morbidity and mortality worldwide. Alcohol intake can lead to changes in gut microbiota composition, even before liver disease development. These alterations worsen with advancing disease and could be complicit in disease progression. Microbial function, especially related to bile acid metabolism, can modulate alcohol-associated injury even in the presence of cirrhosis and alcoholic hepatitis. Microbiota changes might also alter brain function, and the gut–brain axis might be a potential target to reduce alcoholic relapse risk. Gut microbiota manipulation including probiotics, faecal microbial transplant and antibiotics has been studied in alcoholic liver disease with varying success. Further investigation of the modulation of the gut–liver axis is relevant, as most of these patients are not candidates for liver transplantation. This Review focuses on clinical studies involving the gut microbiota in patients with alcoholic liver disease across the spectrum from alcoholic fatty liver to cirrhosis and alcoholic hepatitis. Specific alterations in the gut–liver–brain axis that are complicit in the interactions between the gut microbiota and alcohol addiction are also reviewed.
Key points
-
Alcohol affects many organ systems, but alcoholic liver disease develops in selected patients and ranges from simple steatosis to inflammation, cirrhosis and alcoholic hepatitis.
-
Gut microbiota composition and function, especially bile acid physiology, are affected throughout the spectrum of alcohol use disorder, and these changes can improve after alcohol cessation in patients without alcoholic liver disease.
-
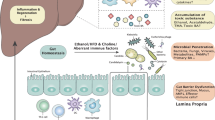
In patients who have substantial liver fibrosis, gut microbial changes occur in parallel to liver injury, with an increase in endotoxin-producing and a reduction in autochthonous bacterial taxa, which continue through active drinking in cirrhosis until alcoholic hepatitis.
-
Functional microbial changes, in particular, hepatic bile acid production and bacterial biotransformation, are altered in parallel with the disease stages and differ between actively drinking patients with cirrhosis and those with alcoholic hepatitis.
-
Alcohol use disorder can also affect the gut–brain axis, which could potentiate further misuse and affective disorders and hasten the development of hepatic encephalopathy.
-
Strategies that address both alcohol cessation and microbiota alteration are needed for meaningful improvement in the natural history of this multifaceted disorder.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rehm, J., Samokhvalov, A. V. & Shield, K. D. Global burden of alcoholic liver diseases. J. Hepatol. 59, 160–168 (2013).
World Health Organization. Global status on alcohol and health 2014. WHO http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf (2014).
Mathurin, P. & Bataller, R. Trends in the management and burden of alcoholic liver disease. J. Hepatol. 62, S38–S46 (2015).
Yoon, Y. & Chen, C. M. Surveillance report #105. NIAAA https://pubs.niaaa.nih.gov/publications/surveillance105/Cirr13.pdf (2016).
Mills, S. J. & Harrison, S. A. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr. Gastroenterol. Rep. 7, 32–36 (2005).
Singal, A. K., Bataller, R., Ahn, J., Kamath, P. S. & Shah, V. H. ACG clinical guideline: alcoholic liver disease. Am. J. Gastroenterol. 113, 175–194 (2018).
National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. NIAAA https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics (2018).
Zakhari, S. & Li, T. K. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology 46, 2032–2039 (2007).
Mutlu, E. A. et al. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G966–G978 (2012).
Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 148, 30–36 (2015).
Soyka, M. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of substance use and related disorders, part 1: alcoholism. World J. Biol. Psychiatry 9, 6–23 (2008).
Moos, R. H. & Moos, B. S. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 101, 212–222 (2006).
Rosa, H., Silverio, A. O., Perini, R. F. & Arruda, C. B. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am. J. Gastroenterol. 95, 1290–1293 (2000).
Ahluwalia, V. et al. The etiology of cirrhosis is a strong determinant of brain reserve: a multimodal magnetic resonance imaging study. Liver Transpl. 21, 1123–1132 (2015).
Martin, P., DiMartini, A., Feng, S., Brown, R. Jr & Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 59, 1144–1165 (2014).
Altamirano, J. et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: prediction and impact on long-term survival. Hepatology 66, 1842–1853 (2017).
Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Sokol, H. et al. Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048 (2016).
Bajaj, J. S. et al. Fungal dysbiosis in cirrhosis. Gut 67, 1146–1154 (2018).
Yang, A. M. et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Invest. 127, 2829–2841 (2017).
Hooks, K. B. & O’Malley, M. A. Dysbiosis and its discontents. mBio 8, e01492–17 (2017).
Rodriguez, J. M. et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050 (2015).
Vandeputte, D. et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62 (2016).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Bajaj, J. S. et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 68, 234–247 (2018).
Iebba, V. et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 39, 1–12 (2016).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480 (2016).
