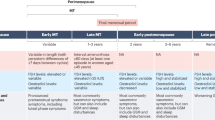
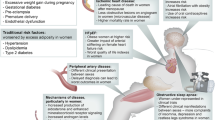
Abstract
The menopausal transition is an impactful period in women’s lives, when the risk of cardiovascular disease is accelerated. Similarly, diabetes mellitus profoundly impacts cardiovascular risk. However, the interplay between menopause and diabetes mellitus has not been adequately studied. The menopausal transition is accompanied by metabolic changes that predispose to diabetes mellitus, particularly type 2 diabetes mellitus (T2DM), as menopause results in increased risk of upper body adipose tissue accumulation and increased incidence of insulin resistance. Equally, diabetes mellitus can affect ovarian ageing, potentially causing women with type 1 diabetes mellitus and early-onset T2DM to experience menopause earlier than women without diabetes mellitus. Earlier age at menopause has been associated with a higher risk of T2DM later in life. Menopausal hormone therapy can reduce the risk of T2DM and improve glycaemic control in women with pre-existing diabetes mellitus; however, there is not enough evidence to support the administration of menopausal hormone therapy for diabetes mellitus prevention or control. This Review critically appraises studies published within the past few years on the interaction between diabetes mellitus and menopause and addresses all clinically relevant issues, such as the effect of menopause on the development of T2DM, and the management of both menopause and diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report. cdc.gov https://www.cdc.gov/diabetes/data/statistics-report/index.html (2020).
Mauvais-Jarvis, F., Manson, J. A. E., Stevenson, J. C. & Fonseca, V. A. Menopausal hormone therapy and type 2 diabetes prevention: Evidence, mechanisms, and clinical implications. Endocr. Rev. 38, 173–188 (2017). This review describes the mechanisms by which menopausal hormone therapy reduces the risk of incident T2DM.
Park, S. U., Walsh, L. & Berkowitz, K. M. Mechanisms of ovarian aging. Reproduction 162, R19–R33 (2021).
Anagnostis, P. et al. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur. J. Endocrinol. 180, 41–50 (2019).
Paschou, S. et al. Diabetes in menopause: risks and management. Curr. Vasc. Pharmacol. 17, 556–563 (2018).
Vryonidou, A., Paschou, S. A., Muscogiuri, G., Orio, F. & Goulis, D. G. Mechanisms in endocrinology: metabolic syndrome through the female life cycle. Eur. J. Endocrinol. 173, R153–R163 (2015).
LeBlanc, E. S. et al. Reproductive history and risk of type 2 diabetes mellitus in postmenopausal women: findings from the Women’s Health Initiative. Menopause 24, 64–72 (2017). Women with a shorter reproductive lifespan (<30 years) have a 37% higher risk of T2DM than women with a normal reproductive lifespan (36–40 years).
Tatulashvili, S. et al. Gonadal hormonal factors before menopause and incident type 2 diabetes in women: a 22-year follow-up of 83 799 women from the E3N cohort study. J. Diabetes 13, 330–338 (2021).
Slopien, R. et al. Menopause and diabetes: EMAS clinical guide. Maturitas 117, 6–10 (2018). This clinical guide describes the individualization of menopausal hormone therapy in women with diabetes mellitus.
World Health Organization. Diabetes. WHO.int https://www.who.int/news-room/fact-sheets/detail/diabetes (2021).
Paschou, S. A. et al. Therapeutic strategies for type 2 diabetes mellitus in women after menopause. Maturitas 126, 69–72 (2019).
Lambrinoudaki, I. et al. Premature ovarian insufficiency: a toolkit for the primary care physician. Maturitas 147, 53–63 (2021).
Skurnick, J. H., Weiss, G., Goldsmith, L. T., Santoro, N. & Crawford, S. Longitudinal changes in hypothalamic and ovarian function in perimenopausal women with anovulatory cycles: relationship with vasomotor symptoms. Fertil. Steril. 91, 1127–1134 (2009).
Freedman, R. R. Physiology of hot flashes. Am. J. Hum. Biol. 13, 453–464 (2001).
