Abstract
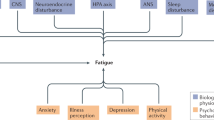
Fatigue is an important clinical problem in patients with IBD, affecting nearly 50% of patients in clinical remission and > 80% of those with active disease. The resulting decrease in quality of life and impaired work productivity and functioning contribute markedly to the societal costs of fatigue. However, despite the burden and effects of fatigue, little is known about its aetiology and pathophysiology, which impairs our ability to effectively treat this symptom. Here, we review the theories behind the development of fatigue in IBD and the role of contributing factors, including nutritional deficiency, inflammation and altered metabolism. We also explore the potential role of the gut microbiome in mediating fatigue and other psychological symptoms through the gut–brain axis. We discuss the efficacy of nutrient repletion and various psychological and pharmacological interventions on relieving fatigue in patients with IBD and expand the discussion to non-IBD-related fatigue when evidence exists. Finally, we present a therapeutic strategy for the management of fatigue in IBD and call for further mechanistic and clinical research into this poorly studied symptom.
Key points
-
Fatigue is one of the most frequently reported concerns of patients with IBD and can result in a decrease in quality of life and impaired work productivity.
-
Fatigue in IBD is multifactorial, with contributions from active inflammation, nutritional deficiency, altered metabolism and psychological comorbidity.
-
Emerging evidence also suggests a possible role for bidirectional communication between the gut and central nervous system (the gut–brain axis) in mediating fatigue.
-
The multidimensionality of contributing factors could imply that the mechanism of fatigue is not uniform in all patients and that there might be different subtypes of fatigue.
-
The multidimensionality of fatigue suggests the existence of different subtypes that respond to different interventions.
-
Studies conducted in the past few years suggest a potential role for psychological interventions, physical activity and microbiome-directed therapies for relief of fatigue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Danese, S. et al. Anaemia from a patient perspective in inflammatory bowel disease: results from the European Federation of Crohn’s and Ulcerative Colitis Association’s online survey. Eur. J. Gastroenterol. Hepatol. 26, 1385–1391 (2014).
Bjornsson, E. et al. Fatigue in patients with primary sclerosing cholangitis. Scand. J. Gastroenterol. 39, 961–968 (2004).
Ricci, J. A. et al. Fatigue in the U. S. workforce: prevalence and implications for lost productive work time. J. Occup. Environ. Med. 49, 1–10 (2007).
Lonnfors, S. et al. IBD and health-related quality of life — discovering the true impact. J. Crohns Colitis 8, 1281–1286 (2014).
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196 (2012).
Czuber-Dochan, W. et al. Healthcare professionals’ perceptions of fatigue experienced by people with IBD. J. Crohns Colitis 8, 835–844 (2014).
Romberg-Camps, M. J. et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm. Bowel Dis. 16, 2137–2147 (2010).
Jelsness-Jorgensen, L. P. et al. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment. Pharmacol. Ther. 33, 106–114 (2011).
Czuber-Dochan, W. et al. The experience of fatigue in people with inflammatory bowel disease: an exploratory study. J. Adv. Nurs. 69, 1987–1999 (2013).
Markowitz, A. J. & Rabow, M. W. Palliative management of fatigue at the close of life: “it feels like my body is just worn out”. JAMA 298, 217 (2007).
Narayanan, V. & Koshy, C. Fatigue in cancer: a review of literature. Indian J. Palliat Care 15, 19–25 (2009).
Walker, E. A., Katon, W. J. & Jemelka, R. P. Psychiatric disorders and medical care utilization among people in the general population who report fatigue. J. Gen. Intern. Med. 8, 436–440 (1993).
Cathebras, P. J. et al. Fatigue in primary care: prevalence, psychiatric comorbidity, illness behavior, and outcome. J. Gen. Intern. Med. 7, 276–286 (1992).
Cullen, W., Kearney, Y. & Bury, G. Prevalence of fatigue in general practice. Ir. J. Med. Sci. 171, 10–12 (2002).
Gallagher, A. M. et al. Incidence of fatigue symptoms and diagnoses presenting in UK primary care from 1990 to 2001. J. R. Soc. Med. 97, 571–575 (2004).
Schappert, S. M. Vital and health statistics. National ambulatory medical care survey: 1989 summary. CDC https://www.cdc.gov/nchs/data/series/sr_13/sr13_110.pdf (1992).