Hughes, H. K., Rose, D. & Ashwood, P. The gut microbiota and dysbiosis in autism spectrum disorders. Curr. Neurol. Neurosci. Rep. 18, 81 (2018).
Ni, J., Wu, G. D., Albenberg, L. & Tomov, V. T. Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584 (2017).
Bajaj, J. S., Betrapally, N. S. & Gillevet, P. M. Decompensated cirrhosis and microbiome interpretation. Nature 525, E1–E2 (2015).
Young, V. B. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ 356, j831 (2017).
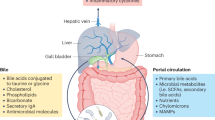
Ridlon, J. M., Harris, S. C., Bhowmik, S., Kang, D. J. & Hylemon, P. B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7, 22–39 (2016).
Gillevet, P., Sikaroodi, M., Keshavarzian, A. & Mutlu, E. A. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem. Biodivers. 7, 1065–1075 (2010).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Segal, Z. V. et al. Antidepressant monotherapy versus sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch. Gen. Psychiatry 67, 1256–1264 (2010).
Vancamelbeke, M. & Vermeire, S. The intestinal barrier: a fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 11, 821–834 (2017).
Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
Bajaj, J. S. & Hylemon, P. B. Gut-liver axis alterations in alcoholic liver disease: are bile acids the answer? Hepatology 67, 2074–2075 (2018).
Wiest, R., Albillos, A., Trauner, M., Bajaj, J. S. & Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103 (2017).
Bala, S., Marcos, M., Gattu, A., Catalano, D. & Szabo, G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLOS ONE 9, e96864 (2014).
Voigt, R. M. et al. Diurnal variations in intestinal barrier integrity and liver pathology in mice: implications for alcohol binge. Am. J. Physiol. Gastrointest. Liver Physiol. 314, G131–G141 (2018).
de Timary, P., Leclercq, S., Starkel, P. & Delzenne, N. A dysbiotic subpopulation of alcohol-dependent subjects. Gut Microbes 6, 388–391 (2015).
Bull-Otterson, L. et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLOS ONE 8, e53028 (2013).
Wang, Y. et al. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 179, 2866–2875 (2011).
Chen, P. et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 148, 203–214 (2015).
Shao, T. et al. Intestinal HIF-1alpha deletion exacerbates alcoholic liver disease through inducing intestinal dysbiosis and barrier dysfunction. J. Hepatol. 69, 886–895 (2018).
Forsyth, C. B. et al. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 43, 163–172 (2009).
Nath, B. et al. Hepatocyte-specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology 53, 1526–1537 (2011).
Bajaj, J. S. et al. Continued alcohol misuse in human cirrhosis is associated with an impaired gut-liver axis. Alcohol. Clin. Exp. Res. 41, 1857–1865 (2017).
Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 60, 940–947 (2014).
Kakiyama, G. et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G929–G937 (2014).
Wang, L. et al. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 421, 44–53 (2015).
Grant, B. F., Dufour, M. C. & Harford, T. C. Epidemiology of alcoholic liver disease. Semin. Liver Dis. 8, 12–25 (1988).
Forsyth, C. B., Voigt, R. M., Burgess, H. J., Swanson, G. R. & Keshavarzian, A. Circadian rhythms, alcohol and gut interactions. Alcohol 49, 389–398 (2015).
Couch, R. D. et al. Alcohol induced alterations to the human fecal VOC metabolome. PLOS ONE 10, e0119362 (2015).
Leclercq, S. et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl Acad. Sci. USA 111, E4485–E4493 (2014).
Lucey, M. R., Mathurin, P. & Morgan, T. R. Alcoholic hepatitis. N. Engl. J. Med. 360, 2758–2769 (2009).
Hartmann, P. et al. Modulation of the intestinal bile acid-FXR-FGF15 axis improves alcoholic liver disease in mice. Hepatology 67, 2150–2166 (2017).
Dubinkina, V. B. et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 5, 141 (2017).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Bajaj, J. S. et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 62, 1260–1271 (2015).
Raghava, K. V., Shivananda, H., Mundinamane, D., Boloor, V. & Thomas, B. Evaluation of periodontal status in alcoholic liver cirrhosis patients: a comparative study. J. Contemp. Dent. Pract. 14, 179–182 (2013).
Bajaj, J. S. et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G168–G175 (2012).
Han, S. H. et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur. J. Gastroenterol. Hepatol. 27, 1300–1306 (2015).
Dhiman, R. K. et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 147, 1327–1337 (2014).
Tuomisto, S. et al. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 14, 40 (2014).
Llopis, M. et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 65, 830–839 (2016).
Grander, C. et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, 891–901 (2018).
Brandl, K. et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J. Hepatol. 69, 396–405 (2018).