Rance, N. E., Dacks, P. A., Mittelman-Smith, M. A., Romanovsky, A. A. & Krajewski-Hall, S. J. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front. Neuroendocrinol. 34, 211–227 (2013).
Padilla, S. L., Johnson, C. W., Barker, F. D., Patterson, M. A. & Palmiter, R. D. A neural circuit underlying the generation of hot flushes. Cell Rep. 24, 271–277 (2018).
Freedman, R. R. Menopausal hot flashes: mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 142, 115–120 (2014).
Berendsen, H. H. The role of serotonin in hot flushes. Maturitas 36, 155–164 (2000).
Sipe, K. et al. Serotonin 2A receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res. 1028, 191–202 (2004).
Davis, S. R. et al. Menopause. Nat. Rev. Dis. Prim. 1, 15004 (2015).
[No authors listed]. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause 27, 976–992 (2020).
Rozenberg, S. et al. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos. Int. 31, 2271–2286 (2020).
Gabet, A., Danchin, N., Juillière, Y. & Olié, V. Acute coronary syndrome in women: rising hospitalizations in middle-aged French women, 2004–14. Eur. Heart J. 38, 1060–1065 (2017).
Institute for Health Metrics and Evaluation. GBD: Results. IHME http://ghdx.healthdata.org/gbd-results-tool (2021).
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016).
Maas, A. H. E. M. et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 42, 967–984 (2021).
InterLACE Study Team. Variations in reproductive events across life: a pooled analysis of data from 505 147 women across 10 countries. Hum. Reprod. 34, 881–893 (2019).
Tal, R. et al. AMH highly correlates with cumulative live birth rate in women with diminished ovarian reserve independent of age. J. Clin. Endocrinol. Metab. 106, 2754–2766 (2021).
de Kat, A. C. et al. Can menopause prediction be improved with multiple AMH measurements? Results from the prospective Doetinchem Cohort Study. J. Clin. Endocrinol. Metab. 104, 5024–5031 (2019).
Santoro, N., Roeca, C., Peters, B. A. & Neal-Perry, G. The menopause transition: signs, symptoms, and management options. J. Clin. Endocrinol. Metab. 106, 1–15 (2021).
Dorman, J. S. et al. Menopause in type 1 diabetic women: is it premature? Diabetes 50, 1857–1862 (2001).
Brand, J. S. et al. Diabetes and onset of natural menopause: results from the European Prospective Investigation into Cancer and Nutrition. Hum. Reprod. 30, 1491–1498 (2015). This study evaluates the effect of pre-existing diabetes mellitus on the timing of natural menopause.
Yi, Y. et al. Women with type 1 diabetes (T1D) experience a shorter reproductive period compared with nondiabetic women: the Pittsburgh Epidemiology of Diabetes Complications (EDC) study and the Study of Women’s Health Across the Nation (SWAN). Menopause 28, 634–641 (2021).
Paschou, S. A. et al. Menstrual disorders and androgen-related traits in young women with type : a clinical study. Endocr. Pract. 26, 1269–1276 (2020).
Sjöberg, L. et al. Menopause in women with type 1 diabetes. Menopause 18, 158–163 (2011).
Yi, Y. et al. Association of age at diabetes complication diagnosis with age at natural menopause in women with type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study. J. Diabetes Complicat. 35, 107832 (2021).
Yarde, F. et al. Age at menopause in women with type 1 diabetes mellitus: the OVADIA study. Hum. Reprod. 30, 441–446 (2015).
Kim, C. et al. Effect of glycemic treatment and microvascular complications on menopause in women with type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort. Diabetes Care 37, 701–708 (2014).
Khalil, N. et al. Menopausal bone changes and incident fractures in diabetic women: a cohort study. Osteoporos. Int. 22, 1367–1376 (2011).
Monterrosa-Castro, A. et al. Type II diabetes mellitus and menopause: a multinational study. Climacteric 16, 663–672 (2013).
Gold, E. B. et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am. J. Epidemiol. 178, 70–83 (2013).
Aydin, Z. D. Determinants of age at natural menopause in the Isparta Menopause and Health Study: premenopausal body mass index gain rate and episodic weight loss. Menopause 17, 494–505 (2010).