Basu, N. et al. Fatigue is associated with excess mortality in the general population: results from the EPIC-Norfolk study. BMC Med. 14, 122 (2016).
Cohen, B. L. et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment. Pharmacol. Ther. 39, 811–822 (2014).
Singh, S. et al. Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 9, 769–775 (2011).
Villoria, A. et al. Fatigue in out-patients with inflammatory bowel disease: prevalence and predictive factors. PLOS ONE 12, e0181435 (2017).
Minderhoud, I. M. et al. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. Am. J. Gastroenterol. 98, 1088–1093 (2003).
Huppertz-Hauss, G. et al. Fatigue in a population-based cohort of patients with inflammatory bowel disease 20 years after diagnosis: the IBSEN study. Scand. J. Gastroenterol. 52, 351–358 (2017).
Levenstein, S. et al. Cross-cultural variation in disease-related concerns among patients with inflammatory bowel disease. Am. J. Gastroenterol. 96, 1822–1830 (2001).
Tench, C. M. et al. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology 39, 1249–1254 (2000).
Hewlett, S. et al. Self-management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann. Rheum. Dis. 70, 1060–1067 (2011).
Segal, B. et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjogren’s syndrome. Arthritis Rheum. 59, 1780–1787 (2008).
Dernis-Labous, E., Messow, M. & Dougados, M. Assessment of fatigue in the management of patients with ankylosing spondylitis. Rheumatology 42, 1523–1528 (2003).
Husted, J. A. et al. Occurrence and correlates of fatigue in psoriatic arthritis. Ann. Rheum. Dis. 68, 1553–1558 (2009).
Smets, E. M. et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 39, 315–325 (1995).
Tinsley, A. et al. Validation of the functional assessment of chronic illness therapy-fatigue (FACIT-F) in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 34, 1328–1336 (2011).
Cella, D. et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J. Pain Symptom Manage. 24, 547–561 (2002).
Cella, D. et al. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J. Rheumatol 32, 811–819 (2005).
Czuber-Dochan, W. et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J. Crohns Colitis 8, 1398–1406 (2014).
Norton, C. et al. Assessing fatigue in inflammatory bowel disease: comparison of three fatigue scales. Aliment. Pharmacol. Ther. 42, 203–211 (2015).
Bower, J. E. et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 64, 604–611 (2002).
Collado-Hidalgo, A. et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 12, 2759–2766 (2006).
Kallen, K. J. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim. Biophys. Acta 1592, 323–343 (2002).
Smith, A. J. & Humphries, S. E. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 20, 43–59 (2009).
Bower, J. E. et al. Cytokine genetic variations and fatigue among patients with breast cancer. J. Clin. Oncol. 31, 1656–1661 (2013).
Spangelo, B. L. et al. Role of the cytokines in the hypothalamic-pituitary-adrenal and gonadal axes. Neuroimmunomodulation 2, 299–312 (1995).
Joyce, J. C. et al. Identification of symptom domains in ulcerative colitis that occur frequently during flares and are responsive to changes in disease activity. Health Qual. Life Outcomes 6, 69 (2008).
Szigethy, E. et al. Depressive symptoms and inflammatory bowel disease in children and adolescents: a cross-sectional study. J. Pediatr. Gastroenterol. Nutr. 39, 395–403 (2004).
Graff, L. A. et al. Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clin. Gastroenterol. Hepatol. 11, 1140–1146 (2013).
Vogelaar, L. et al. Fatigue in patients with inflammatory bowel disease is associated with distinct differences in immune parameters. Clin. Exp. Gastroenterol. 10, 83–90 (2017).
Borren, N. Z. et al. Fatigue in quiescent inflammatory bowel disease is associated with low GM-CSF and metabolomic alterations [abstract 533]. Gastroenterology 152 (Suppl. 1), S124–S125 (2017).
Testa, A. et al. The burden of anaemia in patients with inflammatory bowel diseases. Dig. Liver Dis. 48, 267–270 (2016).
Bager, P. et al. The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand. J. Gastroenterol. 46, 304–309 (2011).
Weiss, G. & Gasche, C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica 95, 175–178 (2010).
Jelsness-Jorgensen, L. P. et al. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm. Bowel Dis. 17, 1564–1572 (2011).
