Abstract
Obesity is caused by a long-term difference between energy intake and expenditure — an imbalance that is seemingly easily restored by increasing exercise and reducing caloric consumption. However, as simple as this solution appears, for many people, losing excess weight is difficult to achieve and even more difficult to maintain. The reason for this difficulty is that energy intake and expenditure, and by extension body weight, are regulated through complex hormonal, neural and metabolic mechanisms that are under the influence of many environmental factors and internal responses. Adding to this complexity, the microorganisms (microbes) that comprise the gut microbiota exert direct effects on the digestion, absorption and metabolism of food. Furthermore, the gut microbiota exerts a miscellany of protective, structural and metabolic effects both on the intestinal milieu and peripheral tissues, thus affecting body weight by modulating metabolism, appetite, bile acid metabolism, and the hormonal and immune systems. In this Review, we outline historical and recent advances in understanding how the gut microbiota is involved in regulating body weight homeostasis. We also discuss the opportunities, limitations and challenges of using gut microbiota-related approaches as a means to achieve and maintain a healthy body weight.
Key points
-
Two decades ago, pioneering studies showed that prebiotic-induced changes in the gut microbiota affect adipose mass in rats.
-
Numerous dietary factors, such as fat, proteins and fibre, shape the gut microbiota and eventually contribute to the regulation of host energy metabolism through specific microbial metabolites.
-
So far, no proof has been provided that one specific bacteria or group of bacteria can predict the onset of obesity or body weight loss in humans.
-
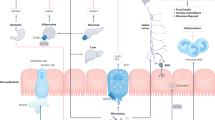
The regulation of host energy metabolism is under the influence of many metabolites produced and/or modified by the gut microbiota, and some of these molecules might become drug candidates.
-
Demonstrating whether particular microbiota compositions are beneficial or detrimental for body weight management remains a challenge and requires future studies to design personalized, targeted modulation of microbiota.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412 (1995).
Gibson, G. R. et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Bäckhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104, 979–984 (2007).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102, 11070–11075 (2005).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Kirk, R. G. “Life in a germ-free world”: isolating life from the laboratory animal to the bubble boy. Bull. Hist. Med. 86, 237–275 (2012).
Evrard, E., Hoet, P. P., Eyssen, H., Charlier, H. & Sacquet, E. Faecal lipids in germ-free and conventional rats. Br. J. Exp. Pathol. 45, 409–414 (1964).
Hoet, P. P. & Eyssen, H. Steatorrhoea in rats with an intestinal Cul-De-Sac. Gut 5, 309–314 (1964).
Goodlad, R. A. et al. Plasma enteroglucagon, gastrin and peptide YY in conventional and germ-free rats refed with a fibre-free or fibre-supplemented diet. Q. J. Exp. Physiol. 74, 437–442 (1989).
Daubioul, C. A., Taper, H. S., De Wispelaere, L. D. & Delzenne, N. M. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese zucker rats. J. Nutr. 130, 1314–1319 (2000).
Daubioul, C. et al. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J. Nutr. 132, 967–973 (2002).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Brown, A. J. et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003).
Nilsson, N. E., Kotarsky, K., Owman, C. & Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 303, 1047–1052 (2003).
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Rastelli, M., Cani, P. D. & Knauf, C. The gut microbiome influences host endocrine functions. Endocr. Rev. 40, 1271–1284 (2019).
Kok, N. N. et al. Insulin, glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide and insulin-like growth factor I as putative mediators of the hypolipidemic effect of oligofructose in rats. J. Nut. 128, 1099–1103 (1998).
Cani, P. D., Dewever, C. & Delzenne, N. M. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br. J. Nutr. 92, 521–526 (2004).
Cani, P. D., Neyrinck, A. M., Maton, N. & Delzenne, N. M. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes. Res 13, 1000–1007 (2005).
Cani, P. D. et al. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55, 1484–1490 (2006).