Fogle, R. H., Stanczyk, F. Z., Zhang, X. & Paulson, R. J. Ovarian androgen production in postmenopausal women. J. Clin. Endocrinol. Metab. 92, 3040–3043 (2007).
Goossens, G. H., Jocken, J. W. E. & Blaak, E. E. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 17, 47–66 (2021).
Carr, M. C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 88, 2404–2411 (2003).
Greendale, G. A. et al. Changes in body composition and weight during the menopause transition. JCI Insight 4, e124865 (2019).
Samargandy, S. et al. Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: the SWAN heart study. Menopause 28, 626–633 (2021).
Pu, D., Tan, R., Yu, Q. & Wu, J. Metabolic syndrome in menopause and associated factors: a meta-analysis. Climacteric 20, 583–591 (2017).
Lovejoy, J. C., Champagne, C. M., De Jonge, L., Xie, H. & Smith, S. R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 32, 949–958 (2008).
Lee, C. G. et al. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J. Clin. Endocrinol. Metab. 94, 1104–1110 (2009).
Guthrie, J. R., Dennerstein, L. & Dudley, E. C. Weight gain and the menopause: a 5-year prospective study. Climacteric 2, 205–211 (1999).
Rogers, N. H., Perfield, J. W. II, Strissel, K. J., Obin, M. S. & Greenberg, A. S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150, 2161–2168 (2009).
Heine, P. A., Taylor, J. A., Iwamoto, G. A., Lubahn, D. B. & Cooke, P. S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc. Natl Acad. Sci. USA 97, 12729–12734 (2000).
Ainslie, D. A. et al. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int. J. Obes. Relat. Metab. Disord. 25, 1680–1688 (2001).
Geraci, A. et al. Sarcopenia and menopause: the role of estradiol. Front. Endocrinol. 12, 682012 (2021).
Stute, P. et al. Management of depressive symptoms in peri- and postmenopausal women: EMAS position statement. Maturitas 131, 91–101 (2020).
de Mutsert, R. et al. Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: the Netherlands Epidemiology of Obesity Study. Metab. Syndr. Relat. Disord. 16, 54–63 (2018).
Janssen, I., Powell, L. H., Kazlauskaite, R. & Dugan, S. A. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity 18, 604–610 (2010).
Zhu, L. et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62, 424–434 (2013).
Tiano, J. P. & Mauvais-Jarvis, F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat. Rev. Endocrinol. 8, 342–351 (2012).
Mauvais-Jarvis, F. Role of sex steroids in β cell function, growth, and survival. Trends Endocrinol. Metab. 27, 844–855 (2016).
Crețu, D., Cernea, S., Onea, C. R. & Pop, R.-M. Reproductive health in women with type 2 diabetes mellitus. Hormones 19, 291–300 (2020).
Matthews, K. A., Gibson, C. J., El Khoudary, S. R. & Thurston, R. C. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: Study of Women’s Health Across the Nation. J. Am. Coll. Cardiol. 62, 191–200 (2013).
Matthews, K. A. et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J. Am. Coll. Cardiol. 54, 2366–2373 (2009).
Park, S. K. et al. Association between changes in oestradiol and follicle-stimulating hormone levels during the menopausal transition and risk of diabetes. Diabet. Med. 34, 531–538 (2017).
Brand, J. S. et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 36, 1012–1019 (2013). This study demonstrates that premature ovarian insufficiency increases the risk of T2DM by 32%.
Shen, T. Y., Strong, C. & Yu, T. Age at menopause and mortality in Taiwan: a cohort analysis. Maturitas 136, 42–48 (2020).
Heianza, Y. et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care 36, 4007–4014 (2013).
Appiah, D., Winters, S. J. & Hornung, C. A. Bilateral oophorectomy and the risk of incident diabetes in postmenopausal women. Diabetes Care 37, 725–733 (2014).
Mishra, S. R., Waller, M., Chung, H.-F. & Mishra, G. D. Epidemiological studies of the association between reproductive lifespan characteristics and risk of type 2 diabetes and hypertension: a systematic review. Maturitas 155, 14–23 (2022).