Goldenberg, B. A. et al. Is iron deficiency in the absence of anemia associated with fatigue in inflammatory bowel disease? Am. J. Gastroenterol. 108, 1392–1397 (2013).
Bager, P. et al. Fatigue in out-patients with inflammatory bowel disease is common and multifactorial. Aliment. Pharmacol. Ther. 35, 133–141 (2012).
Bermejo, F. et al. Should we monitor vitamin B12 and folate levels in Crohn’s disease patients? Scand. J. Gastroenterol. 48, 1272–1277 (2013).
Huijts, M. et al. Association of vitamin B12 deficiency with fatigue and depression after lacunar stroke. PLOS ONE 7, e30519 (2012).
Briani, C. et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients 5, 4521–4539 (2013).
Kabbani, T. A. et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. Am. J. Gastroenterol. 111, 712–719 (2016).
Torki, M. et al. Vitamin D deficiency associated with disease activity in patients with inflammatory bowel diseases. Dig. Dis. Sci. 60, 3085–3091 (2015).
Ulitsky, A. et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J. Parenter Enteral Nutr. 35, 308–316 (2011).
Frigstad, S. O. et al. Vitamin D deficiency in inflammatory bowel disease: prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 52, 100–106 (2017).
Frigstad, S. O. et al. Fatigue is not associated with Vitamin D deficiency in IBD patients. World J. Gastroenterol. 24, 3293–3301 (2017).
Narula, N. et al. Impact of high-dose vitamin D3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig. Dis. Sci. 62, 448–455 (2017).
Walker, J. R. et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am. J. Gastroenterol. 103, 1989–1997 (2008).
Vogelaar, L. et al. Determinants of fatigue in Crohn’s disease patients. Eur. J. Gastroenterol. Hepatol. 25, 246–251 (2013).
Ranjbaran, Z. et al. Impact of sleep disturbances in inflammatory bowel disease. J. Gastroenterol. Hepatol. 22, 1748–1753 (2007).
Graff, L. A. et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm. Bowel Dis. 17, 1882–1889 (2011).
Irwin, M. R. et al. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatry 64, 538–540 (2008).
Meier-Ewert, H. K. et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 43, 678–683 (2004).
Shearer, W. T. et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J. Allergy Clin. Immunol. 107, 165–170 (2001).
Ananthakrishnan, A. N. et al. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin. Gastroenterol. Hepatol. 11, 965–971 (2013).
Emmer, B. J. et al. Brain involvement in rheumatoid arthritis: a magnetic resonance spectroscopy study. Arthritis Rheum. 60, 3190–3195 (2009).
Wang, P. I. et al. Perfusion-weighted MR imaging in cerebral lupus erythematosus. Acad. Radiol 19, 965–970 (2012).
Cutolo, M. et al. Evidence of cerebral hypoperfusion in scleroderma patients. Rheumatology 39, 1366–1373 (2000).
Hamed, S. A. et al. Assessment of biocorrelates for brain involvement in female patients with rheumatoid arthritis. Clin. Rheumatol. 31, 123–132 (2012).
Puri, B. K., Holmes, J. & Hamilton, G. Eicosapentaenoic acid-rich essential fatty acid supplementation in chronic fatigue syndrome associated with symptom remission and structural brain changes. Int. J. Clin. Pract. 58, 297–299 (2004).
Chaudhuri, A. et al. Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome. Neuroreport 14, 225–228 (2003).
van Erp, S. et al. Cerebral magnetic resonance imaging in quiescent Crohn’s disease patients with fatigue. World J. Gastroenterol. 23, 1018–1029 (2017).
van Langenberg, D. R. et al. Objectively measured muscle fatigue in Crohn’s disease: correlation with self-reported fatigue and associated factors for clinical application. J. Crohns Colitis 8, 137–146 (2014).
Lehmann, M. et al. Serum amino acid concentrations in nine athletes before and after the 1993 Colmar ultra triathlon. Int. J. Sports Med. 16, 155–159 (1995).
Mizuno, K. et al. Mental fatigue-induced decrease in levels of several plasma amino acids. J. Neural Transm. 114, 555–561 (2007).
Jin, G. et al. Changes in plasma and tissue amino acid levels in an animal model of complex fatigue. Nutrition 25, 597–607 (2009).