Ciocan, D. et al. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment. Pharmacol. Ther. 48, 961–974 (2018).
Puri, P. et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology 67, 1284–1302 (2018).
Zhong, W. & Zhou, Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J. Gastrointest. Pathophysiol. 5, 514–522 (2014).
Harada, S. et al. Metabolomic profiling reveals novel biomarkers of alcohol intake and alcohol-induced liver injury in community-dwelling men. Environ. Health Prev. Med. 21, 18–26 (2016).
Liang, Q., Wang, C., Li, B. & Zhang, A. Metabolomics of alcoholic liver disease: a clinical discovery study. RSC Adv. 5, 80381–80387 (2015).
Rachakonda, V. et al. Serum metabolomic profiling in acute alcoholic hepatitis identifies multiple dysregulated pathways. PLOS ONE 9, e113860 (2014).
Bajaj, J. S. et al. Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am. J. Gastroenterol. 113, 556–563 (2018).
Gustot, T. et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J. Hepatol. 60, 267–274 (2014).
Addolorato, G., Mirijello, A., Barrio, P. & Gual, A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J. Hepatol. 65, 618–630 (2016).
Davis, B. C. & Bajaj, J. S. Effects of alcohol on the brain in cirrhosis: beyond hepatic encephalopathy. Alcohol. Clin. Exp. Res. 42, 660–667 (2018).
Butterworth, R. F. Thiamine deficiency-related brain dysfunction in chronic liver failure. Metab. Brain Dis. 24, 189–196 (2009).
Cenit, M. C., Sanz, Y. & Codoner-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 23, 5486–5498 (2017).
Temko, J. E. et al. The microbiota, the gut and the brain in eating and alcohol use disorders: a ‘menage a trois’? Alcohol Alcohol. 52, 403–413 (2017).
Xiao, H. W. et al. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 287, 23–30 (2018).
Volpe, G. E. et al. Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J. Stud. Alcohol Drugs 75, 347–357 (2014).
Butterworth, R. F. Pathogenesis of hepatic encephalopathy in cirrhosis: the concept of synergism revisited. Metab. Brain Dis. 31, 1211–1215 (2015).
Bajaj, J. S. The role of microbiota in hepatic encephalopathy. Gut Microbes 5, 397–403 (2014).
Kang, D. J. et al. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice. Hepatology 64, 1232–1248 (2016).
Mathurin, P. et al. Early liver transplantation for severe alcoholic hepatitis. N. Engl. J. Med. 365, 1790–1800 (2011).
Lee, B. P. et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology 155, 422–430 (2018).
Kirpich, I. A. et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42, 675–682 (2008).
Stadlbauer, V. et al. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J. Hepatol. 48, 945–951 (2008).
Kalambokis, G. N. et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin. Gastroenterol. Hepatol. 10, 815–818 (2012).
Vlachogiannakos, J. et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J. Gastroenterol. Hepatol. 28, 450–455 (2013).
Kelly, C. R. et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237 (2015).
Kao, D. et al. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology 63, 339–340 (2016).
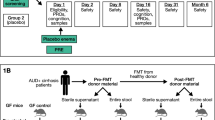
Bajaj, J. S. et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 66, 1727–1738 (2017).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012).
Philips, C. A. et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin. Gastroenterol. Hepatol. 15, 600–602 (2017).
Philips, C. A., Phadke, N., Ganesan, K., Ranade, S. & Augustine, P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J. Gastroenterol. 37, 215–225 (2018).
Kang, D. J. et al. Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatol. Commun. 1, 61–70 (2017).
Wang, L. et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 19, 227–239 (2016).
Chen, Y. et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54, 562–572 (2011).
Kakiyama, G. et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 58, 949–955 (2013).
Acknowledgements
This manuscript was partly supported by VA Merit Review I0CX001076, NCATS R21TR002024 to J.S.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.S.B. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol 16, 235–246 (2019). https://doi.org/10.1038/s41575-018-0099-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0099-1
This article is cited by
-
Non-traumatic osteonecrosis of the femoral head induced by steroid and alcohol exposure is associated with intestinal flora alterations and metabolomic profiles
Journal of Orthopaedic Surgery and Research (2024)
-
Characteristics of microbiome-derived metabolomics according to the progression of alcoholic liver disease
Hepatology International (2024)
-
Characterization of siderophore from probiotic Bacillus spp. strain isolated from traditional fermented food of the Himalaya
Systems Microbiology and Biomanufacturing (2024)
-
Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease
Journal of Translational Medicine (2023)
-
Gegen-Qinlian decoction alleviates anxiety-like behaviors in methamphetamine-withdrawn mice by regulating Akkermansia and metabolism in the colon
Chinese Medicine (2023)