Duncan, A. C. et al. The effect of estradiol and a combined estradiol/progestagen preparation on insulin sensitivity in healthy postmenopausal women. J. Clin. Endocrinol. Metab. 84, 2402–2407 (1999).
Gray, K. E. et al. Vasomotor symptom characteristics: are they risk factors for incident diabetes? Menopause 25, 520–530 (2018). The risk of T2DM is higher in women with severe vasomotor symptoms than in women without severe vasomotor symptoms.
Herber-Gast, G. C. M. & Mishra, G. D. Early severe vasomotor menopausal symptoms are associated with diabetes. Menopause 21, 855–860 (2014).
Thurston, R. C., Chang, Y., Mancuso, P. & Matthews, K. A. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: findings from the Study of Women’s Health Across the Nation. Fertil. Steril. 100, 793–800 (2013).
Reeves, A. N. et al. Symptom clusters predict risk of metabolic-syndrome and diabetes in midlife: the Study of Women’s Health Across the Nation. Ann. Epidemiol. 58, 48–55 (2021).
Katainen, R. E., Engblom, J. R., Siirtola, T. J., Erkkola, R. U. & Polo-Kantola, P. Climacteric symptoms in middle-aged women with chronic somatic diseases. Maturitas 86, 17–24 (2016).
Monteleone, P., Mascagni, G., Giannini, A., Genazzani, A. R. & Simoncini, T. Symptoms of menopause – global prevalence, physiology and implications. Nat. Rev. Endocrinol. 14, 199–215 (2018).
Ma, Y. et al. All-cause, cardiovascular, and cancer mortality rates in postmenopausal white, black, Hispanic, and Asian women with and without diabetes in the United States: the Women’s Health Initiative, 1993–2009. Am. J. Epidemiol. 178, 1533–1541 (2013).
Wang, Y. et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 17, 136 (2019).
Peters, S. A. E., Huxley, R. R. & Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 57, 1542–1551 (2014).
Yetkin, E. et al. Diabetes mellitus and female gender are the strongest predictors of poor collateral vessel development in patients with severe coronary artery stenosis. Angiogenesis 18, 201–207 (2015).
Malmborg, M. et al. Does type 2 diabetes confer higher relative rates of cardiovascular events in women compared with men? Eur. Heart J. 41, 1346–1353 (2020). Despite higher absolute rates of cardiovascular disease in men, women have higher relative rates of cardiovascular disease associated with diabetes mellitus than men.
Angoulvant, D. et al. Impact of gender on relative rates of cardiovascular events in patients with diabetes. Diabetes Metab. 47, 101226 (2021).
Policardo, L. et al. Gender difference in diabetes-associated risk of first-ever and recurrent ischemic stroke. J. Diabetes Complicat. 29, 713–717 (2015).
Bancks, M. P. et al. Sex differences in cardiovascular risk factors before and after the development of type 2 diabetes and risk for incident cardiovascular disease. Diabetes Res. Clin. Pract. 166, 108334 (2020).
Gersh, F. L., O’Keefe, J. H., Lavie, C. J. & Henry, B. M. The renin-angiotensin-aldosterone system in postmenopausal women: the promise of hormone therapy. Mayo Clin. Proc. 96, 3130–3141 (2021).
Yoshida, Y. et al. Early menopause and cardiovascular disease risk in women with or without type 2 diabetes: a pooled analysis of 9,374 postmenopausal women. Diabetes Care 44, 2564–2572 (2021).
Greendale, G. A. et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from The Study of Women’s Health Across the Nation (SWAN). J. Bone Miner. Res. 27, 111–118 (2012).
Anagnostis, P., Bosdou, J. K., Vaitsi, K., Goulis, D. G. & Lambrinoudaki, I. Estrogen and bones after menopause: a reappraisal of data and future perspectives. Hormones 20, 13–21 (2021).
Hough, F. S., Pierroz, D. D., Cooper, C. & Ferrari, S. L. Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur. J. Endocrinol. 174, R127–R138 (2016).
Shanbhogue, V. V., Mitchell, D. M., Rosen, C. J. & Bouxsein, M. L. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 4, 159–173 (2016).