Kume, S. et al. Potential biomarkers of fatigue identified by plasma metabolome analysis in rats. PLOS ONE 10, e0120106 (2015).
Blomstrand, E. et al. Administration of branched-chain amino acids during sustained exercise—effects on performance and on plasma concentration of some amino acids. Eur. J. Appl. Physiol. Occup. Physiol. 63, 83–88 (1991).
Blomstrand, E. et al. Effect of branched-chain amino acid and carbohydrate supplementation on the exercise-induced change in plasma and muscle concentration of amino acids in human subjects. Acta Physiol. Scand. 153, 87–96 (1995).
Hassmen, P. et al. Branched-chain amino acid supplementation during 30-km competitive run: mood and cognitive performance. Nutrition 10, 405–410 (1994).
Sartor, R. B. Microbial influences in inflammatory bowel diseases. Gastroenterology 134, 577–594 (2008).
Missaghi, B. et al. Perturbation of the human microbiome as a contributor to inflammatory bowel disease. Pathogens 3, 510–527 (2014).
Foster, J. A. & McVey Neufeld, K. A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312 (2013).
Nagy-Szakal, D. et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 5, 44 (2017).
Maes, M., Mihaylova, I. & Leunis, J. C. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J. Affect Disord. 99, 237–240 (2007).
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004).
Collins, S. M. & Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136, 2003–2014 (2009).
Ait-Belgnaoui, A. et al. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 26, 510–520 (2014).
Kantak, P. A., Bobrow, D. N. & Nyby, J. G. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol. 25, 71–79 (2014).
Smith, C. J. et al. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G793–G802 (2014).
Vogelaar, L. et al. Physical fitness and physical activity in fatigued and non-fatigued inflammatory bowel disease patients. Scand. J. Gastroenterol. 50, 1357–1367 (2015).
Visser, M. et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 57, M326–M332 (2002).
Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 29, 247–264 (2009).
Zoico, E. & Roubenoff, R. The role of cytokines in regulating protein metabolism and muscle function. Nutr. Rev. 60, 39–51 (2002).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
DeFilippis, E. M. et al. Exercise and self-reported limitations in patients with inflammatory bowel disease. Dig. Dis. Sci. 61, 215–220 (2016).
Lichtenstein, G. R. et al. Infliximab improves quality of life in patients with Crohn’s disease. Inflamm. Bowel Dis. 8, 237–243 (2002).
Loftus, E. V. et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am. J. Gastroenterol. 103, 3132–3141 (2008).
Yennurajalingam, S. & Bruera, E. Cancer-related fatigue, the role of adrenal suppression and steroids: reply to the comments of Eren et al. Support Care Cancer 22, 2601 (2014).
Eren, O. O., Ozturk, M. A. & Oyan, B. Cancer-related fatigue: can it be due to adrenal suppression secondary to high-dose steroids used as antiemetic? Support Care Cancer 22, 2599–2600 (2014).
Moraska, A. R. et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J. Clin. Oncol. 28, 3673–3679 (2010).
Gong, S. et al. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLOS ONE 9, e84391 (2014).
Bruera, E. et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J. Clin. Oncol. 24, 2073–2078 (2006).
Butler, J. M. et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 69, 1496–1501 (2007).
Cueva, J. F. et al. Methylphenidate in the management of asthenia in breast cancer patients treated with docetaxel: results of a pilot study. Invest. New Drugs 30, 688–694 (2012).
Yennurajalingam, S. et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J. Clin. Oncol. 31, 3076–3082 (2013).
Shen, J. et al. Excessive daytime sleepiness and fatigue in depressed patients and therapeutic response of a sedating antidepressant. J. Affect Disord. 134, 421–426 (2011).
Marin, H. & Menza, M. A. Specific treatment of residual fatigue in depressed patients. Psychiatry 1, 12–18 (2004).
Wu, S. et al. Interventions for post-stroke fatigue. Cochrane Database Syst. Rev. 7, CD007030 (2015).
Vercoulen, J. H. et al. Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet 347, 858–861 (1996).
Choi-Kwon, S. et al. Fluoxetine is not effective in the treatment of post-stroke fatigue: a double-blind, placebo-controlled study. Cerebrovasc. Dis. 23, 103–108 (2007).