Delzenne, N. M., Cani, P. D., Daubioul, C. & Neyrinck, A. M. Impact of inulin and oligofructose on gastrointestinal peptides. Br. J. Nutr. 93, S157–S161 (2005).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Duncan, S. H. et al. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 32, 1720–1724 (2008).
Khachatryan, Z. A. et al. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS One 3, e3064 (2008).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Zoetendal, E. G., Akkermans, A. D. & De Vos, W. M. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64, 3854–3859 (1998).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Daoust, L. et al. Gnotobiotic mice housing conditions critically influence the phenotype associated with transfer of faecal microbiota in a context of obesity. Gut https://doi.org/10.1136/gutjnl-2021-326475 (2022).
Cani, P. D. & Knauf, C. Gnotobiotic mice housing conditions makes the difference in the context of obesity! Gut https://doi.org/10.1136/gutjnl-2022-328532 (2022).
Hasan, N. & Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 7, e7502 (2019).
Hu, S. et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 28, 415–431.e4 (2018).
Suriano, F. et al. Fat and not sugar as the determining factor for gut microbiota changes, obesity and related metabolic disorders in mice. Am. J. Physiol. Endocrinol. Metab. https://doi.org/10.1152/ajpendo.00141.2022 (2022).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Cani, P. D. et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50, 2374–2383 (2007).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med 1, 6ra14 (2009).
Hildebrandt, M. A. et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137, 1716–1724 (2009).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Carvalho, B. M. et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 55, 2823–2834 (2012).
Ding, S. et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One 5, e12191 (2010).
Rabot, S. et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 24, 4948–4959 (2010).
Caesar, R., Tremaroli, V., Kovatcheva-Datchary, P., Cani, P. D. & Bäckhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 22, 658–668 (2015).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Dewulf, E. M. et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62, 1112–1121 (2013).
Everard, A. et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60, 2775–2786 (2011).
Cani, P. D., Depommier, C., Derrien, M., Everard, A. & de Vos, W. M. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 19, 625–637 (2022).
Wolters, M. et al. Dietary fat, the gut microbiota, and metabolic health — a systematic review conducted within the MyNewGut project. Clin. Nutr. 38, 2504–2520 (2019).
Bartlett, A. & Kleiner, M. Dietary protein and the intestinal microbiota: an understudied relationship. iScience 25, 105313 (2022).
Beaumont, M. et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 106, 1005–1019 (2017).
Choi, B. S. et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat. Commun. 12, 3377 (2021).
Holmes, A. J. et al. Diet-microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 25, 140–151 (2017).
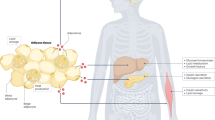
Anhe, F. F. et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2, 233–242 (2020).
Massier, L. et al. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut 69, 1796–1806 (2020).
Velmurugan, G., Dinakaran, V., Rajendhran, J. & Swaminathan, K. Blood microbiota and circulating microbial metabolites in diabetes and cardiovascular disease. Trends Endocrinol. Metab. 31, 835–847 (2020).
Cani, P. D. & Van Hul, M. Microbial signatures in metabolic tissues: a novel paradigm for obesity and diabetes? Nat. Metab. 2, 211–212 (2020).
Regnier, M., Van Hul, M., Knauf, C. & Cani, P. D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 248, R67–R82 (2021).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455 (2020).
Canfora, E. E., Jocken, J. W. & Blaak, E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591 (2015).
van Deuren, T., Blaak, E. E. & Canfora, E. E. Butyrate to combat obesity and obesity-associated metabolic disorders: Current status and future implications for therapeutic use. Obes. Rev. 23, e13498 (2022).
Chambers, E. S. et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut 68, 1430–1438 (2019).
Byrne, C. S. et al. Effects of inulin propionate ester incorporated into palatable food products on appetite and resting energy expenditure: a randomised crossover study. Nutrients 11, 861 (2019).
Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Makishima, M. et al. Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002).
Ihunnah, C. A., Jiang, M. & Xie, W. Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim. Biophys. Acta 1812, 956–963 (2011).