Hanley, D. et al. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross-sectional results from the Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 18, 784–790 (2003).
Chen, F. P., Kuo, S. F., Lin, Y. C., Fan, C. M. & Chen, J. F. Status of bone strength and factors associated with vertebral fracture in postmenopausal women with type 2 diabetes. Menopause 26, 182–188 (2019).
Bonds, D. E. et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 91, 3404–3410 (2006). Women with diabetes mellitus have 20% higher fracture risk than women without diabetes mellitus, independently of baseline BMD.
Paul, J. et al. Do proximal hip geometry, trabecular microarchitecture, and prevalent vertebral fractures differ in postmenopausal women with type 2 diabetes mellitus? A cross-sectional study from a teaching hospital in southern India. Osteoporos. Int. 32, 1585–1593 (2021).
Dytfeld, J. & Michalak, M. Type 2 diabetes and risk of low-energy fractures in postmenopausal women: meta-analysis of observational studies. Aging Clin. Exp. Res. 29, 301–309 (2017).
Janghorbani, M., Feskanich, D., Willett, W. C. & Hu, F. Prospective study of diabetes and risk of hip fracture: the Nurses’ Nealth Study. Diabetes Care 29, 1573–1578 (2006).
Almutlaq, N., Neyman, A. & DiMeglio, L. A. Are diabetes microvascular complications risk factors for fragility fracture? Curr. Opin. Endocrinol. Diabetes Obes. 28, 354–359 (2021).
Thong, E. P. et al. The diabetes-fracture association in women with type 1 and type 2 diabetes is partially mediated by falls: a 15-year longitudinal study. Osteoporos. Int. 32, 1175–1184 (2021).
Stuenkel, C. A. et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 3975–4011 (2015).
Margolis, K. L. et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47, 1175–1187 (2004). Menopausal hormone therapy reduces the risk of incident T2DM in women without T2DM at baseline.
Kanaya, A. M. et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 138, 1–9 (2003).
Espeland, M. A. et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. Diabetes Care 21, 1589–1595 (1998).
Salpeter, S. R. et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes, Obes. Metab. 8, 538–554 (2006).
Manson, J. A. E. et al. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Ann. Epidemiol. 2, 665–673 (1992).
De Lauzon-Guillain, B. et al. Menopausal hormone therapy and new-onset diabetes in the French Etude Epidemiologique de Femmes de la Mutuelle Générale de l’Education Nationale (E3N) cohort. Diabetologia 52, 2092–2100 (2009).
Mattiasson, I., Rendell, M., Törnquist, C., Jeppsson, S. & Hulthén, U. L. Effects of estrogen replacement therapy on abdominal fat compartments as related to glucose and lipid metabolism in early postmenopausal women. Horm. Metab. Res. 34, 583–588 (2002).
Armeni, E. & Lambrinoudaki, I. Androgens and cardiovascular disease in women and men. Maturitas 104, 54–72 (2017).
Kim, J. E. et al. Associations of postmenopausal hormone therapy with metabolic syndrome among diabetic and non-diabetic women. Maturitas 121, 76–82 (2019).
Brussaard, H. E., Gevers Leuven, J. A., Frölich, M., Kluft, C. & Krans, H. M. J. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia 40, 843–849 (1997).
Friday, K. E., Dong, C. & Fontenot, R. U. Conjugated equine estrogen improves glycemic control and blood lipoproteins in postmenopausal women with type 2 diabetes 1. J. Clin. Endocrinol. Metab. 86, 48–52 (2001).
Andersson, B. et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus 1. J. Clin. Endocrinol. Metab. 82, 638–643 (1997).
Kim, J.-E. et al. Effects of menopausal hormone therapy on cardiovascular diseases and type 2 diabetes in middle-aged postmenopausal women: analysis of the Korea National Health Insurance Service Database. Menopause 28, 1225–1232 (2021).
Mackay, L., Kilbride, L., Adamson, K. A. & Chisholm, J. Hormone replacement therapy for women with type 1 diabetes mellitus. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD008613.pub2 (2013).