Mikocka-Walus, A. A. et al. The role of antidepressants in the management of inflammatory bowel disease (IBD): a short report on a clinical case-note audit. J. Psychosom. Res. 72, 165–167 (2012).
Mikocka-Walus, A. et al. Fluoxetine for maintenance of remission and to improve quality of life in patients with Crohn’s disease: a pilot randomized placebo-controlled trial. J. Crohns Colitis 11, 509–514 (2017).
Bivard, A. et al. MIDAS (modafinil in debilitating fatigue after stroke): a randomized, double-blind, placebo-controlled, cross-over trial. Stroke 48, 1293–1298 (2017).
White, P. D., Chalder, T. & Sharpe, M. The planning, implementation and publication of a complex intervention trial for chronic fatigue syndrome: the PACE trial. BJPsych Bull. 39, 24–27 (2015).
Larun, L. et al. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 4, CD003200 (2017).
McCombie, A. M., Mulder, R. T. & Gearry, R. B. Psychotherapy for inflammatory bowel disease: a review and update. J. Crohns Colitis 7, 935–949 (2013).
Vogelaar, L. et al. Solution focused therapy: a promising new tool in the management of fatigue in Crohn’s disease patients psychological interventions for the management of fatigue in Crohn’s disease. J. Crohns Colitis 5, 585–591 (2011).
Vogelaar, L. et al. Fatigue management in patients with IBD: a randomised controlled trial. Gut 63, 911–918 (2014).
Zick, S. M. et al. Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res. Treat. 161, 299–310 (2017).
Hill, C. et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Rao, A. V. et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 1, 6 (2009).
Sullivan, A., Nord, C. E. & Evengard, B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr. J. 8, 4 (2009).
Singh, P. K. et al. Role of Lactobacillus acidophilus loaded floating beads in chronic fatigue syndrome: behavioral and biochemical evidences. Neurogastroenterol. Motil. 24, 366 (2012).
Samsel, A. & Seneff, S. Glyphosate, pathways to modern diseases III: manganese, neurological diseases, and associated pathologies. Surg. Neurol. Int. 6, 45 (2015).
Galland, L. The gut microbiome and the brain. J. Med. Food 17, 1261–1272 (2014).
Steenbergen, L. et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 48, 258–264 (2015).
Huang, R., Wang, K. & Hu, J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients 8, E483 (2016).
Scholten, A. M. et al. Surplus vitamin B12 use does not reduce fatigue in patients with Irritable Bowel Syndrome or inflammatory bowel disease: a randomized double-blind placebo-controlled trial. Clin. Nutr. ESPEN 23, 48–53 (2018).
Miheller, P. et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm. Bowel Dis. 15, 1656–1662 (2009).
Jorgensen, S. P. et al. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 32, 377–383 (2010).
Yang, L. et al. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin. Transl Gastroenterol. 4, e33 (2013).
Lima, G. L. et al. Vitamin D supplementation in adolescents and young adults with juvenile systemic lupus erythematosus for improvement in disease activity and fatigue scores: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res. (Hoboken) 68, 91–98 (2016).
Wells, C. W. et al. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm. Bowel Dis. 12, 123–130 (2006).
Verdon, F. et al. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ 326, 1124 (2003).
Bilski, J. et al. Moderate exercise training attenuates the severity of experimental rodent colitis: the importance of crosstalk between adipose tissue and skeletal muscles. Mediators Inflamm. 2015, 605071 (2015).
van Weert, E. et al. Cancer-related fatigue: predictors and effects of rehabilitation. Oncologist 11, 184–196 (2006).
Vermaete, N. et al. Physical activity, physical fitness and the effect of exercise training interventions in lymphoma patients: a systematic review. Ann. Hematol. 92, 1007–1021 (2013).
van den Berg-Emons, R. J. et al. Fatigue after liver transplantation: effects of a rehabilitation program including exercise training and physical activity counseling. Phys. Ther. 94, 857–865 (2014).
Puetz, T. W., Flowers, S. S. & O’Connor, P. J. A randomized controlled trial of the effect of aerobic exercise training on feelings of energy and fatigue in sedentary young adults with persistent fatigue. Psychother. Psychosom. 77, 167–174 (2008).
Nathan, I. et al. Exercise in individuals with inflammatory bowel disease. Gastroenterol. Nurs. 36, 437–442 (2013).