Wagner, M. et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42, 420–430 (2005).
Kawamata, Y. et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 (2003).
McGlone, E. R. & Bloom, S. R. Bile acids and the metabolic syndrome. Ann. Clin. Biochem. 56, 326–337 (2019).
Tu, J., Wang, Y., Jin, L. & Huang, W. Bile acids, gut microbiota and metabolic surgery. Front. Endocrinol. 13, 929530 (2022).
Ridlon, J. M., Kang, D. J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Ridlon, J. M., Harris, S. C., Bhowmik, S., Kang, D. J. & Hylemon, P. B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7, 22–39 (2016).
Duparc, T. et al. Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut 66, 620–632 (2017).
Lin, H., An, Y., Tang, H. & Wang, Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J. Agric. Food Chem. 67, 3624–3632 (2019).
Li, J. Y. et al. Secondary bile acids mediate high-fat diet-induced upregulation of R-spondin 3 and intestinal epithelial proliferation. JCI Insight 7, e148309 (2022).
de Aguiar Vallim, T. Q., Tarling, E. J. & Edwards, P. A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 17, 657–669 (2013).
Li, M. et al. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 13, 2060 (2022).
van Nierop, F. S. et al. Effects of acute dietary weight loss on postprandial plasma bile acid responses in obese insulin resistant subjects. Clin. Nutr. 36, 1615–1620 (2017).
Cani, P. D. et al. Endocannabinoids–at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 12, 133–143 (2016).
Busquets-Garcia, A., Bolanos, J. P. & Marsicano, G. Metabolic messengers: endocannabinoids. Nat. Metab. 4, 848–855 (2022).
Muccioli, G. G. et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 6, 392 (2010).
Suriano, F. et al. Exploring the endocannabinoidome in genetically obese (ob/ob) and diabetic (db/db) mice: links with inflammation and gut microbiota. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1867, 159056 (2022).
Manca, C. et al. Germ-free mice exhibit profound gut microbiota-dependent alterations of intestinal endocannabinoidome signaling. J. Lipid Res. 61, 70–85 (2020).
Suriano, F. et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: two sides of the same coin. Microbiome 9, 147 (2021).
Geurts, L. et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat. Commun. 6, 6495 (2015).
Everard, A. et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 10, 457 (2019).
Rastelli, M. et al. Intestinal NAPE-PLD contributes to short-term regulation of food intake via gut-to-brain axis. Am. J. Physiol. Endocrinol. Metab. 319, E647–E657 (2020).
Cohen, L. J. et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 549, 48–53 (2017).
Ye, L. et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 29, 179–196.e9 (2021).
Bhattarai, Y. et al. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe 23, 775–785.e5 (2018).
Chimerel, C. et al. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 9, 1202–1208 (2014).
Gao, K., Mu, C. L., Farzi, A. & Zhu, W. Y. Tryptophan metabolism: a link between the gut microbiota and brain. Adv. Nutr. 11, 709–723 (2020).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Natividad, J. M. et al. Impaired Aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 28, 737–749.e4 (2018).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Postal, B. G. et al. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol. Metab. 39, 101007 (2020).
Anhe, F. F., Barra, N. G., Cavallari, J. F., Henriksbo, B. D. & Schertzer, J. D. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 36, 109691 (2021).
Roager, H. M. & Christensen, L. H. Personal diet-microbiota interactions and weight loss. Proc. Nutr. Soc. 81, 243–254 (2022).
Puchalska, P. & Crawford, P. A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 (2017).
Kolb, H. et al. Ketone bodies: from enemy to friend and guardian angel. BMC Med. 19, 313 (2021).
Hersant, H. & Grossberg, G. The ketogenic diet and Alzheimer’s disease. J. Nutr. Health Aging 26, 606–614 (2022).
Dilliraj, L. N. et al. The evolution of ketosis: potential impact on clinical conditions. Nutrients 14, 3613 (2022).