Depypere, H. et al. A randomized trial on the effect of oral combined estradiol and drospirenone on glucose and insulin metabolism in healthy menopausal women with a normal oral glucose tolerance test. Maturitas 138, 36–41 (2020).
Nie, L. et al. C-reactive protein mediates the effect of serum progesterone on obesity for men and postmenopausal women in Henan rural cohort study. J. Inflamm. Res. 14, 633–644 (2021).
Godsland, I. F. et al. Effects of low and high dose oestradiol and dydrogesterone therapy on insulin and lipoprotein metabolism in healthy postmenopausal women. Clin. Endocrinol. 60, 541–549 (2004).
Davidson, M. H. et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch. Intern. Med. 160, 3315–3325 (2000).
Shufelt, C. L. & Manson, J. E. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J. Clin. Endocrinol. Metab. 106, 1245–1254 (2021). Transdermal oestrogens pose a lower risk of venous thromboembolism than oral oestrogens and micronized progesterone does not oppose the beneficial effects of oestrogens on cardiovascular risk factors.
Hulley, S. et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 280, 605–613 (1998).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Anderson, G. L. et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291, 1701–1712 (2004).
Manson, J. A. E. et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 310, 1353–1368 (2013).
Boardman, H. M. P. et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane database Syst. Rev. https://doi.org/10.1002/14651858.CD002229.pub4 (2015).
Hodis, H. N. et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N. Engl. J. Med. 374, 1221–1231 (2016). Menopausal hormone therapy within 6 years of the final menstrual period attenuates the progression of carotid atherosclerosis.
Santen, R. J. Use of cardiovascular age for assessing risks and benefits of menopausal hormone therapy. Menopause 24, 589–595 (2017).
Rovinski, D., Ramos, R. B., Fighera, T. M., Casanova, G. K. & Spritzer, P. M. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb. Res. 168, 83–95 (2018).
Paschou, S. & Papanas, N. Type 2 diabetes mellitus and menopausal hormone therapy: an update. Diabetes Ther. 10, 2313–2320 (2019).
Cosentino, F. et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 41, 255–323 (2020).
Wang, B. et al. Unmasking fracture risk in type 2 diabetes: the association of longitudinal glycemic hemoglobin level and medications. J. Clin. Endocrinol. Metab. 107, e1390–e1401 (2022).
Almourani, R., Chinnakotla, B., Patel, R., Kurukulasuriya, L. R. & Sowers, J. Diabetes and cardiovascular disease: an update. Curr. Diab. Rep. 19, 161 (2019).
American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes 2021. Diabetes Care 44, S111–S124 (2021).
Sousa, G. R. et al. Glycemic control, cardiac autoimmunity, and long-term risk of cardiovascular disease in type 1 diabetes mellitus. Circulation 139, 730–743 (2019).
Giugliano, D., Maiorino, M. I., Bellastella, G., Chiodini, P. & Esposito, K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J. Am. Heart Assoc. 8, e012356 (2019).
Hidayat, K., Fang, Q.-L., Shi, B.-M. & Qin, L.-Q. Influence of glycemic control and hypoglycemia on the risk of fracture in patients with diabetes mellitus: a systematic review and meta-analysis of observational studies. Osteoporos. Int. 32, 1693–1704 (2021).
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 3, 866–875 (2015).
Wing, R. R. et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 369, 145–154 (2013).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 63, 2985–3023 (2014).
Ghaemi, F. et al. Effects of a Mediterranean diet on the development of diabetic complications: a longitudinal study from the nationwide diabetes report of the National Program for Prevention and Control of Diabetes (NPPCD 2016-2020). Maturitas 153, 61–67 (2021).
Elhayany, A., Lustman, A., Abel, R., Attal-Singer, J. & Vinker, S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes. Metab. 12, 204–209 (2010).
Upadhyay, J. et al. Pharmacotherapy of type 2 diabetes: an update. Metab.: Clin. Exp. 78, 13–42 (2018).
Buse, J. B. et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 43, 487–493 (2020).
Singh, A. K. & Singh, R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: a systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab. Syndr. 14, 181–187 (2020).
Raparelli, V. et al. Sex differences in cardiovascular effectiveness of newer glucose-lowering drugs added to metformin in type 2 diabetes mellitus. J. Am. Heart Assoc. 9, e012940 (2020).