Chan, D. et al. Inflammatory bowel disease and exercise: results of a Crohn’s and Colitis UK survey. Frontline Gastroenterol. 5, 44–48 (2014).
Minderhoud, I. M., Samsom, M. & Oldenburg, B. Crohn’s disease, fatigue, and infliximab: is there a role for cytokines in the pathogenesis of fatigue? World J. Gastroenterol. 13, 2089–2093 (2007).
Romkens, T. E. et al. High prevalence of fatigue in inflammatory bowel disease: a case control study. J. Crohns Colitis 5, 332–337 (2011).
Grimstad, T. et al. Fatigue in newly diagnosed inflammatory bowel disease. J. Crohns Colitis 9, 725–730 (2015).
Hashash, J. G. et al. Quality of sleep and coexistent psychopathology have significant impact on fatigue burden in patients with inflammatory bowel disease. J. Clin. Gastroenterol. 52, 423–430 (2018).
Guyatt, G. et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 96, 804–810 (1989).
Jelsness-Jørgensen, L.-P. The Fatigue Questionnaire has a good test-retest profile in IBD. Aliment. Pharmacol. Ther. 35, 621–622 (2012).
Tack, B. Dimensions and correlates of fatigue in older adults with rheumatoid arthritis. Thesis, School of Nursing, Univ. California (1991).
Chalder, T. et al. Development of a fatigue scale. J. Psychosom. Res. 37, 147–153 (1993).
Vercoulen, J. H. et al. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 38, 383–392 (1994).
Fisk, J. D. et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin. Infect. Dis. 18 (Suppl. 1), S79–S83 (1994).
Kalaitzakis, E. et al. Quality of life in short-bowel syndrome: impact of fatigue and gastrointestinal symptoms. Scand. J. Gastroenterol. 43, 1057–1065 (2008).
Piche, T. et al. Impact of functional bowel symptoms on quality of life and fatigue in quiescent Crohn disease and irritable bowel syndrome. Neurogastroenterol. Motil. 22, 626 (2010).
Banovic, I., Gilibert, D. & Cosnes, J. Crohn’s disease and fatigue: constancy and co-variations of activity of the disease, depression, anxiety and subjective quality of life. Psychol. Health Med. 15, 394–405 (2010).
Bol, Y. et al. The contribution of disease severity, depression and negative affectivity to fatigue in multiple sclerosis: a comparison with ulcerative colitis. J. Psychosom. Res. 69, 43–49 (2010).
Lesage, A. C. et al. Results of a national survey on quality of life in inflammatory bowel diseases. Clin. Res. Hepatol. Gastroenterol. 35, 117–124 (2011).
Yellen, S. B. et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manage. 13, 63–74 (1997).
Piper, B. F. et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol. Nurs. Forum 25, 677–684 (1998).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.J.v.d.W. has served on advisory boards for Celltrion and Pfizer. She is supported by research funding from Pfizer, Takeda and Tramedico and has received a speaker fee from Takeda and Jansen. A.N.A. has served on advisory boards for Abbvie, Merck and Takeda. He is supported by research funding from the Crohn’s and Colitis Foundation, NIH and Pfizer. N.Z.B. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borren, N.Z., van der Woude, C.J. & Ananthakrishnan, A.N. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol 16, 247–259 (2019). https://doi.org/10.1038/s41575-018-0091-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0091-9
This article is cited by
-
Notoginsenoside R1 promotes Lgr5+ stem cell and epithelium renovation in colitis mice via activating Wnt/β-Catenin signaling
Acta Pharmacologica Sinica (2024)
-
Depression and active disease are the major risk factors for fatigue and sleep disturbance in inflammatory bowel disease with consequent poor quality of life: Analysis of the interplay between psychosocial factors from the developing world
Indian Journal of Gastroenterology (2024)
-
Profile and quality of life of the adult population in good health according to the level of vitality: European NHWS cross sectional analysis
BMC Public Health (2023)
-
Qualitative and psychometric evaluation of the PROMIS®-Fatigue SF-7a scale to assess fatigue in patients with moderately to severely active inflammatory bowel disease
Journal of Patient-Reported Outcomes (2023)
-
Mindfulness-Based Cognitive Therapy for Fatigue in Patients with Inflammatory Bowel Disease: Results of a Randomized Controlled Trial
Mindfulness (2023)