Qi, J. et al. Metagenomics reveals that intravenous injection of beta-hydroxybutyric acid (BHBA) disturbs the nasopharynx microflora and increases the risk of respiratory diseases. Front. Microbiol. 11, 630280 (2020).
Ang, Q. Y. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 181, 1263–1275.e16 (2020).
Van Hul, M. et al. Comparison of the effects of soluble corn fiber and fructooligosaccharides on metabolism, inflammation and gut microbiome of high-fat diet fed mice. Am. J. Physiol. Endocrinol. Metab. 319, E779–E791 (2020).
Anhe, F. F. et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64, 872–883 (2015).
Leclercq, S. et al. Gut microbiota-induced changes in beta-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep. 33, 108238 (2020).
Ellenbroek, J. H. et al. Long-term ketogenic diet causes glucose intolerance and reduced beta- and alpha-cell mass but no weight loss in mice. Am. J. Physiol. Endocrinol. Metab. 306, E552–E558 (2014).
Yuasa, M. et al. Consumption of a low-carbohydrate and high-fat diet (the ketogenic diet) exaggerates biotin deficiency in mice. Nutrition 29, 1266–1270 (2013).
Belda, E. et al. Impairment of gut microbial biotin metabolism and host biotin status in severe obesity: effect of biotin and prebiotic supplementation on improved metabolism. Gut 71, 2463–2480 (2022).
Menotti, A. & Puddu, P. E. How the Seven Countries Study contributed to the definition and development of the Mediterranean diet concept: a 50-year journey. Nutr. Metab. Cardiovasc. Dis. 25, 245–252 (2015).
Hidalgo-Mora, J. J. et al. The Mediterranean diet: a historical perspective on food for health. Maturitas 132, 65–69 (2020).
Sofi, F., Cesari, F., Abbate, R., Gensini, G. F. & Casini, A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ 337, a1344 (2008).
De Filippis, F. et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821 (2016).
Khalili, H. et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut 69, 1637–1644 (2020).
Tsigalou, C. et al. Gut microbiome and Mediterranean diet in the context of obesity. Current knowledge, perspectives and potential therapeutic targets. Metab. Open 9, 100081 (2021).
Meslier, V. et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69, 1258–1268 (2020).
Cani, P. D. & Van Hul, M. Mediterranean diet, gut microbiota and health: when age and calories do not add up! Gut 69, 1167–1168 (2020).
Reynolds, A. et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 393, 434–445 (2019).
Hiel, S. et al. Link between gut microbiota and health outcomes in inulin -treated obese patients: lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin. Nutr. 39, 3618–3628 (2020).
Rodriguez, J. et al. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut 69, 1975–1987 (2020).
Rodriguez, J. et al. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes 12, 1810530 (2020).
Myhrstad, M. C. W., Tunsjo, H., Charnock, C. & Telle-Hansen, V. H. Dietary fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients 12, 859 (2020).
Makki, K., Deehan, E. C., Walter, J. & Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018).
Vinelli, V. et al. Effects of dietary fibers on short-chain fatty acids and gut microbiota composition in healthy adults: a systematic review. Nutrients 14, 2559 (2022).
Rastall, R. A. et al. Structure and function of non-digestible carbohydrates in the gut microbiome. Benef. Microbes 13, 95–168 (2022).
Ozkul, C., Yalinay, M. & Karakan, T. Structural changes in gut microbiome after Ramadan fasting: a pilot study. Benef. Microbes 11, 227–233 (2020).
Ozkul, C., Yalinay, M. & Karakan, T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: a preliminary study on intermittent fasting. Turk. J. Gastroenterol. 30, 1030–1035 (2019).
Su, J. et al. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am. J. Clin. Nutr. 113, 1332–1342 (2021).
Ali, I. et al. Ramadan fasting leads to shifts in human gut microbiota structured by dietary composition. Front. Microbiol. 12, 642999 (2021).
Welton, S. et al. Intermittent fasting and weight loss: systematic review. Can. Fam. Physician 66, 117–125 (2020).
Lowe, D. A. et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern. Med. 180, 1491–1499 (2020).