O’Donoghue, M. L. et al. The efficacy and safety of dapagliflozin in women and men with type 2 diabetes mellitus. Diabetologia 64, 1226–1234 (2021).
Zinman, B. et al. Empagliflozin in women with type 2 diabetes and cardiovascular disease – an analysis of EMPA-REG OUTCOME®. Diabetologia 61, 1522–1527 (2018).
Rådholm, K., Zhou, Z., Clemens, K., Neal, B. & Woodward, M. Effects of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes in women versus men. Diabetes Obes. Metab. 22, 263–266 (2020).
Simkin-Silverman, L. R., Wing, R. R., Boraz, M. A. & Kuller, L. H. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann. Behav. Med. 26, 212–220 (2003).
Wu, L. et al. Effects of lifestyle intervention improve cardiovascular disease risk factors in community-based menopausal transition and early postmenopausal women in China. Menopause 21, 1263–1268 (2014).
Wilding, J. P. H., Overgaard, R. V., Jacobsen, L. V., Jensen, C. B. & le Roux, C. W. Exposure-response analyses of liraglutide 3.0 mg for weight management. Diabetes Obes. Metab. 18, 491–499 (2016).
Davies, M. J. et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 314, 687–699 (2015).
Petri, K. C. C., Ingwersen, S. H., Flint, A., Zacho, J. & Overgaard, R. V. Exposure-response analysis for evaluation of semaglutide dose levels in type 2 diabetes. Diabetes Obes. Metab. 20, 2238–2245 (2018).
Paschou, S. A. et al. Type 2 diabetes and osteoporosis: a guide to optimal management. J. Clin. Endocrinol. Metab. 102, 3621–3634 (2017).
Bonds, D. E. et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 49, 459–468 (2006).
Pentti, K. et al. Hormone therapy protects from diabetes: the Kuopio osteoporosis risk factor and prevention study. Eur. J. Endocrinol. 160, 979–983 (2009).
Gartlehner, G. et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women evidence report and systematic review for the US Preventive Services Task Force. JAMA 318, 2234–2249 (2017).
Prentice, R. L. et al. Dual-outcome intention-to-treat analyses in the Women’s Health Initiative randomized controlled hormone therapy trials. Am. J. Epidemiol. 189, 972–981 (2020).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.A.P. has participated in clinical trials sponsored by NovoNordisk, Sanofi and Eli Lilly, and has received honoraria for advisory board membership or lectures from NovoNordisk, Sanofi, Bausch Health and Abbott. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Michael A. Nauck; Martha Hickey, who co-reviewed with Sarah Price; and the other, anonymous reviewer(s) for their contribution to the peer review of this work
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
A search was undertaken in the electronic databases PubMed (MEDLINE), Scopus, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) for English-language publications through to 31 December 2021 using the following search terms: menopause; menopausal hormone therapy; diabetes mellitus; type 1 diabetes; type 2 diabetes; diabetes therapy; obesity; cardiovascular disease; cardiovascular risk factors. The literature search focused on systematic reviews and meta-analyses, randomized controlled trials (RCTs) and position statements. Further references, after a manual search in key journals in the fields of endocrinology, diabetology, menopause, cardiology and angiology, were also included.
Rights and permissions
About this article
Cite this article
Lambrinoudaki, I., Paschou, S.A., Armeni, E. et al. The interplay between diabetes mellitus and menopause: clinical implications. Nat Rev Endocrinol 18, 608–622 (2022). https://doi.org/10.1038/s41574-022-00708-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-022-00708-0
This article is cited by
-
The 100 top-cited articles in menopausal syndrome: a bibliometric analysis
Reproductive Health (2024)
-
Insights and implications of sexual dimorphism in osteoporosis
Bone Research (2024)
-
Menopausal Hormone Therapy in Women with Type 2 Diabetes Mellitus: An Updated Review
Diabetes Therapy (2024)
-
Gonadal dysfunction in women with diabetes mellitus
Endocrine (2024)
-
Recognizing the importance of ovarian aging research
Nature Aging (2022)