Hjorth, M. F. et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int. J. Obes. 43, 149–157 (2019).
Cani, P. D. & Van Hul, M. Do diet and microbes really ‘PREDICT’ cardiometabolic risks? Nat. Rev. Endocrinol. 17, 259–260 (2021).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Murphy, E. F. et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59, 1635–1642 (2010).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Franz, M. J. et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 107, 1755–1767 (2007).
Son, J. W. & Kim, S. Comprehensive review of current and upcoming anti-obesity drugs. Diabetes Metab. J. 44, 802–818 (2020).
Wu, H. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858 (2017).
de la Cuesta-Zuluaga, J. et al. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40, 54–62 (2017).
Jin, J. et al. Distinctive gut microbiota in patients with overweight and obesity with dyslipidemia and its responses to long-term orlistat and ezetimibe intervention: a randomized controlled open-label trial. Front. Pharm. 12, 732541 (2021).
Jin, J. et al. Orlistat and ezetimibe could differently alleviate the high-fat diet-induced obesity phenotype by modulating the gut microbiota. Front. Microbiol. 13, 908327 (2022).
Ke, J. et al. Orlistat-induced gut microbiota modification in obese mice. Evid. Based Complement. Altern. Med. 2020, 9818349 (2020).
Wang, L., Li, P., Tang, Z., Yan, X. & Feng, B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci. Rep. 6, 33251 (2016).
Wang, Z. et al. Gut microbiome differences between metformin- and liraglutide-treated T2DM subjects. Endocrinol. Diabetes Metab. 1, e00009 (2018).
Mulla, C. M., Middelbeek, R. J. W. & Patti, M. E. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. N. Y. Acad. Sci. 1411, 53–64 (2018).
Valenti, V., Cienfuegos, J. A., Becerril Manas, S. & Fruhbeck, G. Mechanism of bariatric and metabolic surgery: beyond surgeons, gastroenterologists and endocrinologists. Rev. Esp. Enferm. Dig. 112, 229–233 (2020).
Anhe, F. F. et al. Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity. Gut https://doi.org/10.1136/gutjnl-2022-328185 (2022).
Gutierrez-Repiso, C. et al. Predictive role of gut microbiota in weight loss achievement after bariatric surgery. J. Am. Coll. Surg. 234, 861–871 (2022).
Ben Izhak, M. et al. Projection of gut microbiome pre- and post-bariatric surgery to predict surgery outcome. mSystems 6, e0136720 (2021).
Crommen, S., Mattes, A. & Simon, M. C. Microbial adaptation due to gastric bypass surgery: the nutritional impact. Nutrients 12, 1199 (2020).
Debedat, J., Clement, K. & Aron-Wisnewsky, J. Gut microbiota dysbiosis in human obesity: impact of bariatric surgery. Curr. Obes. Rep. 8, 229–242 (2019).
Tremaroli, V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238 (2015).
Dao, M. C. et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am. J. Physiol. Endocrinol. Metab. 317, E446–E459 (2019).
Lau, E. et al. Gut microbiota changes after metabolic surgery in adult diabetic patients with mild obesity: a randomised controlled trial. Diabetol. Metab. Syndr. 13, 56 (2021).
Saffouri, G., Pardi, D., Kashyap, P. & Khanna, S. Body mass index changes after fecal microbiota transplant for recurrent Clostridium difficile infection: 216. Am. J. Gastroenterol. 111, S103 (2016).
Zhang, Z. et al. Impact of fecal microbiota transplantation on obesity and metabolic syndrome — a systematic review. Nutrients 11, 2291 (2019).
Proenca, I. M. et al. Fecal microbiota transplantation improves metabolic syndrome parameters: systematic review with meta-analysis based on randomized clinical trials. Nutr. Res. 83, 1–14 (2020).
Yu, E. W. et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med 17, e1003051 (2020).
Hartstra, A. V. et al. Infusion of donor feces affects the gut-brain axis in humans with metabolic syndrome. Mol. Metab. 42, 101076 (2020).
Benitez-Paez, A., Hartstra, A. V., Nieuwdorp, M. & Sanz, Y. Species- and strain-level assessment using rrn long-amplicons suggests donor’s influence on gut microbial transference via fecal transplants in metabolic syndrome subjects. Gut Microbes 14, 2078621 (2022).
Wilson, B. C. et al. Strain engraftment competition and functional augmentation in a multi-donor fecal microbiota transplantation trial for obesity. Microbiome 9, 107 (2021).
Ng, S. C. et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut 71, 716–723 (2022).
Mocanu, V. et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat. Med. 27, 1272–1279 (2021).
Thaiss, C. A. et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540, 544–551 (2016).
Rinott, E. et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology 160, 158–173.e10 (2021).
Udayappan, S. et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2, 16009 (2016).
Koopen, A. et al. Duodenal Anaerobutyricum soehngenii infusion stimulates GLP-1 production, ameliorates glycaemic control and beneficially shapes the duodenal transcriptome in metabolic syndrome subjects: a randomised double-blind placebo-controlled cross-over study. Gut 71, 1577–1587 (2022).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7 (2012).
Huber-Ruano, I. et al. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome 10, 135 (2022).
Pujo, J. et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 70, 1088–1097 (2021).
Romani-Perez, M. et al. Holdemanella biformis improves glucose tolerance and regulates GLP-1 signaling in obese mice. FASEB J. 35, e21734 (2021).
Gomez Del Pulgar, E. M., Benitez-Paez, A. & Sanz, Y. Safety assessment of bacteroides uniformis CECT 7771, a symbiont of the gut microbiota in infants. Nutrients 12, 551 (2020).
Romani-Perez, M., Agusti, A. & Sanz, Y. Innovation in microbiome-based strategies for promoting metabolic health. Curr. Opin. Clin. Nutr. Metab. Care 20, 484–491 (2017).
Rasmussen, T. S. et al. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut 69, 2122–2130 (2020).
Shkoporov, A. N. et al. Reproducible protocols for metagenomic analysis of human faecal phageomes. Microbiome 6, 68 (2018).
Yang, K. et al. Alterations in the gut virome in obesity and type 2 diabetes mellitus. Gastroenterology 161, 1257–1269.e13 (2021).
Shareefdeen, H. & Hill, C. The gut virome in health and disease: new insights and associations. Curr. Opin. Gastroenterol. 38, 549–554 (2022).
Acknowledgements
P.D.C. is research director at FRS-FNRS (Fonds de la Recherche Scientifique) and is the recipient of grants from FNRS (Projet de Recherche PDR-convention: FNRS T.0030.21, CDR-convention: J.0027.22, FRFS-WELBIO: WELBIO-CR-2022A-02, EOS: program no. 40007505) and ARC (action de recherche concertée: ARC19/24-096) and La Caixa (NeuroGut).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
P.D.C. is an inventor on patent applications dealing with the use of specific bacteria and components in the treatment of different diseases. P.D.C. was co-founder of The Akkermansia Company SA and Enterosys. M.V.H. declares no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Yolanda Sanz, Jonathan Schertzer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Hul, M., Cani, P.D. The gut microbiota in obesity and weight management: microbes as friends or foe?. Nat Rev Endocrinol 19, 258–271 (2023). https://doi.org/10.1038/s41574-022-00794-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-022-00794-0
This article is cited by
-
Gut microbiota in overweight and obesity: crosstalk with adipose tissue
Nature Reviews Gastroenterology & Hepatology (2024)
-
Resisting weight gain with prebiotic fibre
Nature Metabolism (2024)
-
New dawn of ginsenosides: regulating gut microbiota to treat metabolic syndrome
Phytochemistry Reviews (2024)
-
Impact of metformin and Dysosmobacter welbionis on diet-induced obesity and diabetes: from clinical observation to preclinical intervention
Diabetologia (2024)
-
A comprehensive review of genetic causes of obesity
World Journal of Pediatrics (2024)